Imidazolium-Quaternized Poly(2,6-Dimethyl-1,4-Phenylene Oxide)/Zeolitic Imidazole Framework-8 Composite Membrane as Polymer Electrolyte for Fuel-Cell Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zeolitic Imidazole Framework-8
2.3. Bromination of Poly(2,6-Dimethyl-1,4-Phenylene Oxide) (Br-PPO)
2.4. Fabrication of the Organic–Inorganic Composite Membrane (ImPPO/ZIF-8)
2.5. Membrane Characterization
2.6. Water Uptake (WU), Swelling Ratio (SR), and Contact Angle (CA)
2.7. Ion Exchange Capacity (IEC)
2.8. Ionic Conductivity (IC) and Membrane Alkaline Stability
2.9. Membrane Electrode Assembly and Fuel Cell Performance
3. Results and Discussion
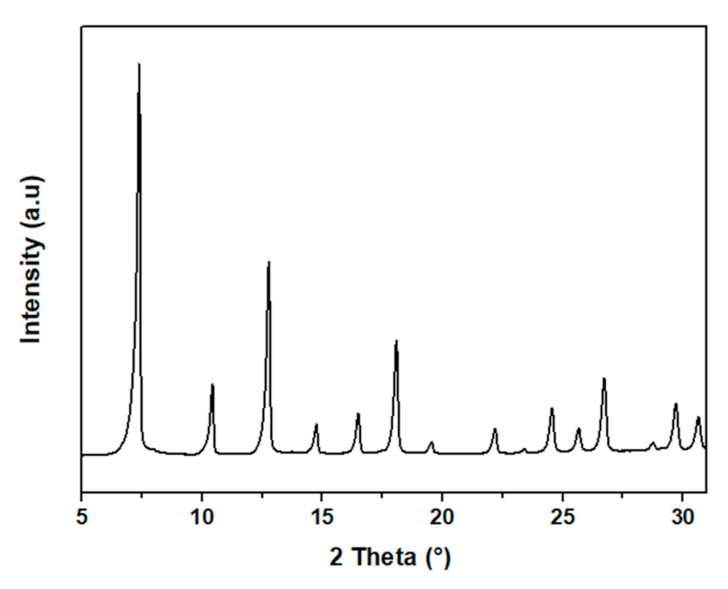
3.1. Synthesis of Zeolitic Imidazole Framework-8
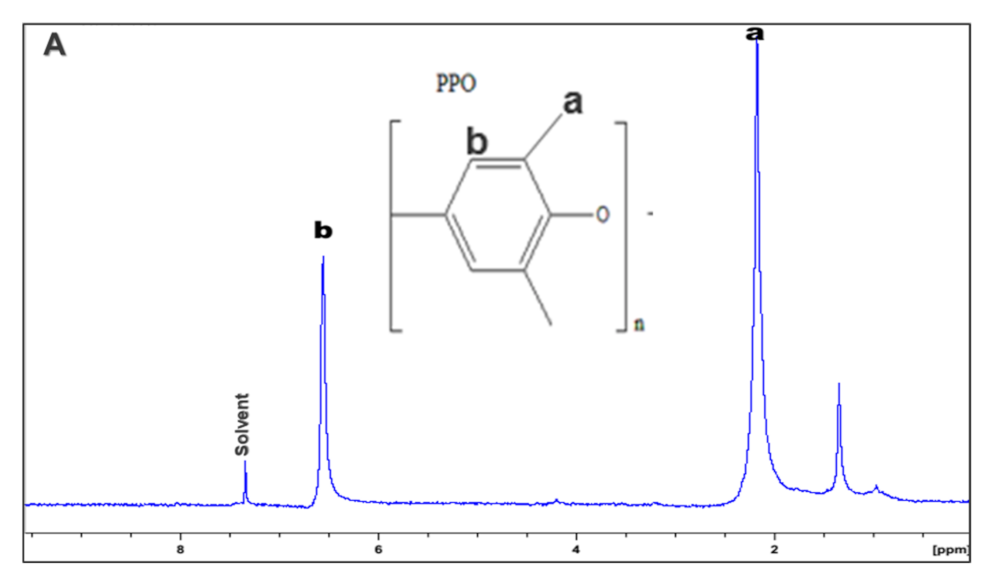
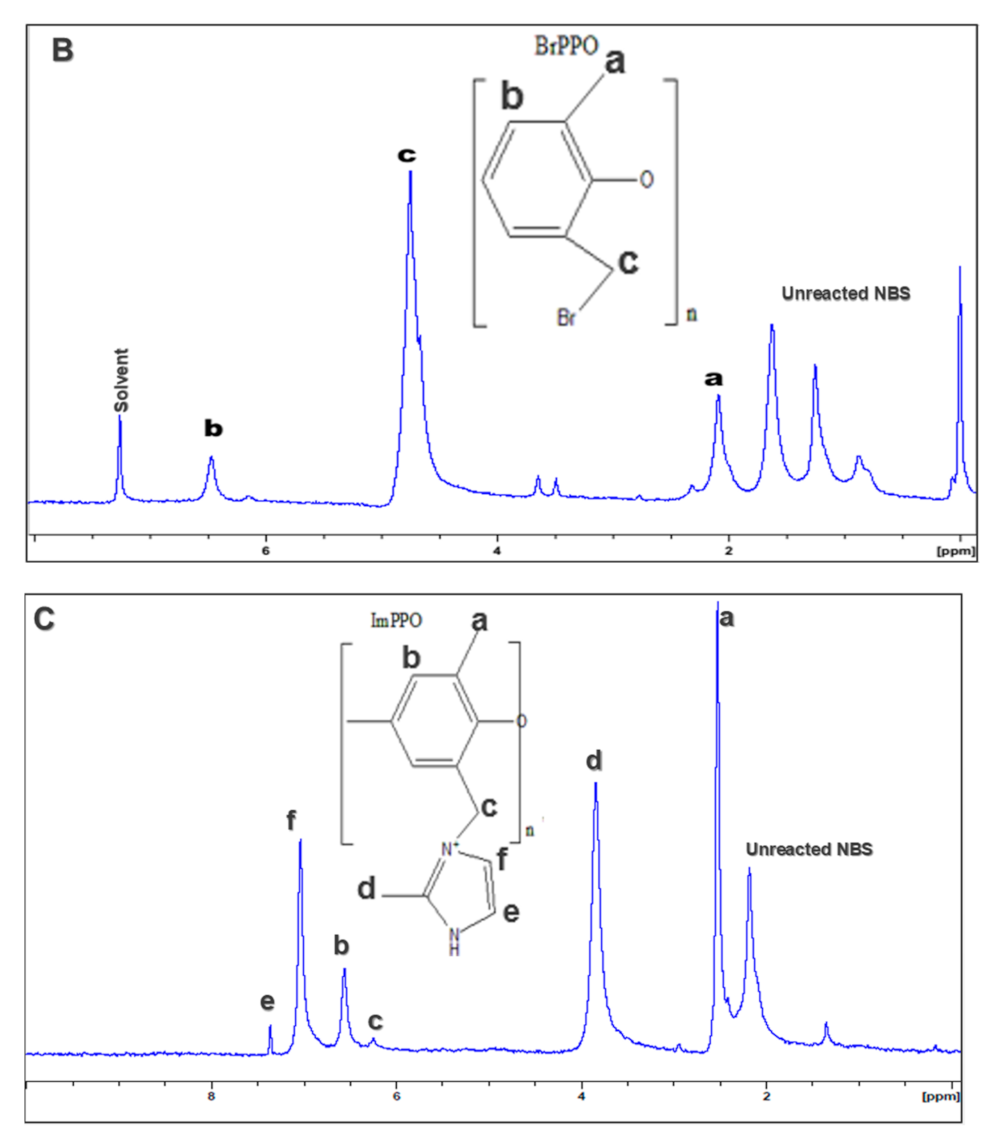
3.2. Proton Nuclear Magnetic Resonance (NMR) of the Membranes
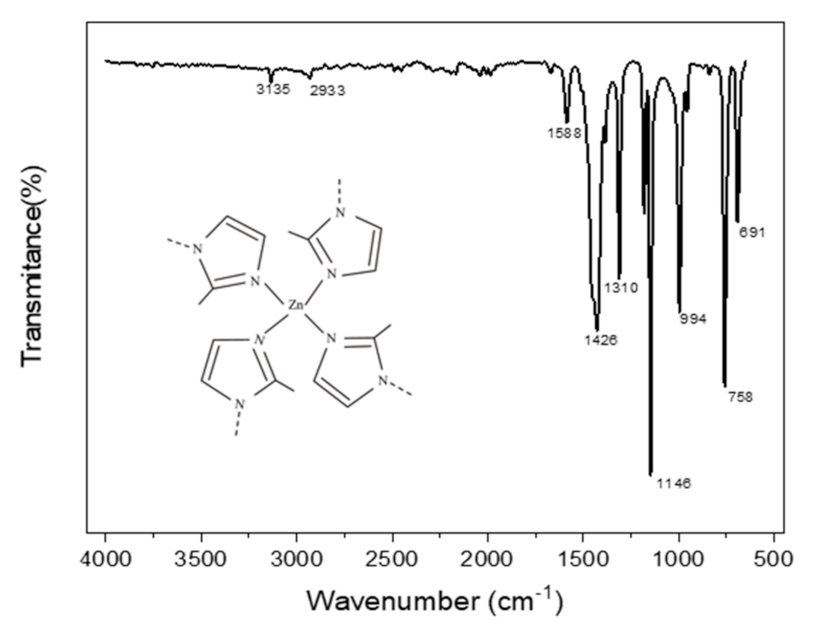
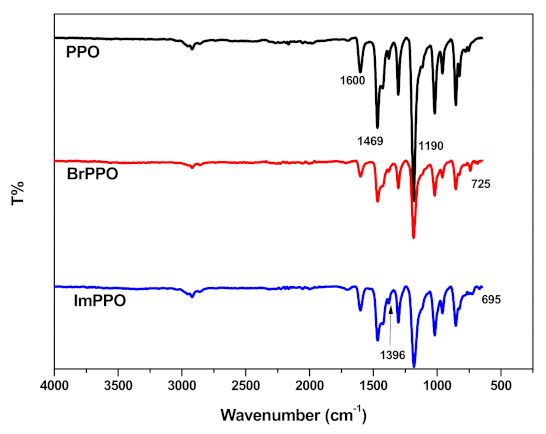
3.3. Fourier Transform Infrared (FTIR) Spectroscopy of the Membranes
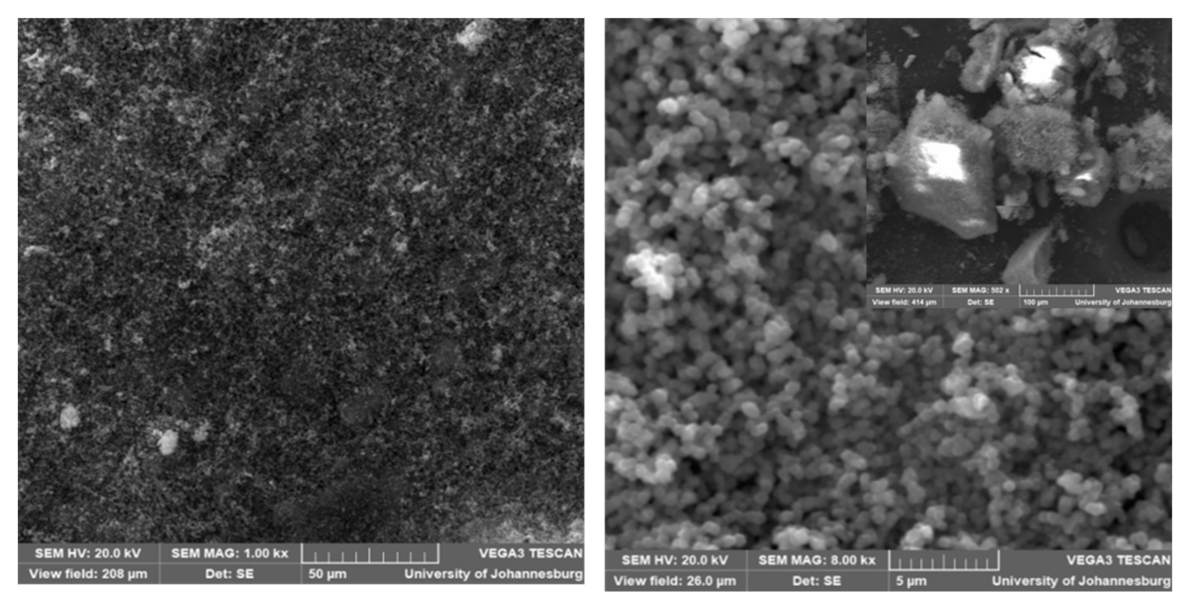
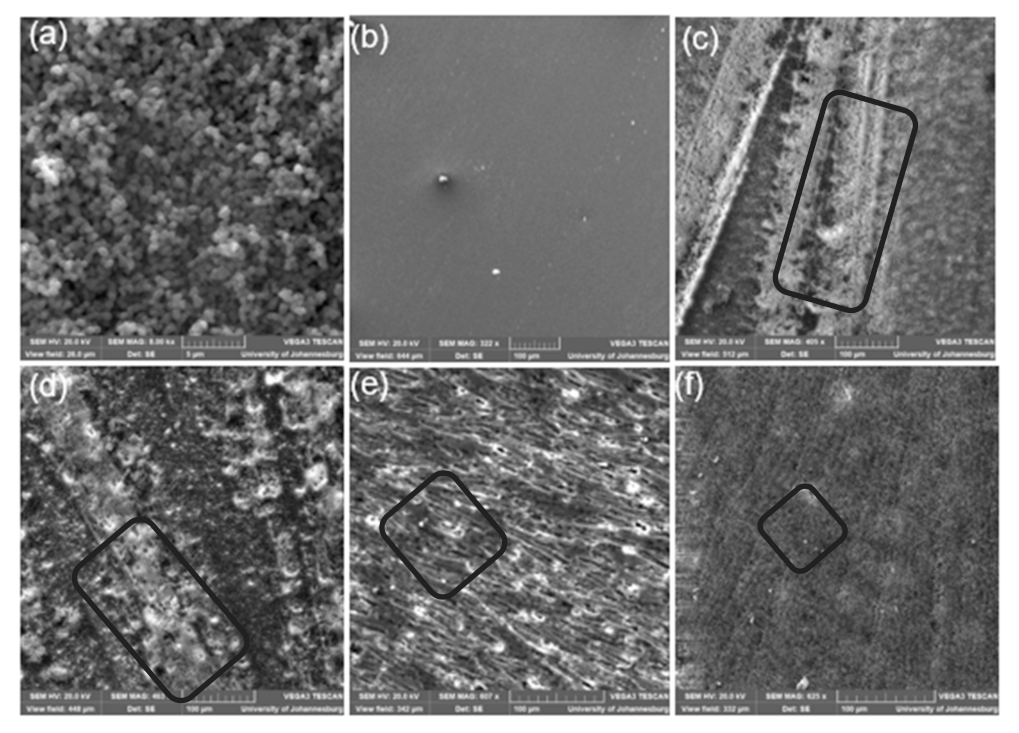
3.4. Surface Morphology of Zif-8 and All the Membranes
3.5. Water Uptake (WU) and Contact Angle (CA)
3.6. Ion Exchange Capacity (IEC) and Ion Conductivity (IC)
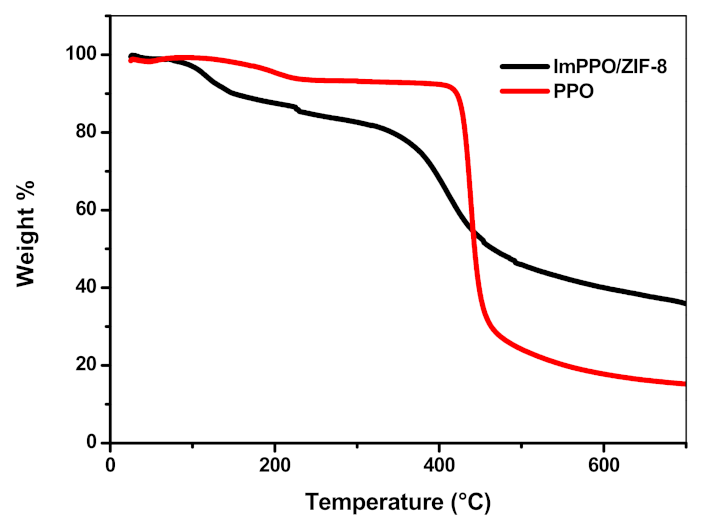
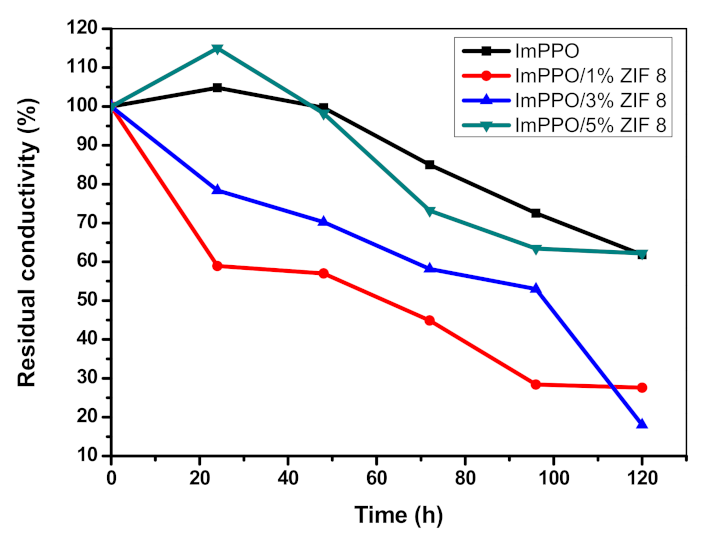
3.7. Thermal and Chemical Stability of ImPPO and ImPPO/Zif-8
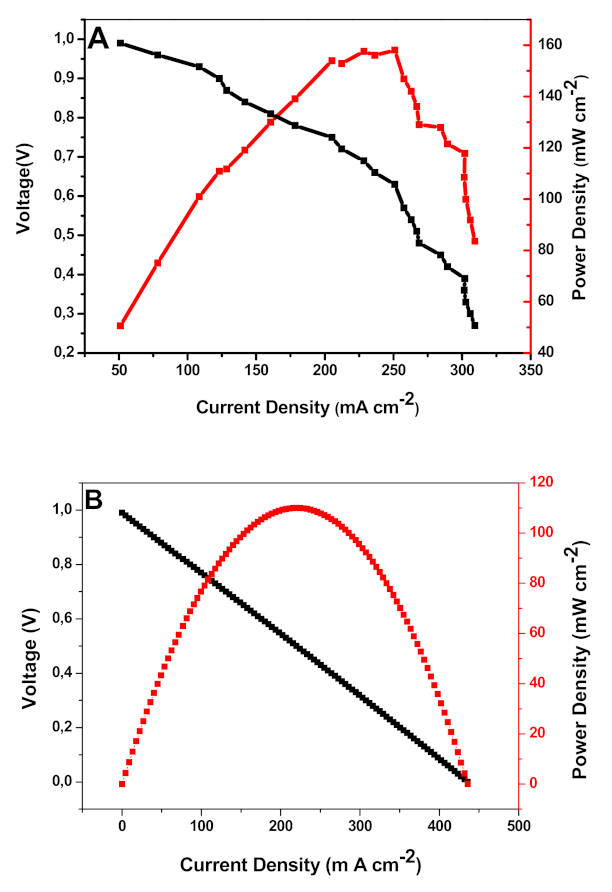
3.8. Polarization Curve of IMPPO/ZIF-8 Anion Exchange Membrane (AEM)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Herve Bang, Y.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Hsu, P.; Hu, T.; Kumar, S.R.; Chang, C.; Wu, K.C.; Tung, K.; Lue, S.J. Highly zeolite-loaded polyvinyl alcohol composite membranes for alkaline fuel-cell electrolytes. Polymers 2018, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhang, R.; Chen, W. Graphene-Supported Nanoelectrocatalysts for Fuel Cells: Synthesis, Properties, and Applications. Chem. Rev. 2014, 114, 5117–5160. [Google Scholar] [CrossRef]
- Msomi, P.F.; Nonjola, P.T.; Ndungu, P.G.; Ramontja, J. Poly (2, 6-dimethyl-1, 4-phenylene)/polysulfone anion exchange membrane blended with TiO2 with improved water uptake for alkaline fuel cell application. Int. J. Hydrogen Energy 2020, 45, 29465–29476. [Google Scholar] [CrossRef]
- Tanç, B.; Arat, H.T.; Baltacıoğlu, E.; Aydın, K. Overview of the next quarter century vision of hydrogen fuel cell electric vehicles. Int. J. Hydrogen Energy 2019, 44, 10120–10128. [Google Scholar] [CrossRef]
- Anahidzade, N.; Dinari, M.; Abdolmaleki, A.; Tadavani, K.F.; Zhiani, M. Enhancement of Hydroxide Conduction by Incorporation of Metal–Organic Frameworks into a Semi-Interpenetrating Network. Energy Fuels 2019, 33, 5749–5760. [Google Scholar] [CrossRef]
- Hagesteijn, K.; Jiang, S.; Ladewig, B. A review of the synthesis and characterization of anion exchange membranes. J. Mater. Sci. 2018, 53, 11131–11150. [Google Scholar] [CrossRef] [Green Version]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.N.; Wilberforce, T.; El Hassan, Z.; Thompson, J.; Ramadan, M.; Olabi, A.G. Fuel cell membranes—Pros and cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, L. Preparation and properties of organic–inorganic alkaline hybrid membranes for direct methanol fuel cell application. Solid State Ion. 2014, 255, 96–103. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Q.L.; Zhu, A.M.; Huang, S.M.; Zeng, Q.H. Performance of organic–inorganic hybrid anion-exchange membranes for alkaline direct methanol fuel cells. J. Power Sources 2009, 186, 328–333. [Google Scholar] [CrossRef]
- Msomi, P.; Nonjola, P.; Ndungu, P.; Ramontja, J. Quaternized poly (2,6 dimethyl–1,4 phenylene oxide)/polysulfone blended anion exchange membrane for alkaline fuel cells application. Mater. Today Proc. 2018, 5, 10496–10504. [Google Scholar] [CrossRef]
- He, X.; He, G.; Gang, M.; Li, Z.; Yin, Y.; Cao, L.; Zhang, B.; Wu, H.; Jiang, Z. Highly conductive and robust composite anion exchange membranes by incorporating quaternized MIL-101(Cr). Sci. Bull. 2017, 62, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Jiang, J.; Liu, Q.; Xie, Z.; Zhai, J. Hybrid nanochannel membrane based on polymer/MOF for high-performance salinity gradient power generation. Nano Energy 2018, 53, 643–649. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Chen, Y.; Xiong, C.; He, G.; Cao, Y.; Wu, H.; Guiver, M.D.; Jiang, Z. Constructing efficient ion nanochannels in alkaline anion exchange membranes by the in situ assembly of a poly(ionic liquid) in metal–organic frameworks. J. Mater. Chem. A 2016, 4, 2340–2348. [Google Scholar] [CrossRef]
- Wu, B.; Ge, L.; Yu, D.; Hou, L.; Li, Q.; Yang, Z.; Xu, T. Cationic metal–organic framework porous membranes with high hydroxide conductivity and alkaline resistance for fuel cells. J. Mater. Chem. A 2016, 4, 14545–14549. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Hao, Y.; He, R.; He, X. Preparation and investigation of various imidazolium-functionalized poly(2,6-dimethyl-1,4-phenylene oxide) anion exchange membranes. Electrochim. Acta 2016, 207, 112–119. [Google Scholar] [CrossRef]
- Hari Gopi, K.; Dhavale, V.M.; Bhat, S.D. Development of polyvinyl alcohol/chitosan blend anion exchange membrane with mono and di quaternizing agents for application in alkaline polymer electrolyte fuel cells. Mater. Sci. Energy Technol. 2019, 2, 194–202. [Google Scholar] [CrossRef]
- Feng, T.; Lin, B.; Zhang, S.; Yuan, N.; Chu, F.; Hickner, M.A.; Wang, C.; Zhu, L.; Ding, J. Imidazolium-based organic–inorganic hybrid anion exchange membranes for fuel cell applications. J. Membr. Sci. 2016, 508, 7–14. [Google Scholar] [CrossRef]
- Vega, J.; Andrio, A.; Lemus, A.A.; del Castillo, L.F.; Compañ, V. Conductivity study of Zeolitic Imidazolate Frameworks, Tetrabutylammonium hydroxide doped with Zeolitic Imidazolate Frameworks, and mixed matrix membranes of Polyetherimide/Tetrabutylammonium hydroxide doped with Zeolitic Imidazolate Frameworks for proton conducting applications. Electrochim. Acta 2017, 258, 153–166. [Google Scholar] [CrossRef]
- Liu, L.; He, S.; Zhang, S.; Zhang, M.; Guiver, M.D.; Li, N. 1,2,3-Triazolium-Based Poly(2,6-Dimethyl Phenylene Oxide) Copolymers as Anion Exchange Membranes. ACS Appl. Mater. Interfaces 2016, 8, 4651–4660. [Google Scholar] [CrossRef]
- Rebeck, N.T.; Li, Y.; Knauss, D.M. Poly(phenylene oxide) copolymer anion exchange membranes. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1770–1778. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Son, T.Y.; Kim, H.J.; Nam, S.Y. A facile approach to fabricate poly(2,6-dimethyl-1,4-phenylene oxide) based anion exchange membranes with extended alkaline stability and ion conductivity for fuel cell applications. J. Membr. Sci. 2019, 591, 117314. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, L.; Sun, N.; Hu, D.; Shen, Q.; Mao, F.; Gao, Q.; Wei, W. Facile and Rapid Preparation of Ag@ZIF-8 for Carboxylation of Terminal Alkynes with CO2 in Mild Conditions. ACS Appl. Mater. Interfaces 2019, 11, 28858–28867. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zuo, P.; Liu, Y.; Yang, Z.; Xu, T. Ion exchange membranes from poly(2,6-dimethyl-1,4-phenylene oxide) and related applications. Sci. China Chem. 2018, 61, 1062–1087. [Google Scholar] [CrossRef]
- Pillai, P.; Dharaskar, S.; Sasikumar, S.; Khalid, M. Zeolitic imidazolate framework-8 nanoparticle: A promising adsorbent for effective fluoride removal from aqueous solution. Appl. Water Sci. 2019, 9, 150. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Mousavi, B.; Luo, Z.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. A 2017, 5, 952–957. [Google Scholar] [CrossRef]
- Nordin, N.A.H.M.; Ismail, A.F.; Misdan, N.; Nazri, N.A.M. Modified ZIF-8 mixed matrix membrane for CO2/CH4 separation. AIP Conf. Proc. 2017, 1891, 020091. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.; Wang, Y.; Kuang, T. ZIF-8-Based Membranes for Carbon Dioxide Capture and Separation. ACS Sustain. Chem. Eng. 2017, 5, 11204–11214. [Google Scholar] [CrossRef]
- Abbasi, A.; Hosseini, S.; Somwangthanaroj, A.; Mohamad, A.A.; Kheawhom, S. Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-Based Hydroxide Exchange Separator Membranes for Zinc–Air Battery. Int. J. Mol. Sci. 2019, 20, 3678. [Google Scholar] [CrossRef] [Green Version]
- Sann, E.E.; Pan, Y.; Gao, Z.; Zhan, S.; Xia, F. Highly hydrophobic ZIF-8 particles and application for oil-water separation. Sep. Purif. Technol. 2018, 206, 186–191. [Google Scholar] [CrossRef]
- Chen, N.; Long, C.; Li, Y.; Wang, D.; Zhu, H. A hamburger-structure imidazolium-modified silica/polyphenyl ether composite membrane with enhancing comprehensive performance for anion exchange membrane applications. Electrochim. Acta 2018, 268, 295–303. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, Z.; Wang, X.; Ying, W.; Chen, D.; Liu, S.; Chen, S.; Jiang, Z.; Peng, X. Zwitterion threaded metal–organic framework membranes for direct methanol fuel cells. J. Mater. Chem. A 2018, 6, 19547–19554. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Son, T.Y.; Im, K.S.; Chae, J.E.; Kim, H.J.; Kim, T.H.; Nam, S.Y. Anion Exchange Composite Membranes Composed of Quaternary Ammonium-Functionalized Poly(2,6-dimethyl-1,4-phenylene oxide) and Silica for Fuel Cell Application. ACS Omega 2021, 6, 10168–10179. [Google Scholar] [CrossRef]
- Qian, J.; Sun, F.; Qin, L. Hydrothermal synthesis of zeolitic imidazolate framework-67 (ZIF-67) nanocrystals. Mater. Lett. 2012, 82, 220–223. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Z.; Jiang, J. Fuel Cell Technology for Distributed Generation: An Overview. IEEE Int. Symp. Ind. Electron. 2006, 2, 1613–1618. [Google Scholar]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef]
- Wu, C.; Wu, Y.; Varcoe, J.R.; Poynton, S.D.; Xu, T.; Fu, Y. Novel silica/poly(2,6-dimethyl-1,4-phenylene oxide) hybrid anion-exchange membranes for alkaline fuel cells: Effect of silica content and the single cell performance. J. Power Sources 2010, 195, 3069–3076. [Google Scholar] [CrossRef]


| Membrane | Contact Angle (°) | Water Uptake (%) | Swelling Ratio (%) | ||||
|---|---|---|---|---|---|---|---|
| 25 °C | 60 °C | 80 °C | 24 h | 48 h | 72 h | ||
| ImPPO | 55.27 | 64 | 78 | 83 | 11.1 | 22.2 | 44.4 |
| ImPPO/1% ZIF-8 | 84.83 | 40 | 42 | 73 | 6 | 12 | 26 |
| ImPPO/3% ZIF-8 | 86.83 | 31 | 9 | 35 | 1.9 | 9.4 | 15.1 |
| ImPPO/5% ZIF-8 | 106.73 | 60 | 24 | 14 | 8.5 | 18.6 | 37.1 |
| Membrane | IEC (mmol/g) | IC (mS/cm) |
|---|---|---|
| ImPPO | 3.15 | 1.02 |
| ImPPO/1% ZIF-8 | 3.87 | 2.43 |
| ImPPO/3% ZIF-8 | 4.06 | 1.96 |
| ImPPO/5% ZIF-8 | 1.93 | 1.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letsau, T.T.; Govender, P.P.; Msomi, P.F. Imidazolium-Quaternized Poly(2,6-Dimethyl-1,4-Phenylene Oxide)/Zeolitic Imidazole Framework-8 Composite Membrane as Polymer Electrolyte for Fuel-Cell Application. Polymers 2022, 14, 595. https://doi.org/10.3390/polym14030595
Letsau TT, Govender PP, Msomi PF. Imidazolium-Quaternized Poly(2,6-Dimethyl-1,4-Phenylene Oxide)/Zeolitic Imidazole Framework-8 Composite Membrane as Polymer Electrolyte for Fuel-Cell Application. Polymers. 2022; 14(3):595. https://doi.org/10.3390/polym14030595
Chicago/Turabian StyleLetsau, Thabakgolo T., Penny P. Govender, and Phumlani F. Msomi. 2022. "Imidazolium-Quaternized Poly(2,6-Dimethyl-1,4-Phenylene Oxide)/Zeolitic Imidazole Framework-8 Composite Membrane as Polymer Electrolyte for Fuel-Cell Application" Polymers 14, no. 3: 595. https://doi.org/10.3390/polym14030595
APA StyleLetsau, T. T., Govender, P. P., & Msomi, P. F. (2022). Imidazolium-Quaternized Poly(2,6-Dimethyl-1,4-Phenylene Oxide)/Zeolitic Imidazole Framework-8 Composite Membrane as Polymer Electrolyte for Fuel-Cell Application. Polymers, 14(3), 595. https://doi.org/10.3390/polym14030595







