Fabrication of Nanostructured Polycaprolactone (PCL) Film Using a Thermal Imprinting Technique and Assessment of Antibacterial Function for Its Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
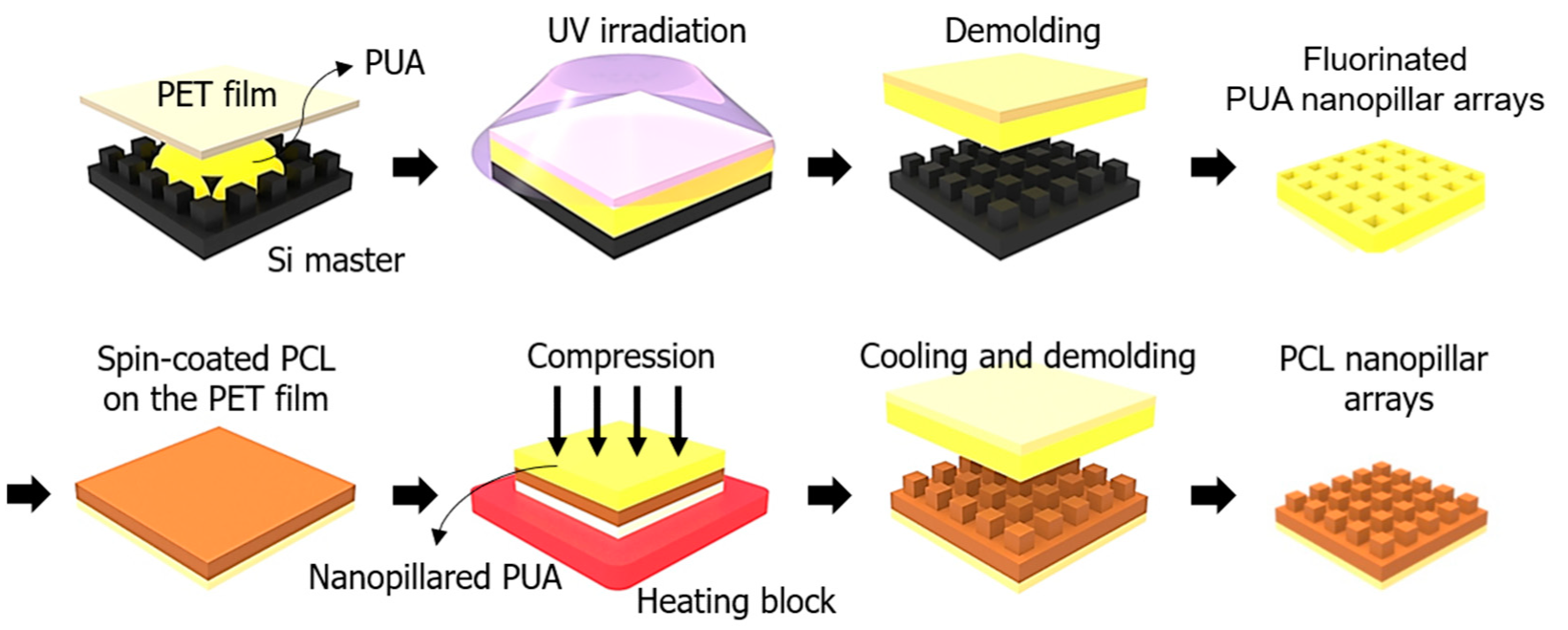
2.2. Fabrication of the PCL-Based Nanopillars
2.3. Scanning Electron Microscopy (SEM)
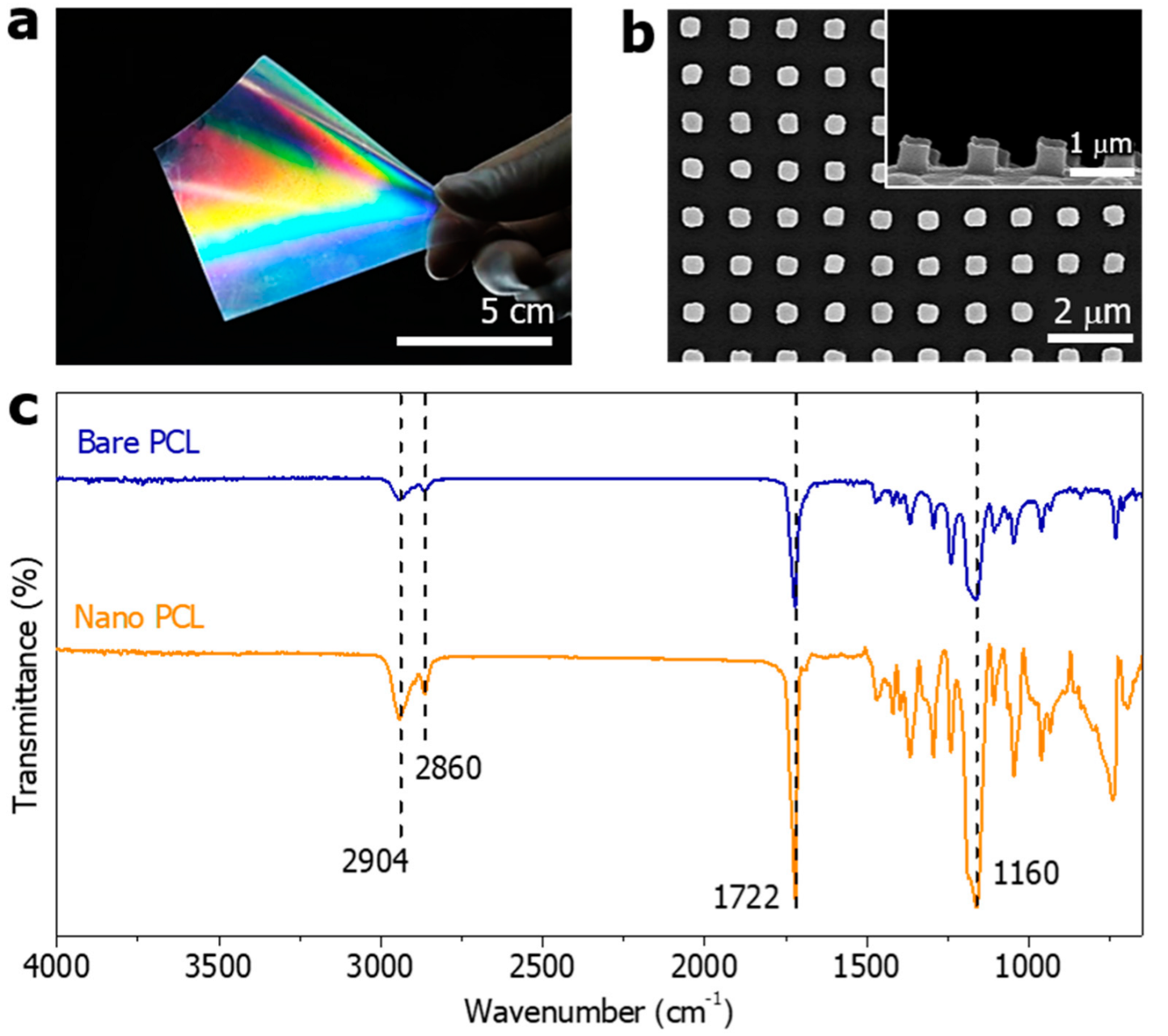
2.4. FT-IR Spectroscopy Analysis
2.5. Measurements of Water Contact Angle and Optical Transmittance
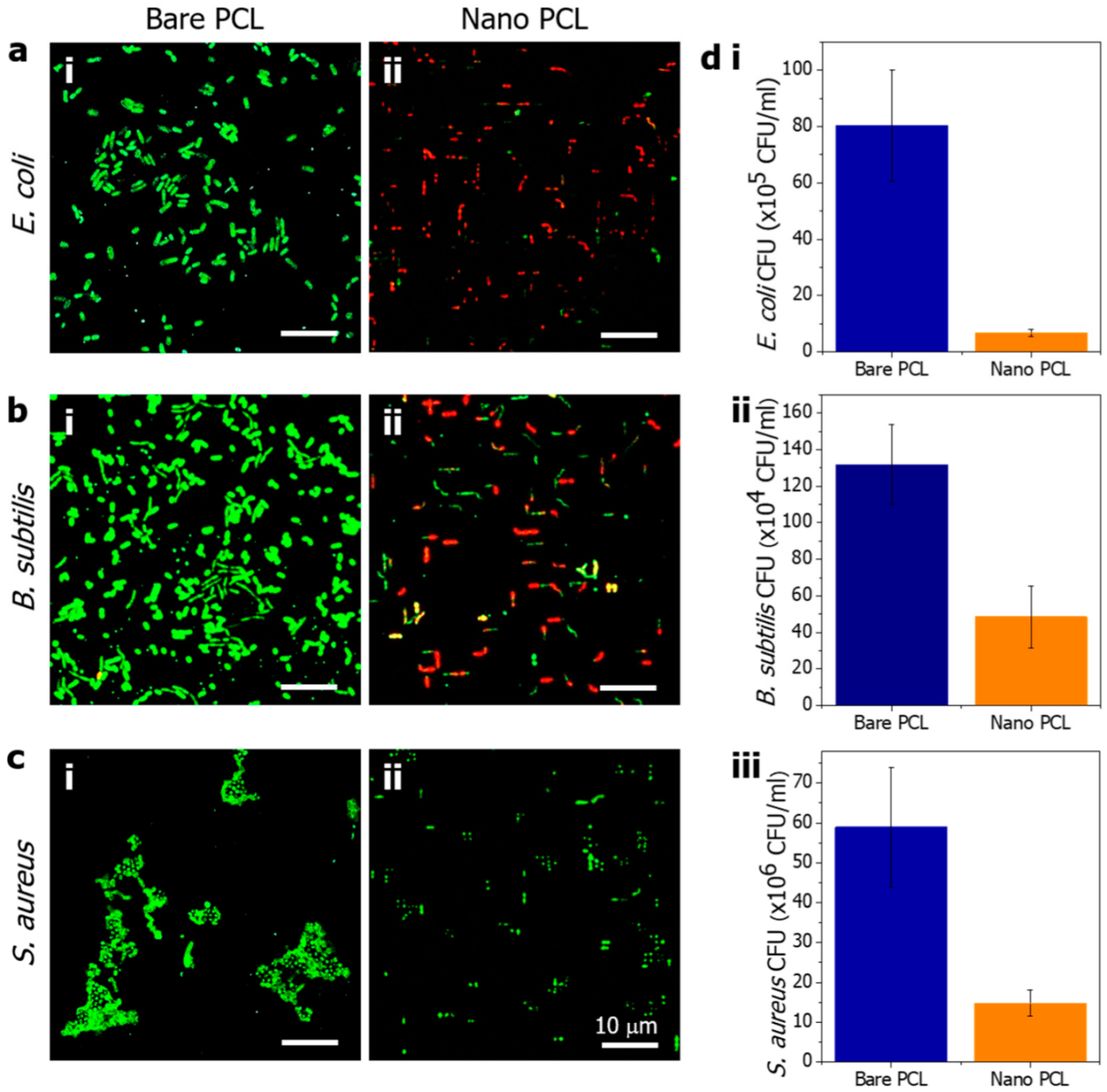
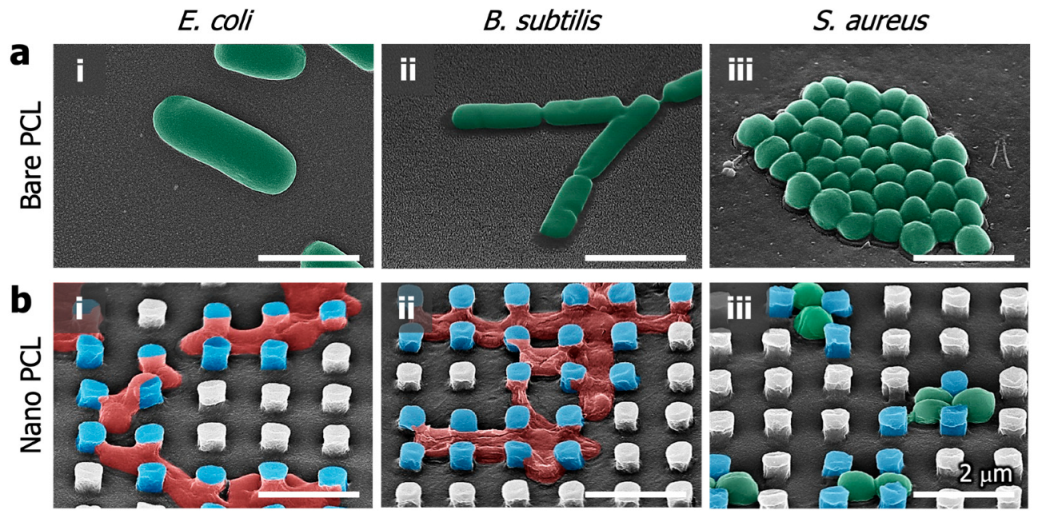
2.6. Antibacterial Tests
3. Results and Discussion
3.1. Surface Characterization of the PCL Nanopillar Arrays by Demolding Temperature
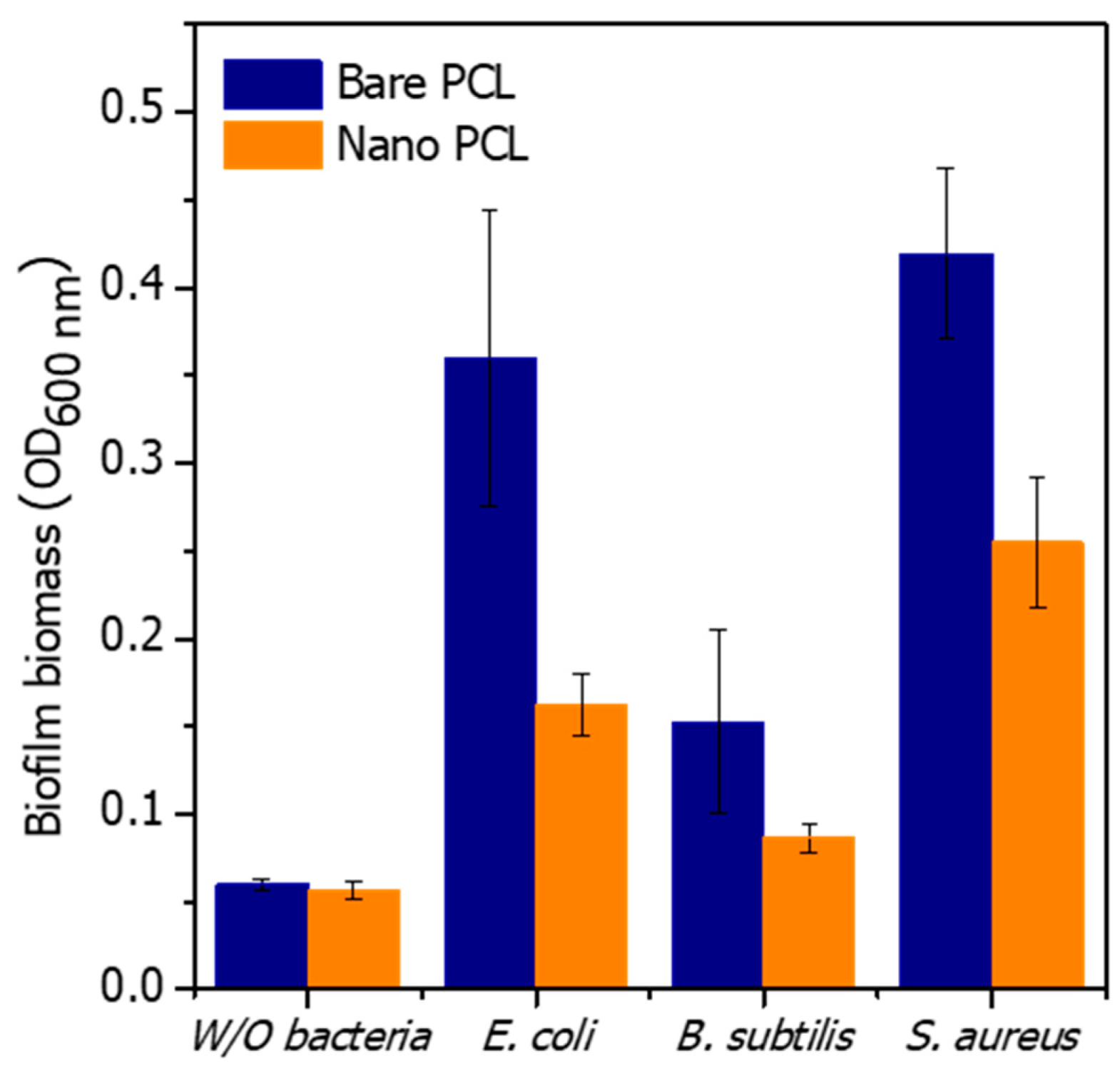
3.2. Antibacterial Evaluation of the PCL Nanopillared Surface
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Festas, A.J.; Ramos, A.; Davim, J.P. Medical Devices Biomaterials—A Review. J. Mater. Des. Appl. 2020, 234, 218–228. [Google Scholar] [CrossRef]
- Jwa, S.-J.; Won, J.-M.; Kim, D.-H.; Kim, K.-B.; Lee, J.-B.; Heo, M.; Shim, K.-S.; Jo, H.-S.; Lee, W.-J.; Roh, T.-S.; et al. Breast Tissue Restoration after the Partial Mastectomy Using Polycaprolactone Scaffold. Polymers 2022, 14, 3817. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaz, A.; Jayasuriya, A.C. Recent Trends in the Application of Widely Used Natural and Synthetic Polymer Nanocomposites in Bone Tissue Regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Davila, S.; González-Rodríguez, L.; Lama, R.; López-Álvarez, M.; Oliveira, A.L.; Serra, J.; Novoa, B.; Figueras, A.; González, P. 3D-Printed PLA Medical Devices: Physicochemical Changes and Biological Response after Sterilisation Treatments. Polymers 2022, 14, 4117. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, S.; Shi, B.; Wang, Y.; Chen, Y.; Wang, X.; Lee, E.S.; Jiang, H.B. Advances in Modification Methods Based on Biodegradable Membranes in Guided Bone/Tissue Regeneration: A Review. Polymers 2022, 14, 871. [Google Scholar] [CrossRef]
- Gunatillake, P.A.; Adhikari, R.; Gadegaard, N. Biodegradable Synthetic Polymers for Tissue Engineering. Eur. Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Shor, L. Novel Fabrication Development for the Application of Polycaprolactone and Composite Polycaprolactone/Hydroxyapitite Scaffolds for Bone Tissue Engineering. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2008. [Google Scholar]
- Muwaffak, Z.; Goyanes, A.; Clark, V.; Basit, A.W.; Hilton, S.T.; Gaisford, S. Patient-Specific 3D Scanned and 3D Printed Antimicrobial Polycaprolactone Wound Dressings. Int. J. Pharm. 2017, 527, 161–170. [Google Scholar] [CrossRef]
- Lin, W.; Ni, Y.; Pang, J. Size Effect-Inspired Fabrication of Konjac Glucomannan/Polycaprolactone Fiber Films for Antibacterial Food Packaging. Int. J. Biol. Macromol. 2020, 149, 853–860. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Zhao, R.; Jin, T.; Zhang, L.; Li, X. Chitosan Surface Modified Electrospun Poly(ε-Caprolactone)/Carbon Nanotube Composite Fibers with Enhanced Mechanical, Cell Proliferation and Antibacterial Properties. Int. J. Biol. Macromol. 2017, 104, 708–715. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. State of the Art in the Antibacterial and Antiviral Applications of Carbon-Based Polymeric Nanocomposites. Int. J. Mol. Sci. 2021, 22, 10511. [Google Scholar] [CrossRef] [PubMed]
- Khunová, V.; Kováčová, M.; Olejniková, P.; Ondreáš, F.; Špitalský, Z.; Ghosal, K.; Berkeš, D. Antibacterial Electrospun Polycaprolactone Nanofibers Reinforced by Halloysite Nanotubes for Tissue Engineering. Polymers 2022, 14, 746. [Google Scholar] [CrossRef] [PubMed]
- Hajduga, M.B.; Bobinski, R.; Dutka, M.; Bujok, J.; Cwiertnia, M.; Pajak, C.; Kurowska, A.; Rajzer, I. The Influence of Graphene Content on the Antibacterial Properties of Polycaprolactone. Int. J. Mol. Sci. 2022, 23, 10899. [Google Scholar] [CrossRef] [PubMed]
- Melo, S.F.; Neves, S.C.; Pereira, A.T.; Borges, I.; Granja, P.L.; Magalhães, F.D.; Gonçalves, I.C. Incorporation of Graphene Oxide into Poly(ɛ-Caprolactone) 3D Printed Fibrous Scaffolds Improves Their Antimicrobial Properties. Mater. Sci. Eng. C 2020, 109, 110537. [Google Scholar] [CrossRef]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart Electrospun Nanofibers Containing PCL/Gelatin/Graphene Oxide for Application in Nerve Tissue Engineering. Mater. Sci. Eng. C 2019, 103, 109768. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, H.; Wang, Y.; Yang, G.; Zhao, H.; Sun, D. Fabrication and Durable Antibacterial Properties of 3D Porous Wet Electrospun RCSC/PCL Nanofibrous Scaffold with Silver Nanoparticles. Appl. Surf. Sci. 2017, 414, 52–62. [Google Scholar] [CrossRef]
- Li, R.; Cheng, Z.; Yu, X.; Wang, S.; Han, Z.; Kang, L. Preparation of Antibacterial PCL/PVP-AgNP Janus Nanofibers by Uniaxial Electrospinning. Mater. Lett. 2019, 254, 206–209. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Electrospun PCL Membranes Incorporated with Biosynthesized Silver Nanoparticles as Antibacterial Wound Dressings. Appl. Nanosci. 2016, 6, 337–344. [Google Scholar] [CrossRef]
- Güneş Çimen, C.; Dündar, M.A.; Demirel Kars, M.; Avcl, A. Enhancement of PCL/PLA Electrospun Nanocomposite Fibers Comprising Silver Nanoparticles Encapsulated with Thymus Vulgaris L. Molecules for Antibacterial and Anticancer Activities. ACS Biomater. Sci. Eng. 2022, 8, 3717–3732. [Google Scholar] [CrossRef]
- Mosallanezhad, P.; Nazockdast, H.; Ahmadi, Z.; Rostami, A. Fabrication and Characterization of Polycaprolactone/Chitosan Nanofibers Containing Antibacterial Agents of Curcumin and ZnO Nanoparticles for Use as Wound Dressing. Front. Bioeng. Biotechnol. 2022, 10, 1027351. [Google Scholar] [CrossRef]
- Zhou, X.; He, J.; Zhou, C. Strategies from Nature: Polycaprolactone-Based Mimetic Antimicrobial Peptide Block Copolymers with Low Cytotoxicity and Excellent Antibacterial Efficiency. Polym. Chem. 2019, 10, 945–953. [Google Scholar] [CrossRef]
- Lan, X.; Liu, Y.; Wang, Y.; Tian, F.; Miao, X.; Wang, H.; Tang, Y. Coaxial Electrospun PVA/PCL Nanofibers with Dual Release of Tea Polyphenols and ε-Poly (L-Lysine) as Antioxidant and Antibacterial Wound Dressing Materials. Int. J. Pharm. 2021, 601, 120525. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yao, T.; Ren, L.; Zhao, Y.; Yuan, X. Antibacterial PCL Electrospun Membranes Containing Synthetic Polypeptides for Biomedical Purposes. Colloids Surf. B Biointerfaces 2018, 172, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Hayles, A.; Hasan, J.; Bright, R.; Palms, D.; Brown, T.; Barker, D.; Vasilev, K. Hydrothermally Etched Titanium: A Review on a Promising Mechano-Bactericidal Surface for Implant Applications. Mater. Today Chem. 2021, 22, 100622. [Google Scholar] [CrossRef]
- Maleki, E.; Mirzaali, M.J.; Guagliano, M.; Bagherifard, S. Analyzing the Mechano-Bactericidal Effect of Nano-Patterned Surfaces on Different Bacteria Species. Surf. Coat. Technol. 2021, 408, 126782. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Crawford, R.J.; Stoodley, P.; Ivanova, E.P. Mechano-Bactericidal Actions of Nanostructured Surfaces. Nat. Rev. Microbiol. 2021, 19, 8–22. [Google Scholar] [CrossRef]
- Tsimbouri, P.M.; Fisher, L.; Holloway, N.; Sjostrom, T.; Nobbs, A.H.; Meek, R.M.D.; Su, B.; Dalby, M.J. Osteogenic and Bactericidal Surfaces from Hydrothermal Titania Nanowires on Titanium Substrates. Sci. Rep. 2016, 6, 36857. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, T.; Li, X.; Cui, Q.; Wu, Q.; Wang, X.; Song, K.; Ge, D. Low-Temperature Hydrothermal Synthesis of Novel 3D Hybrid Nanostructures on Titanium Surface with Mechano-Bactericidal Performance. ACS Biomater. Sci. Eng. 2021, 7, 2268–2278. [Google Scholar] [CrossRef]
- Bright, R.; Fernandes, D.; Wood, J.; Palms, D.; Burzava, A.; Ninan, N.; Brown, T.; Barker, D.; Vasilev, K. Long-Term Antibacterial Properties of a Nanostructured Titanium Alloy Surface: An in Vitro Study. Mater. Today Bio 2022, 13, 100176. [Google Scholar] [CrossRef]
- Jang, K.J.; Kim, S.; Park, S.; Kim, W.; Gwon, Y.; Park, S.; Lim, K.T.; Seonwoo, H.; Kim, J. Lithographically-Fabricated HA-Incorporated PCL Nanopatterned Patch for Tissue Engineering. Appl. Sci. 2020, 10, 2398. [Google Scholar] [CrossRef]
- Kim, D.; Gwon, Y.; Park, S.; Kim, W.; Yun, K.; Kim, J. Eggshell Membrane as a Bioactive Agent in Polymeric Nanotopographic Scaffolds for Enhanced Bone Regeneration. Biotechnol. Bioeng. 2021, 118, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Gwon, Y.; Kim, Y.K.; Park, S.; Kang, S.J.; Park, H.K.; Kim, M.S.; Kim, J. Plasma-Assisted Multiscale Topographic Scaffolds for Soft and Hard Tissue Regeneration. NPJ Regen. Med. 2021, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Csaderova, L.; Martines, E.; Seunarine, K.; Gadegaard, N.; Wilkinson, C.D.W.; Riehle, M.O. A Biodegradable and Biocompatible Regular Nanopattern for Large-Scale Selective Cell Growth. Small 2010, 6, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, I.S.; Schiffman, J.D. Current and Emerging Approaches to Engineer Antibacterial and Antifouling Electrospun Nanofibers. Materials 2018, 11, 1059. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Cho, Y.S.; Park, H.H. PEGDMA-Based Pillar-Shape Nanostructured Antibacterial Films Having Mechanical Robustness. ACS Appl. Bio. Mater. 2022, 5, 3006–3012. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, M.; Jang, H.; Kondaveeti, S.; Sun, K.; Kim, S.; Park, H.H.; Jeong, H.E. Bifunctional Amphiphilic Nanospikes with Antifogging and Antibiofouling Properties. ACS Appl. Mater. Interfaces 2022, 14, 39478–39488. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Sun, K.; Seong, M.; Kang, M.; Park, S.; Hong, S.; Jung, H.; Jang, J.; Kim, J.; Jeong, H.E. Lipid-Hydrogel-Nanostructure Hybrids as Robust Biofilm-Resistant Polymeric Materials. ACS Macro Lett. 2019, 8, 64–69. [Google Scholar] [CrossRef]
- Park, S.; Park, H.H.; Sun, K.; Gwon, Y.; Seong, M.; Kim, S.; Park, T.E.; Hyun, H.; Choung, Y.H.; Kim, J.; et al. Hydrogel Nanospike Patch as a Flexible Anti-Pathogenic Scaffold for Regulating Stem Cell Behavior. ACS Nano 2019, 13, 11181–11193. [Google Scholar] [CrossRef]
- Hawi, S.; Goel, S.; Kumar, V.; Pearce, O.; Ayre, W.N.; Ivanova, E.P. Critical Review of Nanopillar-Based Mechanobactericidal Systems. ACS Appl. Nano Mater. 2021, 5, 1–17. [Google Scholar] [CrossRef]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K.D.V. Bio-Mimicking Nano and Micro-Structured Surface Fabrication for Antibacterial Properties in Medical Implants. J. Nanobiotechnol. 2017, 15, 64. [Google Scholar] [CrossRef]
- Castro, J.; Lima, Â.; Sousa, L.G.V.; Rosca, A.S.; Muzny, C.A.; Cerca, N. Crystal Violet Staining Alone Is Not Adequate to Assess Synergism or Antagonism in Multi-Species Biofilms of Bacteria Associated with Bacterial Vaginosis. Front. Cell Infect. Microbiol. 2022, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Limongi, T.; Dattola, E.; Botta, C.; Coluccio, M.L.; Candeloro, P.; Cucè, M.; Scopacasa, B.; Cantafio, M.E.G.; Critello, C.D.; Pullano, S.A.; et al. Influence of the Fabrication Accuracy of Hot-Embossed PCL Scaffolds on Cell Growths. Front. Bioeng. Biotechnol. 2020, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Selli, F.; Gooneie, A.; ErdoGan, U.H.; Hufenus, R.; Perret, E. Properties, X-ray data and 2D WAXD fitting procedures of meltspun poly(ε-caprolactone). Specifications Table. Data br. 2020, 32, 106223. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Hua, M.; Li, H.; Liu, Y.; Xu, M.; Song, Y. Printability, Shape-Memory, and Mechanical Properties of PHB/PCL/CNFs Composites. J. Appl. Polym. Sci. 2021, 138, 50510. [Google Scholar] [CrossRef]
- Aqil, M.; Abderrahim, B.; Abderrahman, E.; Mohamed, A.; Fatima, T.; Abdesselam, T.; Krim, O. Kinetic Thermal Degradation of Cellulose, Polybutylene Succinate and a Green Composite: Comparative Study. World J. Environ. Eng. 2015, 3, 95–110. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M. Superhydrophobic Surfaces. Curr. Opin. Colloid. Interface Sci. 2006, 11, 193–202. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface Characteristics Influencing Bacterial Adhesion to Polymeric Substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Bruinsma, G.M.; van der Mei, H.C.; Busscher, H.J. Bacterial Adhesion to Surface Hydrophilic and Hydrophobic Contact Lenses. Biomaterials 2001, 22, 3217–3224. [Google Scholar] [CrossRef]
- Bayoudh, S.; Othmane, A.; Bettaieb, F.; Bakhrouf, A.; ben Ouada, H.; Ponsonnet, L. Quantification of the Adhesion Free Energy between Bacteria and Hydrophobic and Hydrophilic Substrata. Mater. Sci. Eng. C 2006, 26, 300–305. [Google Scholar] [CrossRef]
- Dou, X.Q.; Zhang, D.; Feng, C.; Jiang, L. Bioinspired Hierarchical Surface Structures with Tunable Wettability for Regulating Bacteria Adhesion. ACS Nano 2015, 9, 10664–10672. [Google Scholar] [CrossRef]
- Ge, X.; Ren, C.; Ding, Y.; Chen, G.; Lu, X.; Wang, K.; Ren, F.; Yang, M.; Wang, Z.; Li, J.; et al. Micro/Nano-Structured TiO2 Surface with Dual-Functional Antibacterial Effects for Biomedical Applications. Bioact. Mater. 2019, 4, 346–357. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-K.; Jang, S.-J.; Cho, Y.-S.; Park, H.-H. Fabrication of Nanostructured Polycaprolactone (PCL) Film Using a Thermal Imprinting Technique and Assessment of Antibacterial Function for Its Application. Polymers 2022, 14, 5527. https://doi.org/10.3390/polym14245527
Kim H-K, Jang S-J, Cho Y-S, Park H-H. Fabrication of Nanostructured Polycaprolactone (PCL) Film Using a Thermal Imprinting Technique and Assessment of Antibacterial Function for Its Application. Polymers. 2022; 14(24):5527. https://doi.org/10.3390/polym14245527
Chicago/Turabian StyleKim, Hee-Kyeong, Se-Jin Jang, Young-Sam Cho, and Hyun-Ha Park. 2022. "Fabrication of Nanostructured Polycaprolactone (PCL) Film Using a Thermal Imprinting Technique and Assessment of Antibacterial Function for Its Application" Polymers 14, no. 24: 5527. https://doi.org/10.3390/polym14245527
APA StyleKim, H.-K., Jang, S.-J., Cho, Y.-S., & Park, H.-H. (2022). Fabrication of Nanostructured Polycaprolactone (PCL) Film Using a Thermal Imprinting Technique and Assessment of Antibacterial Function for Its Application. Polymers, 14(24), 5527. https://doi.org/10.3390/polym14245527






