Stabilization and Anticancer Enhancing Activity of the Peptide Nisin by Cyclodextrin-Based Nanosponges against Colon and Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of β-Cyclodextrin-Nanosponges
2.2.1. Preparation of Nisin-Loaded β-CDNSs and Coumarin-6 CDNSs
2.2.2. Determination of Nisin Content in β-CDNSs
2.2.3. Size Characterization of Synthesized β-CDNSs
2.2.4. Differential Scanning Calorimetry Analysis
2.2.5. In Vitro Studies of Nisin Release
2.3. Screening of Biological Activity
2.3.1. Cell Culturing Conditions
2.3.2. Cellular Uptake Study
2.3.3. In Vitro Cytotoxicity Assays
2.3.4. MTT (3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyl Tetrazolium Bromide) Assay
2.3.5. Lactate Dehydrogenase (LDH) Assay
2.3.6. Determine the Apoptosis Assay
2.3.7. Tricine-SDS-PAGE
2.4. Statistical Analysis
3. Results
3.1. Characterization Results
3.2. Release Profile
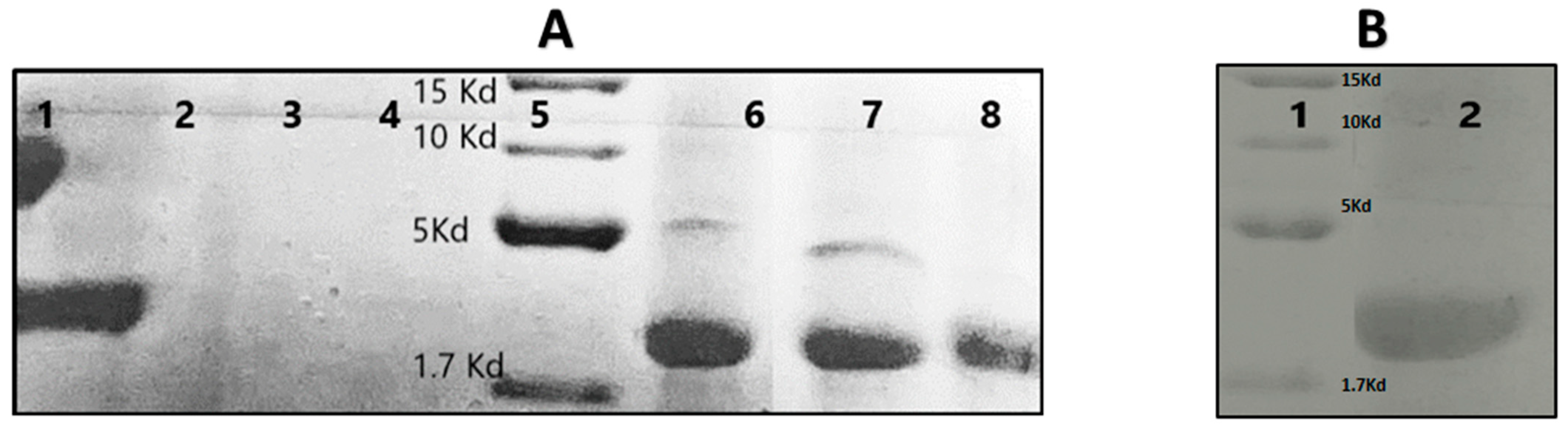
3.3. Tricine-SDS-PAGE of the Nisin-Z Free and Loaded with Nanosponges
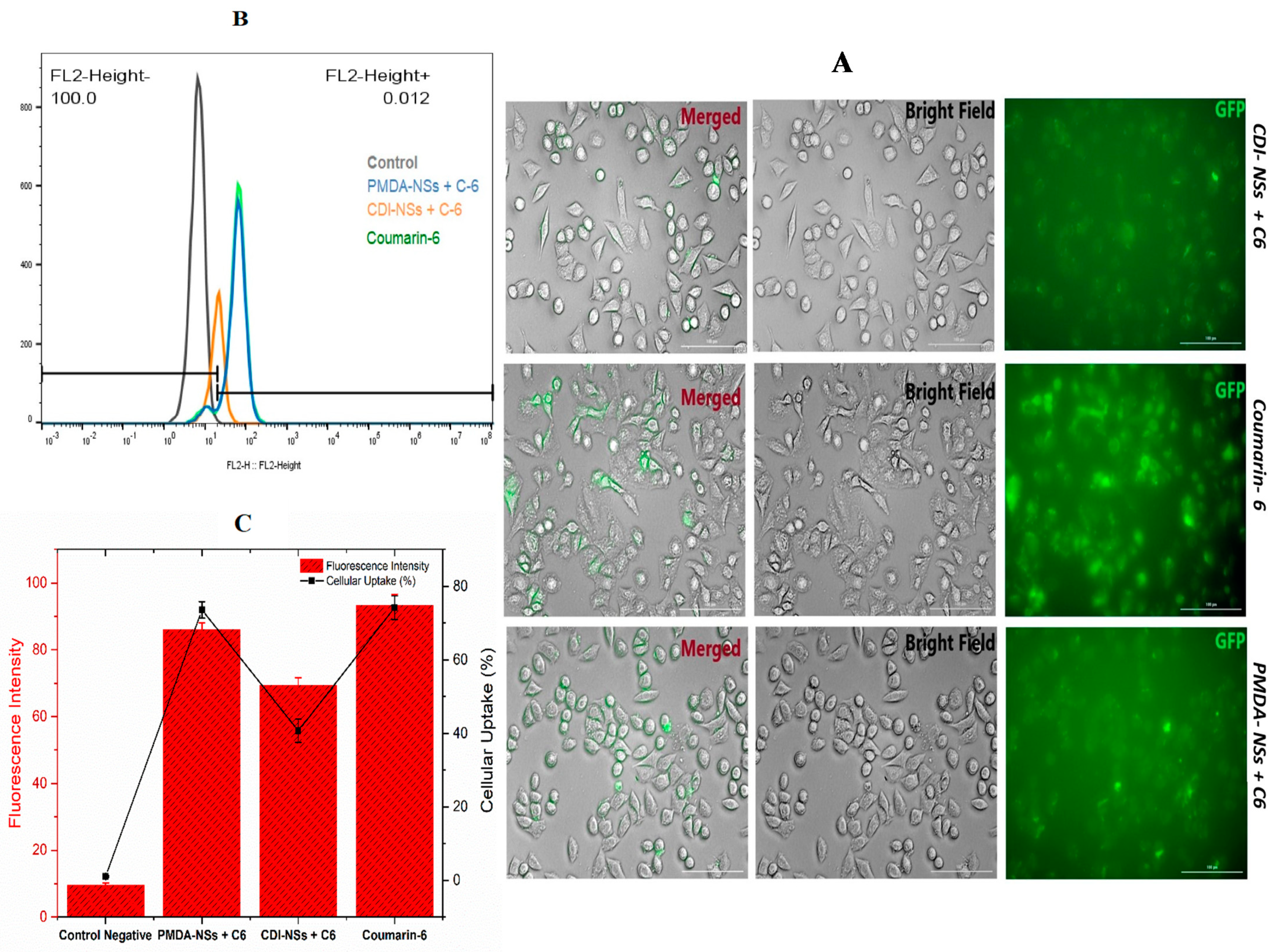
3.4. Intracellular Uptake of Free Coumarin-6 (C-6) in Comparison Coumarin-6-Loaded PMDA-NSs and CDI-NSs
3.5. MTT (3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyl Tetrazolium Bromide) Assay
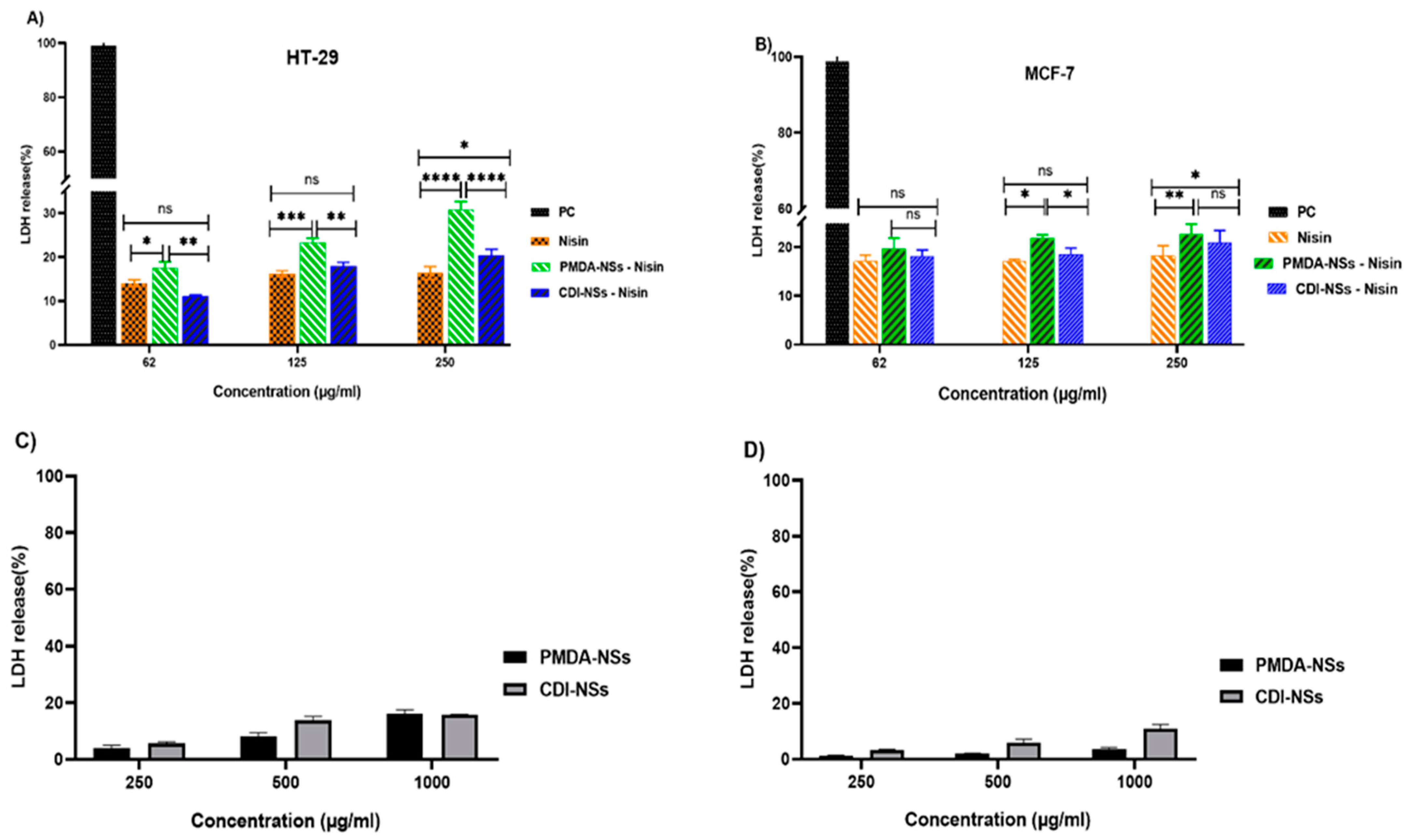
3.6. LDH Release
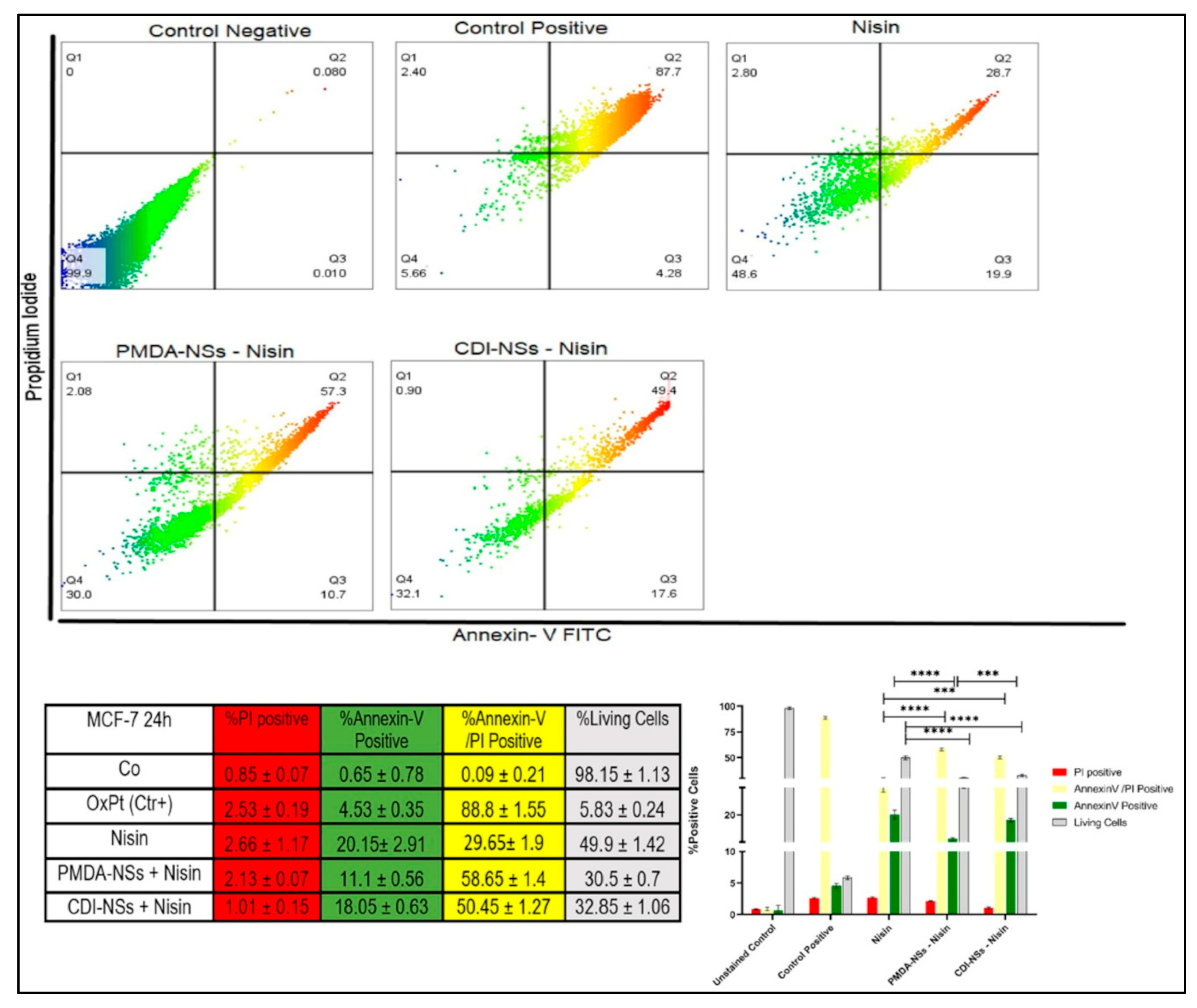
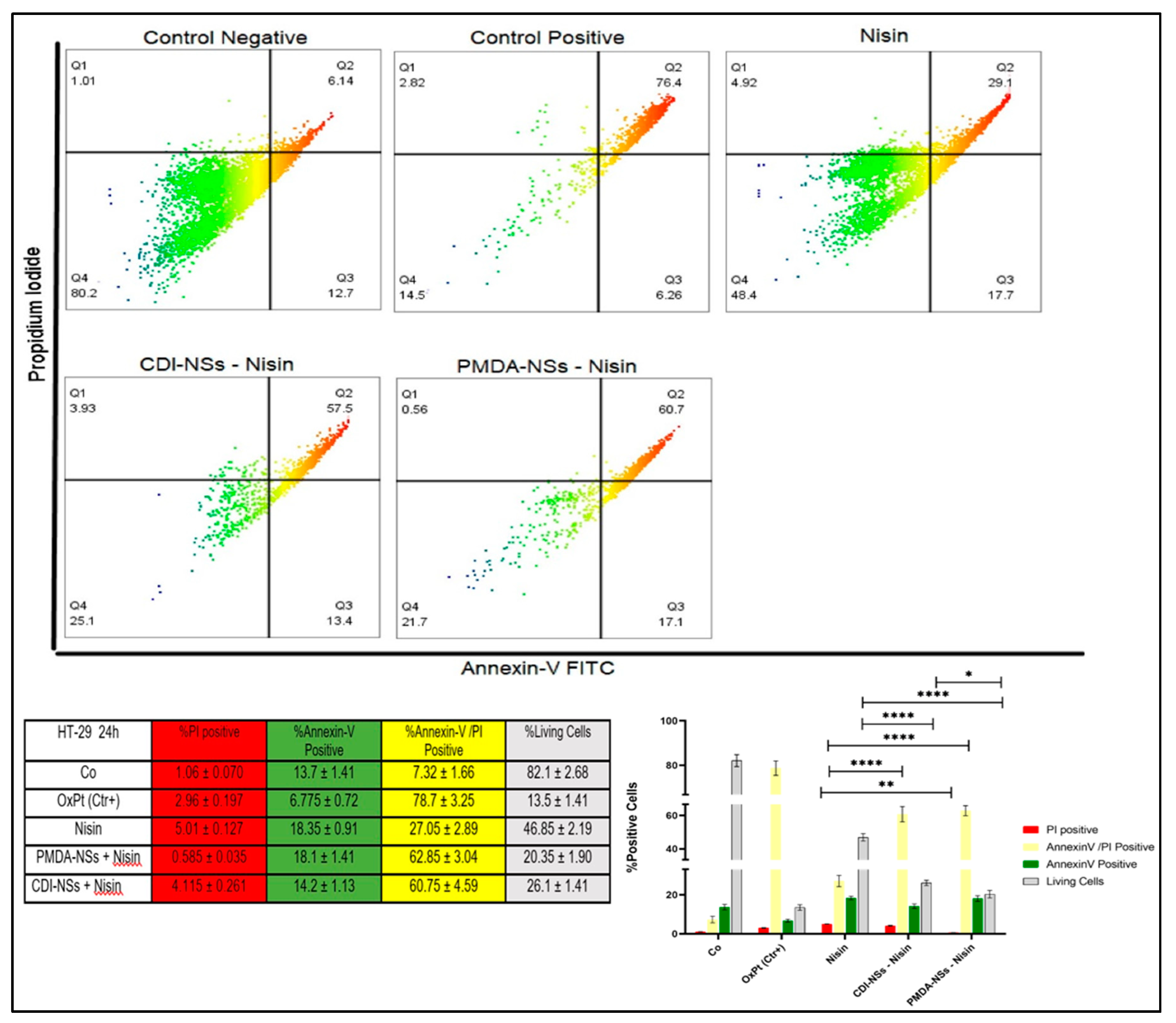
3.7. Detection of Apoptosis and Late Apoptosis/Necrosis with Flow Cytometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlson, L.E.; Waller, A.; Mitchell, A. Screening for Distress and Unmet Needs in Patients With Cancer: Review and Recommendations. J. Clin. Oncol. 2012, 30, 1160–1177. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Lewies, A.; Wentzel, J.F.; Miller, H.C.; du Plessis, L. The antimicrobial peptide nisin Z induces selective toxicity and apoptotic cell death in cultured melanoma cells. Biochimie 2018, 144, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Preet, S.; Pandey, S.; Kaur, K.; Chauhan, S.; Saini, A. Gold nanoparticles assisted co-delivery of nisin and doxorubicin against murine skin cancer. J. Drug Deliv. Sci. Technol. 2019, 53, 101147. [Google Scholar] [CrossRef]
- Shin, J.M.; Gwak, J.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2015, 120, 1449–1465. [Google Scholar] [CrossRef] [Green Version]
- Mulders, J.W.M.; Boerrigter, I.J.; Rollema, H.S.; Siezen, R.J.; de Vos, W.M. Identification and characterization of the lantibiotic nisin Z, a natural nisin variant. Eur. J. Biochem. 1991, 201, 581–584. [Google Scholar] [CrossRef]
- Benech, R.-O.; Kheadr, E.E.; Lacroix, C.; Fliss, I. Antibacterial Activities of Nisin Z Encapsulated in Liposomes or Produced In Situ by Mixed Culture during Cheddar Cheese Ripening. Appl. Environ. Microbiol. 2002, 68, 5607–5619. [Google Scholar] [CrossRef] [Green Version]
- Delves-Broughton, J. Nisin and its application as a food preservative. Int. J. Dairy Technol. 1990, 43, 73–76. [Google Scholar] [CrossRef]
- Moll, G.N.; Clark, J.; Chan, W.C.; Bycroft, B.W.; Roberts, G.C.; Konings, W.N.; Driessen, A.J. Role of transmembrane pH gradient and membrane binding in nisin pore formation. J. Bacteriol. 1997, 179, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, N.E.-S.; Morsy, H.; Abdelgwad, M. The Comparative Effect of Nisin and Thioridazine as Potential Anticancer Agents on Hepatocellular Carcinoma. Rep. Biochem. Mol. Biol. 2021, 9, 452–462. [Google Scholar] [CrossRef]
- Avand, A.; Akbari, V.; Shafizadegan, S. In Vitro Cytotoxic Activity of a Lactococcus lactis Antimicrobial Peptide Against Breast Cancer Cells. Iran. J. Biotechnol. 2018, 16, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Goudarzi, F.; Asadi, A.; Afsharpour, M.; Jamadi, R.H. In Vitro Characterization and Evaluation of the Cytotoxicity Effects of Nisin and Nisin-Loaded PLA-PEG-PLA Nanoparticles on Gastrointestinal (AGS and KYSE-30), Hepatic (HepG2) and Blood (K562) Cancer Cell Lines. AAPS PharmSciTech 2018, 19, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.E.; Ritchie, K.; Kamarajan, P.; Miao, D.; Kapila, Y.L. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC 1. Cancer Med. 2012, 1, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, I.; Breukink, E.; van Kraaij, C.; Kuipers, O.P.; Bierbaum, G.; de Kruijff, B.; Sahl, H.-G. Specific Binding of Nisin to the Peptidoglycan Precursor Lipid II Combines Pore Formation and Inhibition of Cell Wall Biosynthesis for Potent Antibiotic Activity. J. Biol. Chem. 2001, 276, 1772–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dissanayake, S.; Denny, W.A.; Gamage, S.; Sarojini, V. Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. J. Control. Release 2017, 250, 62–76. [Google Scholar] [CrossRef]
- Thundimadathil, J. Cancer Treatment Using Peptides: Current Therapies and Future Prospects. J. Amino Acids 2012, 2012, 967347. [Google Scholar] [CrossRef] [Green Version]
- Becucci, L.; Benci, S.; Nuti, F.; Real-Fernández, F.; Vaezi, Z.; Stella, L.; Venanzi, M.; Rovero, P.; Papini, A.M. Interaction Study of Phospholipid Membranes with an N-Glucosylated β-Turn Peptide Structure Detecting Autoantibodies Biomarkers of Multiple Sclerosis. Membranes 2015, 5, 576–596. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, N.; Mehrnejad, F.; Vaezi, Z.; Sedghi, M.; Asghari, S.M.; Naderi-Manesh, H. Encapsulation of an endostatin peptide in liposomes: Stability, release, and cytotoxicity study. Colloids Surf. B Biointerfaces 2019, 185, 110552. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, M.; Zhou, Y.; Li, L.; Wang, C.; Yuan, Y.; Li, L.; Liao, G.; Bresette, W.; Chen, Y.; et al. A self-assembling peptide hydrogel-based drug co-delivery platform to improve tissue repair after ischemia-reperfusion injury. Acta Biomater. 2019, 103, 102–114. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tsai, T.-L.; Ye, X.-H.; Lin, T.-H. Anti-proliferative effect on a colon adenocarcinoma cell line exerted by a membrane disrupting antimicrobial peptide KL15. Cancer Biol. Ther. 2015, 16, 1172–1183. [Google Scholar] [CrossRef] [Green Version]
- Daniels, T.R.; Bernabeu, E.; Rodríguez, J.A.; Patel, S.; Kozman, M.; Chiappetta, D.A.; Holler, E.; Ljubimova, J.Y.; Helguera, G.; Penichet, M.L. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2012, 1820, 291–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, C.R.; Xu, R.; Iyer, R.; Sankey, E.W.; Liu, A.; Abu-Bonsrah, N.; Sarabia-Estrada, R.; Frazier, J.L.; Sciubba, D.M.; Jallo, G.I. Local delivery methods of therapeutic agents in the treatment of diffuse intrinsic brainstem gliomas. Clin. Neurol. Neurosurg. 2016, 142, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Thipparaboina, R.; Chavan, R.; Kumar, D.; Modugula, S.; Shastri, N.R. Micellar carriers for the delivery of multiple therapeutic agents. Colloids Surf. B Biointerfaces 2015, 135, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Pirisinu, M.; Pham, T.C.; Zhang, D.X.; Hong, T.N.; Nguyen, L.T.; Le, M.T. Extracellular vesicles as natural therapeutic agents and innate drug delivery systems for cancer treatment: Recent advances, current obstacles, and challenges for clinical translation. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Pedrazzo, A.R.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Trotta, F.; Cavalli, R. Characterization and Applications of New Hyper-Cross-Linked Cyclodextrins. Compos. Interfaces 2009, 16, 39–48. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Matencio, A.; Hoti, G.; Monfared, Y.K.; Rezayat, A.; Pedrazzo, A.R.; Caldera, F.; Trotta, F. Cyclodextrin Monomers and Polymers for Drug Activity Enhancement. Polymers 2021, 13, 1684. [Google Scholar] [CrossRef]
- Allahyari, S.; Trotta, F.; Valizadeh, H.; Jelvehgari, M.; Zakeri-Milani, P. Cyclodextrin-based nanosponges as promising carriers for active agents. Expert Opin. Drug Deliv. 2019, 16, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Pastero, L.; Serpe, L.; Trotta, F.; Vavia, P.; Aquilano, D.; Trotta, M.; Zara, G.; Cavalli, R. Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization, stability and cytotoxicity. Eur. J. Pharm. Biopharm. 2010, 74, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Allahyari, S.; Esmailnezhad, N.; Valizadeh, H.; Ghorbani, M.; Jelvehgari, M.; Ghazi, F.; Zakeri-Milani, P. In-vitro characterization and cytotoxicity study of flutamide loaded cyclodextrin nanosponges. J. Drug Deliv. Sci. Technol. 2020, 61, 102275. [Google Scholar] [CrossRef]
- Blay, G.L.; Lacroix, C.; Zihler, A.; Fliss, I. In vitro inhibition activity of Nisin A, Nisin Z, pediocin PA-1 and antibiotics against common intestinal bacteria. Lett. Appl.Microbiol. 2007, 45, 252–257. [Google Scholar] [CrossRef] [PubMed]
- de Arauz, L.J.; Jozala, A.F.; Mazzola, P.G.; Penna, T.C.V. Nisin biotechnological production and application: A review. Trends Food Sci.Technol. 2009, 20, 146–154. [Google Scholar] [CrossRef]
- Huot, E.; Barrena-Gonzalez, C.; Petitdemange, H. Comparative effectiveness of nisin and bacteriocin J46 at different pH values. Lett. Appl. Microbiol. 1996, 22, 76–79. [Google Scholar] [CrossRef]
- Hoti, G.; Caldera, F.; Cecone, C.; Pedrazzo, A.; Anceschi, A.; Appleton, S.; Monfared, Y.; Trotta, F. Effect of the Cross-Linking Density on the Swelling and Rheological Behavior of Ester-Bridged β-Cyclodextrin Nanosponges. Materials 2021, 14, 478. [Google Scholar] [CrossRef]
- Shende, P.; Kulkarni, Y.A.; Gaud, R.; Deshmukh, K.; Cavalli, R.; Trotta, F.; Caldera, F. Acute and Repeated Dose Toxicity Studies of Different β-Cyclodextrin-Based Nanosponge Formulations. J. Pharm. Sci. 2015, 104, 1856–1863. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Matencio, A.; Dhakar, N.K.; Bessone, F.; Musso, G.; Cavalli, R.; Dianzani, C.; García-Carmona, F.; López-Nicolás, J.M.; Trotta, F. Study of oxyresveratrol complexes with insoluble cyclodextrin based nanosponges: Developing a novel way to obtain their complexation constants and application in an anticancer study. Carbohydr. Polym. 2019, 231, 115763. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; Conesa, I.; Muñoz-Sánchez, I.; Laveda-Cano, L.; Cano-Yelo, D.; García-Carmona, F.; López-Nicolás, J.M. Evaluation of juice and milk “food models” fortified with oxyresveratrol and β-Cyclodextrin. FoodHydrocoll. 2020, 98, 105250. [Google Scholar] [CrossRef]
- Appleton, S.L.; Tannous, M.; Argenziano, M.; Muntoni, E.; Rosa, A.C.; Rossi, D.; Caldera, F.; Scomparin, A.; Trotta, F.; Cavalli, R. Nanosponges as protein delivery systems: Insulin, a case study. Int. J. Pharm. 2020, 590, 119888. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, E.; Rezaei, M.; Hosseini, H. Antibacterial activity of plant essential oils and extracts: The role of thyme essential oil, nisin, and their combination to control Listeria monocytogenes inoculated in minced fish meat. Food Control 2014, 35, 177–183. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gossmann, R.; Fahrländer, E.; Hummel, M.; Mulac, D.; Brockmeyer, J.; Langer, K. Comparative examination of adsorption of serum proteins on HSA- and PLGA-based nanoparticles using SDS–PAGE and LC–MS. Eur. J. Pharm. Biopharm. 2015, 93, 80–87. [Google Scholar] [CrossRef]
- Torchilin, V.P.; Lukyanov, A.N. Peptide and protein drug delivery to and into tumors: Challenges and solutions. Drug Discov. Today 2003, 8, 259–266. [Google Scholar] [CrossRef]
- Clemente, N.; Argenziano, M.; Gigliotti, C.L.; Ferrara, B.; Boggio, E.; Chiocchetti, A.; Caldera, F.; Trotta, F.; Benetti, E.; Annaratone, L.; et al. Paclitaxel-Loaded Nanosponges Inhibit Growth and Angiogenesis in Melanoma Cell Models. Front. Pharmacol. 2019, 10, 776. [Google Scholar] [CrossRef] [Green Version]
- Palminteri, M.; Dhakar, N.K.; Ferraresi, A.; Caldera, F.; Vidoni, C.; Trotta, F.; Isidoro, C. Cyclodextrin nanosponge for the GSH-mediated delivery of Resveratrol in human cancer cells. Nanotheranostics 2021, 5, 197–212. [Google Scholar] [CrossRef]
- Swaminathan, S.; Vavia, P.; Trotta, F.; Cavalli, R. Nanosponges Encapsulating Dexamethasone for Ocular Delivery: Formulation Design, Physicochemical Characterization, Safety and Corneal Permeability Assessment. J. Biomed. Nanotechnol. 2013, 9, 998–1007. [Google Scholar] [CrossRef]
- Lembo, D.; Swaminathan, S.; Donalisio, M.; Civra, A.; Pastero, L.; Aquilano, D.; Vavia, P.; Trotta, F.; Cavalli, R. Encapsulation of Acyclovir in new carboxylated cyclodextrin-based nanosponges improves the agent’s antiviral efficacy. Int. J.Pharm. 2013, 443, 262–272. [Google Scholar] [CrossRef]
- Dhakar, N.K.; Matencio, A.; Caldera, F.; Argenziano, M.; Cavalli, R.; Dianzani, C.; Zanetti, M.; López-Nicolás, J.M.; Trotta, F. Comparative Evaluation of Solubility, Cytotoxicity and Photostability Studies of Resveratrol and Oxyresveratrol Loaded Nanosponges. Pharmaceutics 2019, 11, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaminathan, S.; Cavalli, R.; Trotta, F.; Ferruti, P.; Ranucci, E.; Gerges, I.; Manfredi, A.; Marinotto, D.; Vavia, P. In vitro release modulation and conformational stabilization of a model protein using swellable polyamidoamine nanosponges of β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2010, 68, 183–191. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Jiang, W.; Ren, C.; Li, J.; Xin, J.; Li, K. Effective protection and controlled release of insulin by cationic β-cyclodextrin polymers from alginate/chitosan nanoparticles. Int. J. Pharm. 2010, 393, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, W.; Song, L.; Wang, Y.; Jiang, H.; Xian, S.; Ren, Y. Effects of Hydroxylpropyl-β-Cyclodextrin on in Vitro Insulin Stability. Int. J. Mol. Sci. 2009, 10, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Daga, M.; de Graaf, I.A.; Argenziano, M.; Barranco, A.S.M.; Loeck, M.; Al-Adwi, Y.; Cucci, M.A.; Caldera, F.; Trotta, F.; Barrera, G.; et al. Glutathione-responsive cyclodextrin-nanosponges as drug delivery systems for doxorubicin: Evaluation of toxicity and transport mechanisms in the liver. Toxicol. Vitr. 2020, 65, 104800. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Goudarzi, H.; Ghalavand, Z.; Hajikhani, B.; Rafeieiatani, Z.; Hakemi-Vala, M. Anti-proliferative effects of cell wall, cytoplasmic extract of Lactococcus lactis and nisin through down-regulation of cyclin D1 on SW480 colorectal cancer cell line. Iran. J. Microbiol. 2020, 12, 424–430. [Google Scholar] [CrossRef]
- Were, L.M.; Bruce, B.D.; Davidson, P.M.; Weiss, J. Size, Stability, and Entrapment Efficiency of Phospholipid Nanocapsules Containing Polypeptide Antimicrobials. J. Agric. Food Chem. 2003, 51, 8073–8079. [Google Scholar] [CrossRef]
- Fesik, S.W. Promoting apoptosis as a strategy for cancer drug discovery. Nat. Cancer 2005, 5, 876–885. [Google Scholar] [CrossRef]
- Baskić, D.; Popovic, S.; Ristić, P.; Arsenijevic, N. Analysis of cycloheximide-induced apoptosis in human leukocytes: Fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol. Int. 2006, 30, 924–932. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a026716. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, C.M.; Singh, A.T. Apoptosis: A target for anticancer therapy. Int. J. Mol.Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, S.; Ghollasi, M.; Hosseini, H.M. The apoptotic impact of nisin as a potent bacteriocin on the colon cancer cells. Microb. Pathog. 2017, 111, 193–197. [Google Scholar] [CrossRef] [PubMed]




| Carriers | Z-Average (nm) | Zeta Potential (mV) | PDI | EE (%) | DL (%) |
|---|---|---|---|---|---|
| CDI-NSs | 164.3 ± 1.2 | −16.5 ± 0.3 | 0.22 ± 0.04 | - | - |
| CDI-NSs + Nisin | 187.8 ± 2.4 | −13.8 ± 0.3 | 0.41 ± 0.07 | 91 | 22.74 |
| PMDA-NSs | 308 ± 0.9 | −20 ± 0.5 | 0.41 ± 0.02 | - | - |
| PMDA-NSs + Nisin | 369 ± 3.6 | −14.0 ± 0.6 | 0.54 ± 0.09 | 92 | 23.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khazaei Monfared, Y.; Mahmoudian, M.; Cecone, C.; Caldera, F.; Zakeri-Milani, P.; Matencio, A.; Trotta, F. Stabilization and Anticancer Enhancing Activity of the Peptide Nisin by Cyclodextrin-Based Nanosponges against Colon and Breast Cancer Cells. Polymers 2022, 14, 594. https://doi.org/10.3390/polym14030594
Khazaei Monfared Y, Mahmoudian M, Cecone C, Caldera F, Zakeri-Milani P, Matencio A, Trotta F. Stabilization and Anticancer Enhancing Activity of the Peptide Nisin by Cyclodextrin-Based Nanosponges against Colon and Breast Cancer Cells. Polymers. 2022; 14(3):594. https://doi.org/10.3390/polym14030594
Chicago/Turabian StyleKhazaei Monfared, Yousef, Mohammad Mahmoudian, Claudio Cecone, Fabrizio Caldera, Parvin Zakeri-Milani, Adrián Matencio, and Francesco Trotta. 2022. "Stabilization and Anticancer Enhancing Activity of the Peptide Nisin by Cyclodextrin-Based Nanosponges against Colon and Breast Cancer Cells" Polymers 14, no. 3: 594. https://doi.org/10.3390/polym14030594
APA StyleKhazaei Monfared, Y., Mahmoudian, M., Cecone, C., Caldera, F., Zakeri-Milani, P., Matencio, A., & Trotta, F. (2022). Stabilization and Anticancer Enhancing Activity of the Peptide Nisin by Cyclodextrin-Based Nanosponges against Colon and Breast Cancer Cells. Polymers, 14(3), 594. https://doi.org/10.3390/polym14030594











