Synthesis of CuO and PAA-Regulated Silver-Carried CuO Nanosheet Composites and Their Antibacterial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Analytical Methods
2.3. Preparation of CuO
2.4. Preparation of CuO@Ag
2.5. Antimicrobial Activity Testing
3. Results and Discussion
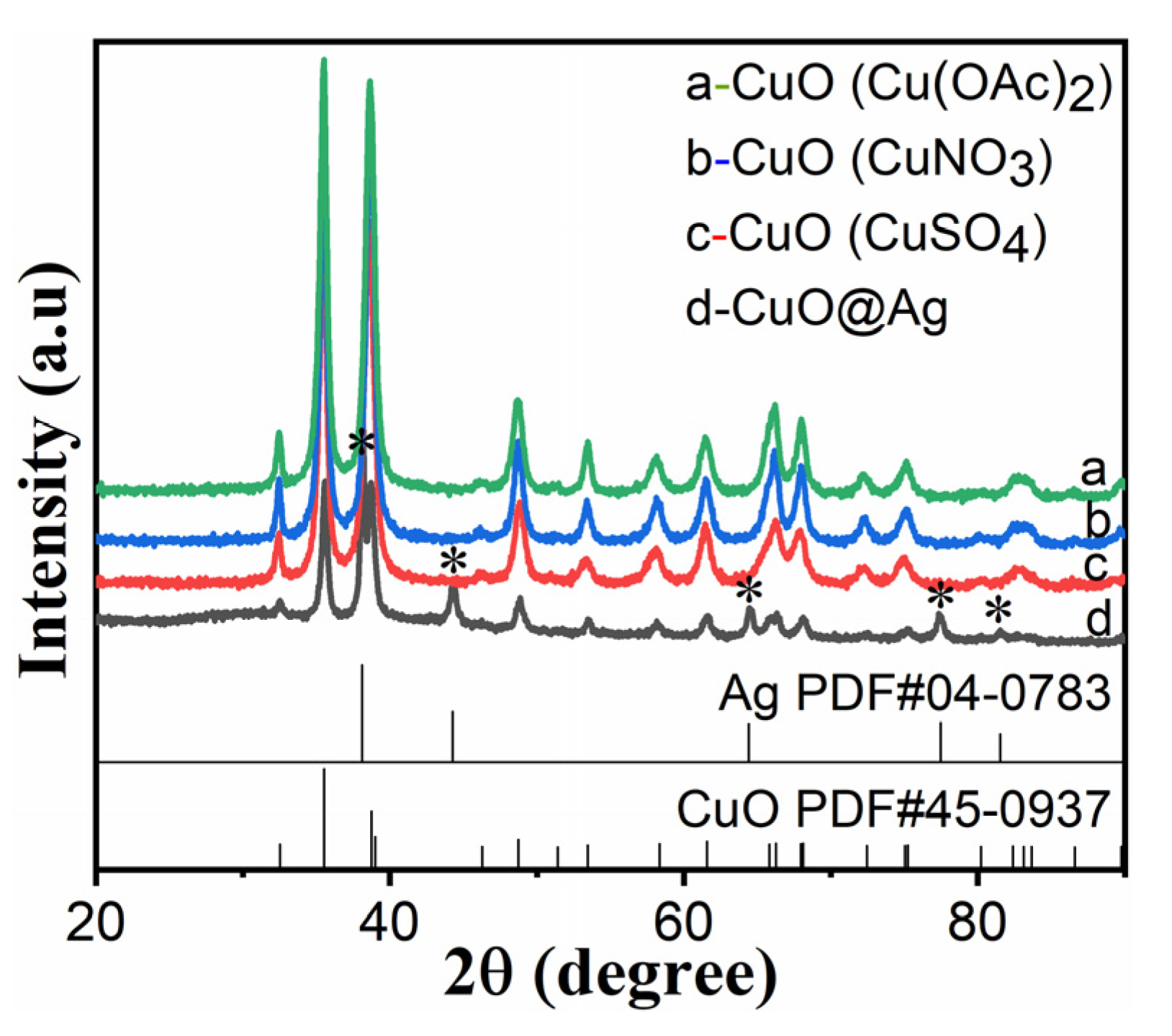
3.1. XRD Patterns of CuO and CuO@Ag Nanosheet Composites
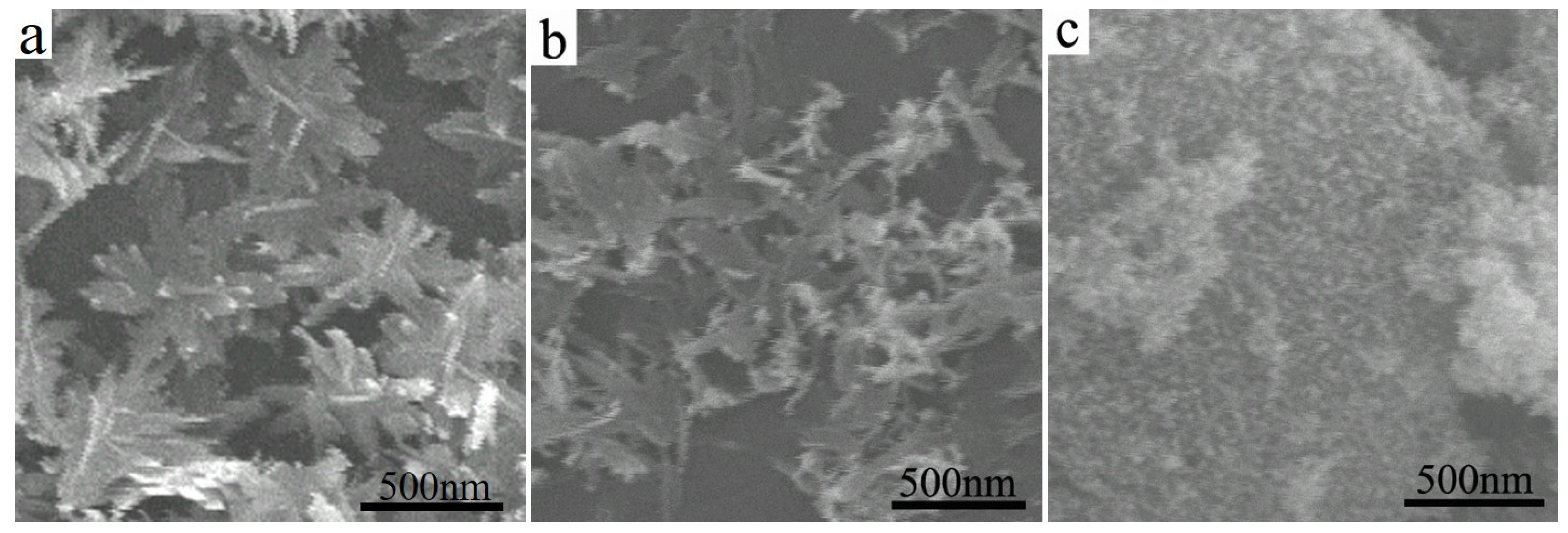
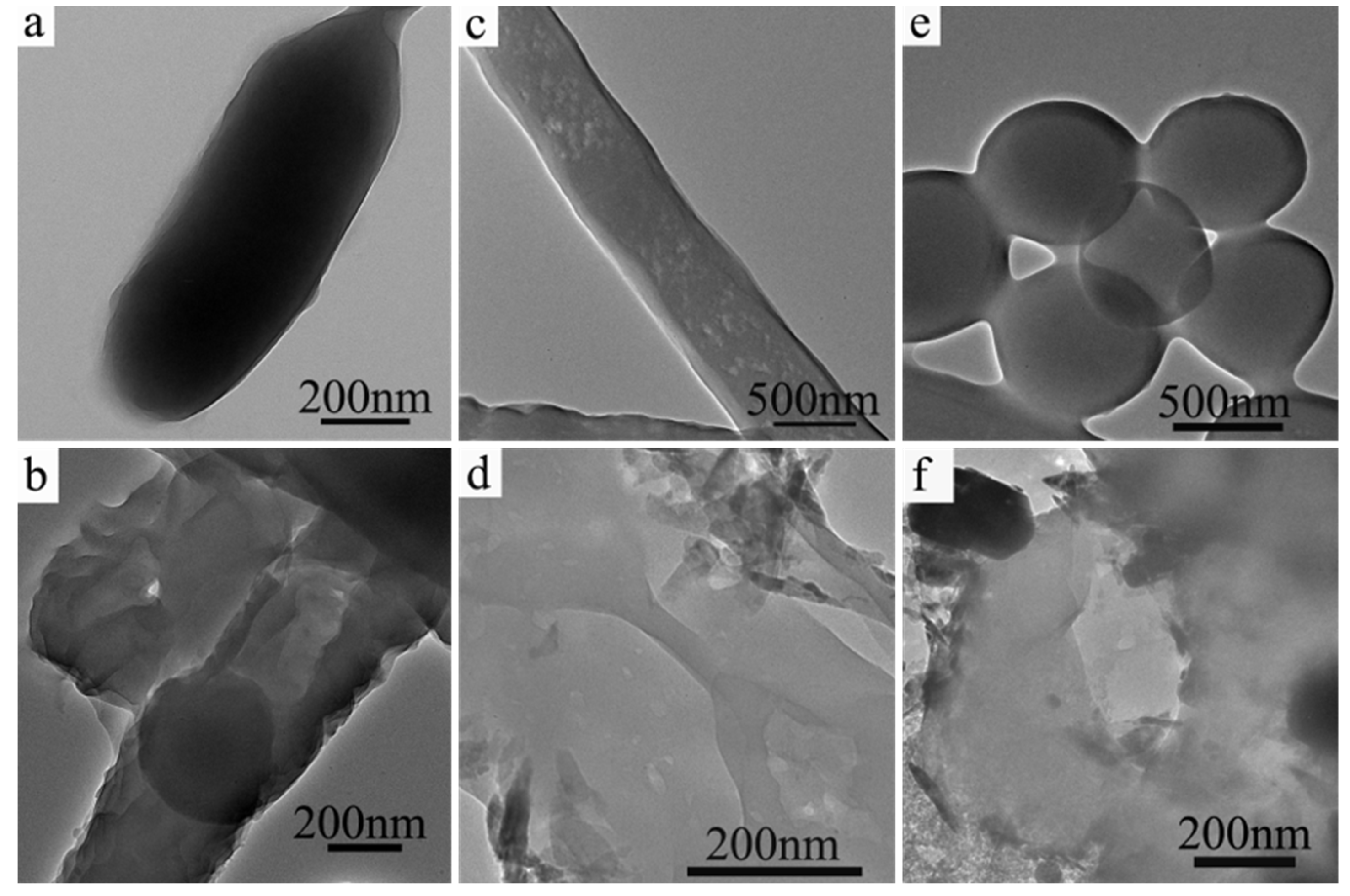
3.2. Morphologies of CuO Nanosheets
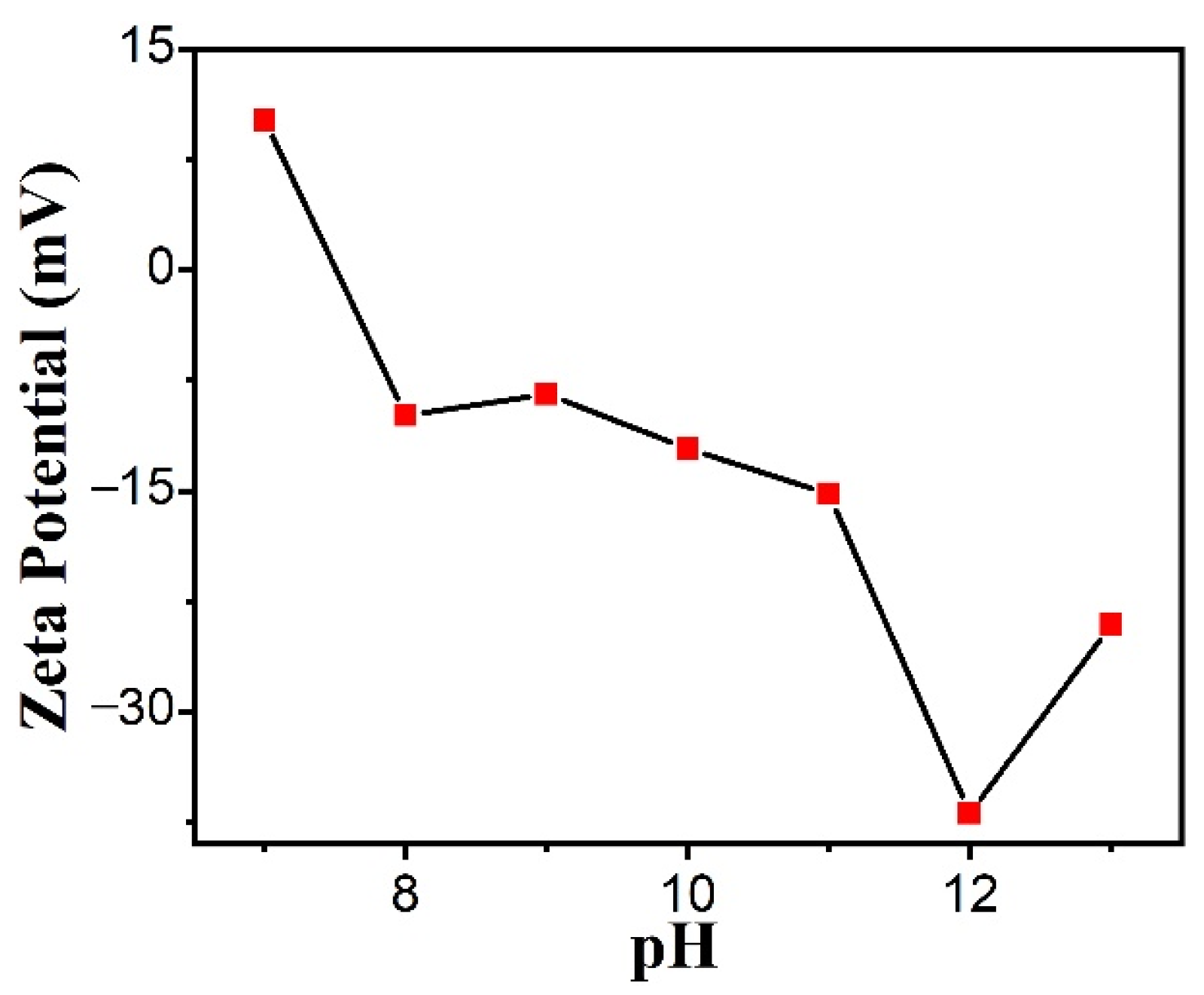
3.3. Zeta Potential of CuO Nanosheets
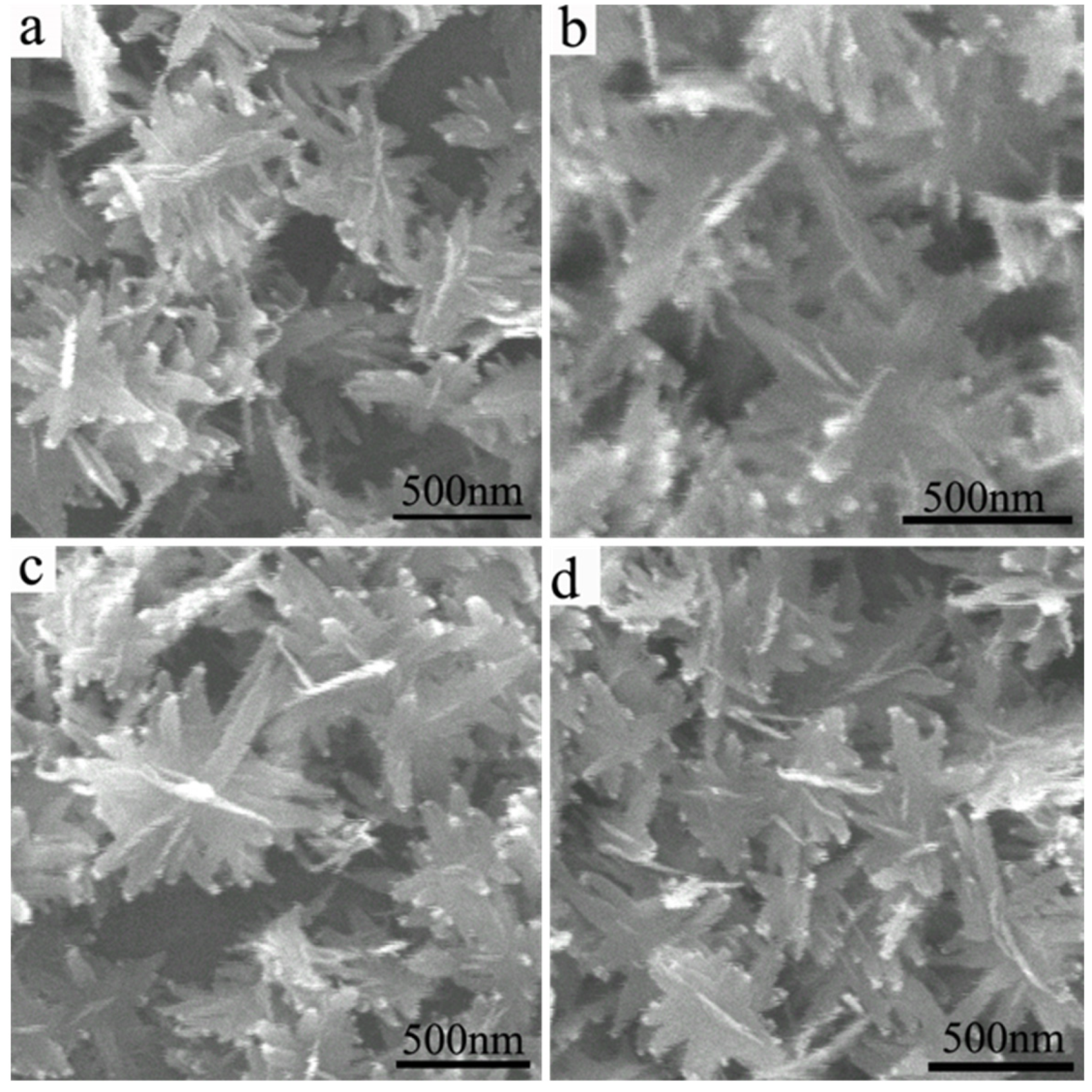
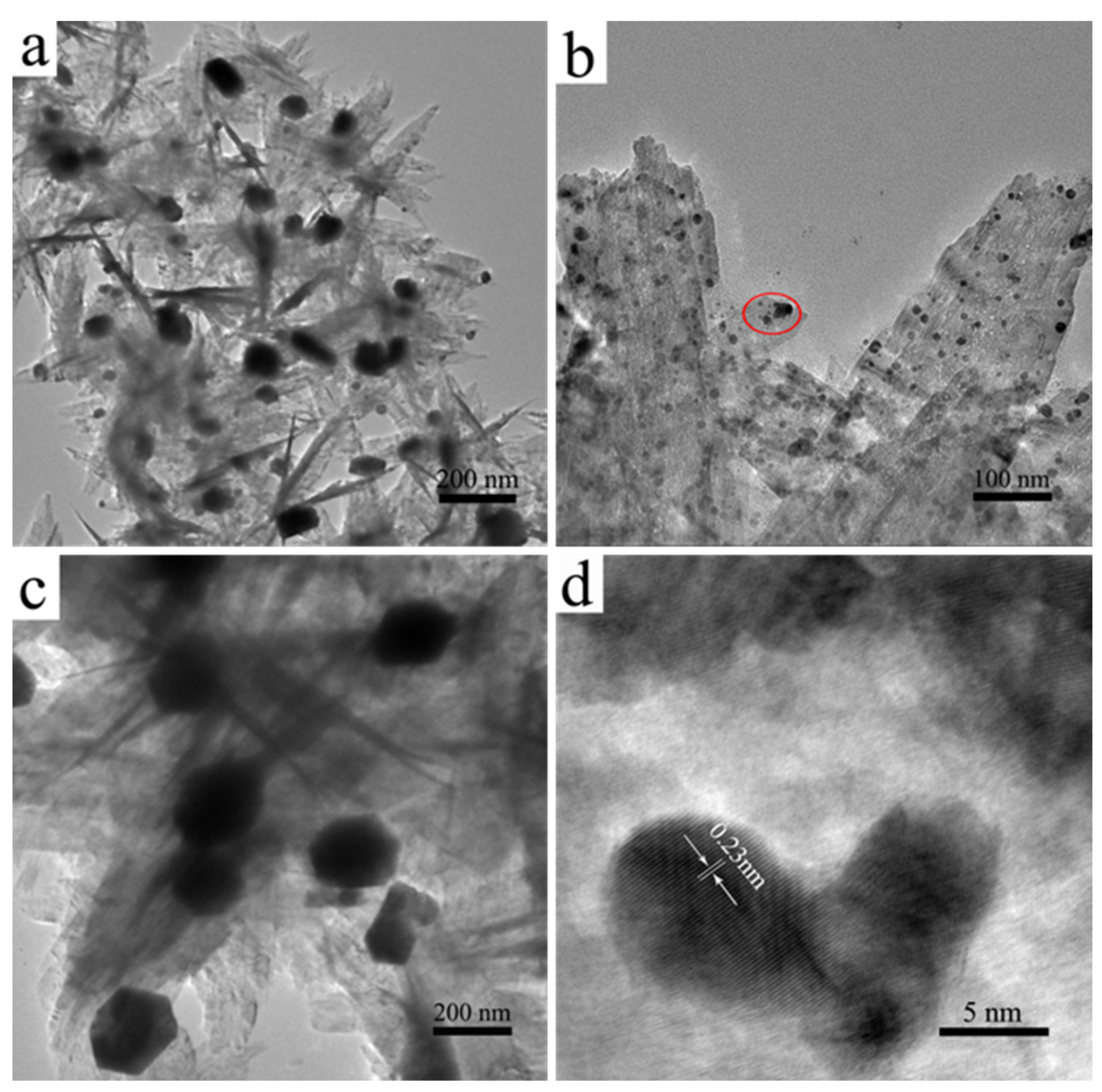
3.4. Morphologies of CuO@Ag Nanosheet Composites
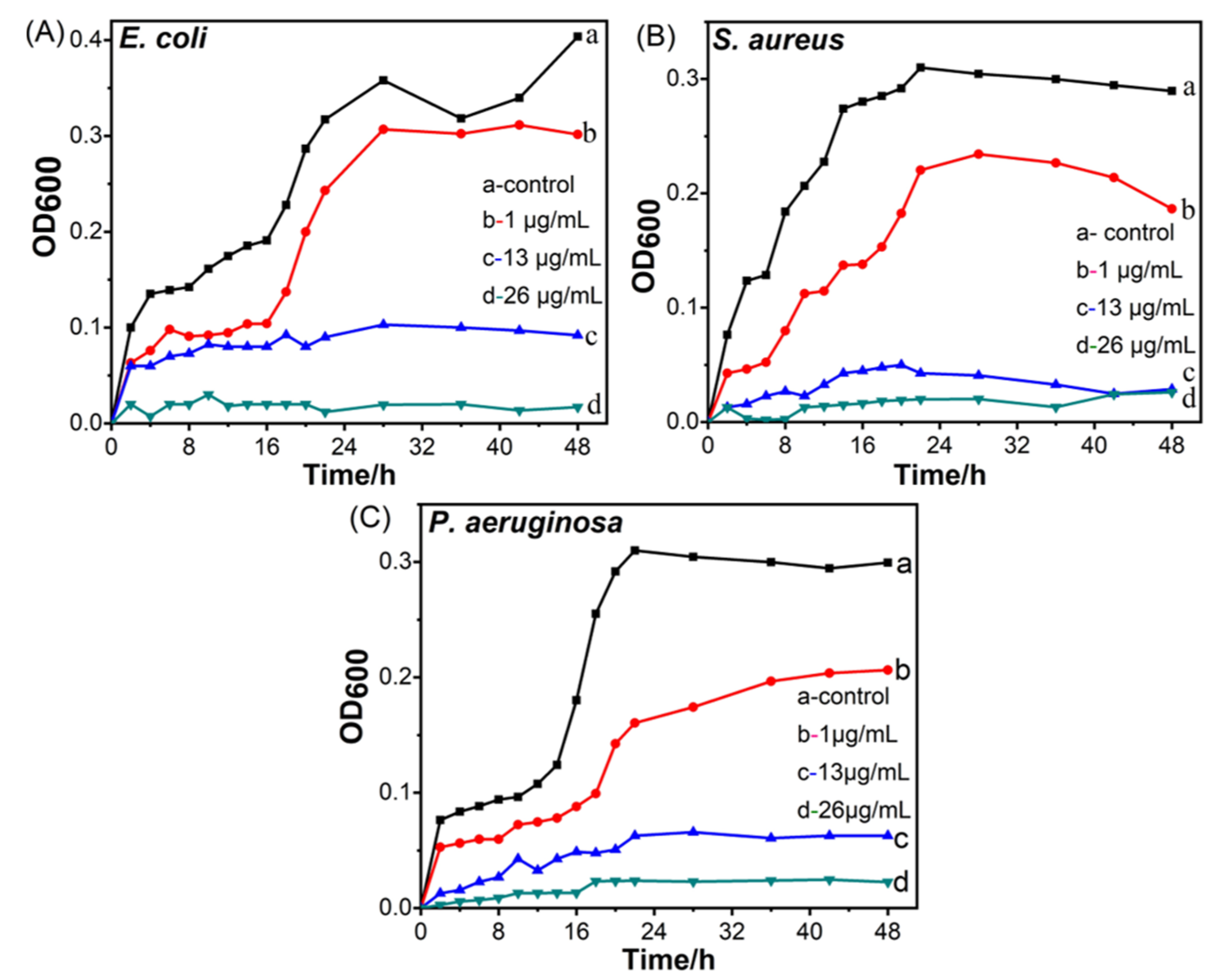
3.5. Antimicrobial Activities of CuO@Ag Nanosheet Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Baarle, D.; Bollaerts, K.; Del Giudice, G.; Lockhart, S.; Luxemburger, C.; Postma, M.J.; Timen, A.; Standaert, B. Preventing infectious diseases for healthy ageing: The VITAL public-private partnership project. Vaccine 2020, 38, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.; Koehler, M.F.T.; Girgis, H.S.; Yan, D.H.; Chen, Y.S.; Chen, Y.; Crawford, J.J.; Durk, M.R.; Higuchi, R.I.; Kang, J.; et al. Optimized arylomycins are a new class of gram-negative antibiotics. Nature 2018, 561, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Thomson, N.; Weill, F.X.; Holt, K.E. Genomic insights into the emergence and spread of antimicrobial-resistant bacterial pathogens. Science 2018, 360, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Luo, Y.; Zheng, Y.F.; Liu, X.M.; Tan, L.; Wu, S.L. Two-dimensional antibacterial materials. Prog. Mater. Sci. 2022, 130, 100976–101116. [Google Scholar] [CrossRef]
- Cave, R.; Misra, R.; Chen, J.Z.; Wang, S.Y.; Mkrtchyan, H.V. Whole genome sequencing revealed new molecular characteristics in multidrug resistant staphylococci recovered from high frequency touched surfaces in London. Sci. Rep. 2019, 9, 9637–9649. [Google Scholar] [CrossRef]
- Agnihotri, S.; Bajaj, G.; Mukherjia, S.; Mukherji, S. Arginine-assisted immobilization of silver nanoparticles on ZnO nanorods: An enhanced and reusable antibacterial substrate without human cell cytotoxicity. Nanoscale 2015, 7, 7415–7429. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Perdikaki, A.; Galeou, A.; Pilatos, G.; Karatasios, I.; Kanellopoulos, N.K.; Prombona, A.; Karanikolos, G.N. Ag and Cu monometallic and Ag/Cu bimetallic nanoparticle-graphene composites with enhanced antibac terial performance. ACS Appl. Mater. Interfaces 2016, 8, 27498–27510. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Govindappa, M.; Manasa, D.J.; Vridhi, V.; Bhoomika, V.; Suryanshi, D.; Ritu, P.; Vinay, B.R. Screening of antibacterial and antioxidant activity of biogenically synthesized silver nanoparticles from alternaria alternata, endophytic fungus of dendrophthoe falcata-a parasitic plant. BioNanoScience 2022, 12, 128–141. [Google Scholar] [CrossRef]
- Chen, P.H.; Yang, Z.; Mai, Z.X.; Huang, Z.Y.; Bie, Y.S.; Wu, S.J.; Dong, X.M.; Fu, X.J.; Ko, F.; Zhang, S.Y.; et al. Electrospun nanofibrous membrane with antibacterial and antiviral properties decorated with myoporum bontioides extract and silver-doped carbon nitride nanoparticles for medical masks application. Sep. Purif. Technol. 2022, 298, 121565–121582. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, N.; Yao, Y.; Zhang, X.; Peng, X.; Zhao, L.; Wang, J.; Peng, L.; Wang, Z.; Mochizuki, K.; et al. Breaking the nanoparticle’s dispersible limit via rotatable surface ligands. Nat. Commun. 2022, 13, 3581–3590. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Li, P.J.; Xie, R.S.; Cao, X.Y.; Su, D.L.; Shan, Y. Biosynthesis of silver nanoparticle composites based on hesperidin and pectin and their synergistic antibacterial mechanism. Int. J. Biol. Macromol. 2022, 214, 220–229. [Google Scholar] [CrossRef]
- Cao, C.J.; Huang, J.C.; Li, L.; Zhao, C.J.; Yao, J.F. Highly dispersed Ag/TiO2 via adsorptive self-assembly for bactericidal application. RSC Adv. 2017, 7, 13347–13352. [Google Scholar] [CrossRef]
- Hou, W.B.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Kumar, P.; Shaikh, A.A.; Kumar, P.; Gupta, V.K.; Dhyani, R.; Sharma, T.K.; Hussain, A.; Gangele, K.; Poluri, K.M.; Rao, K.N.; et al. Double-edged nanobiotic platform with protean functionality: Leveraging the synergistic antibacterial activity of a food-grade peptide to mitigate multidrug-resistant bacterial pathogens. ACS Appl. Mater. Interfaces 2022, 14, 20652–20668. [Google Scholar] [CrossRef]
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the antibacterial mechanism of CuO nanoparticles: Revealing the route of induced oxidative stress. Small 2012, 8, 3326–3337. [Google Scholar] [CrossRef]
- Zhang, H.X.; Ma, J.; Liu, C.; Li, L.; Xu, C.N.; Li, Y.W.; Li, Y.H.; Tian, H.Y. Antibacterial activity of guanidinium-based ionic covalent organic framework anchoring Ag nanoparticles. J. Hazard. Mater. 2022, 435, 128965–128973. [Google Scholar] [CrossRef]
- Peetsch, A.; Greulich, C.; Braun, D.; Stroetges, C.; Rehage, H.; Siebers, B.; Köller, M.; Epple, M. Silver-doped calcium phosphate nanoparticles: Synthesis, characterization, and toxic effects toward mammalian and prokaryotic cells. Coll. Surf. B Biointerfaces 2013, 102, 724–729. [Google Scholar] [CrossRef]
- Cronholm, P.; Karlsson, H.L.; Hedberg, J.; Lowe, T.A.; Winnberg, L.; Elihn, K.; Wallinder, I.O.; Moller, L. Intracellular uptake and toxicity of Ag and CuO nanoparticles: A comparison between nanoparticles and their corresponding metal ions. Small 2012, 9, 970–982. [Google Scholar] [CrossRef]
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Devasconcellos, P.; Bose, S.; Beyenal, H.; Bandyopadhyay, A.; Zirkle, L.G. Antimicrobial particulate silver coatings on stainless steel implants for fracture management. Mater. Sci. Eng. C 2012, 32, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Sjaarda, C.P. Colloidal structure of honey and its influence on antibacterial activity. Compr. Rev. Food. Sci. F 2021, 20, 2063–2080. [Google Scholar] [CrossRef]
- Jin, C.; Rao, S.S.; Xie, J.; Sun, Z.T.; Gao, J.S.; Li, Y.; Li, B.; Liu, S.W.; Liu, L.; Liu, Q.Q.; et al. Enhanced photocatalytic antibacterial performance by hierarchical TiO2/W18O49 Z-scheme heterojunction with Ti3C2Tx-MXene cocatalyst. Chem. Eng. J. 2022, 447, 137369–137379. [Google Scholar] [CrossRef]
- Tan, R.X.; Xie, H.Y.; She, J.Q.; Liang, J.S.; He, H.; Li, J.H.; Fan, Z.Q.; Liu, B. A new approach to fabricate superhydrophobic and antibacterial low density isotropic pyrocarbon by using catalyst free chemical vapor deposition. Carbon 2019, 145, 359–366. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.F.; Chi, W.D.; Yu, C.Y.; Yu, Y.J. Preparation and antibacterial properties of Ag@polydopamine/graphene oxide sheet nanocomposite. Appl. Surf. Sci. 2013, 282, 181–185. [Google Scholar] [CrossRef]
- Yang, F.B.; Zhang, Z.K.; Li, Y.X.; Xiao, C.R.; Zhang, H.; Li, W.; Zhan, L.Z.; Liang, G.Y.; Chang, Y.B.; Ning, C.Y.; et al. In situ construction of black titanium oxide with a multilevel structure on a titanium alloy for photothermal antibacterial therapy. ACS Biomater. Sci. Eng. 2022, 8, 2419–2427. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Effect of Zn salts and hydrolyzing agents on the morphology and antibacterial activity of zinc oxide nanoparticles. Environ. Chem. Lett. 2019, 17, 1105–1109. [Google Scholar] [CrossRef]
- Ali, T.; Warsi, M.F.; Zulfiqar, S.; Sami, A.; Ullah, S.; Rasheed, A.; Alsafari, I.A.; Agboola, P.O.; Shakir, I.; Baig, M.M. Green nickel/nickel oxide nanoparticles for prospective antibacterial and environmental remediation Applications. Ceram. Int. 2022, 48, 8331–8340. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Falamarzi, M.; Gholami, M.R. Synthesis of M/CuO (M = Ag, Au) from Cu based metal organic frameworks for efficient catalytic reduction of p-nitrophenol. Mater. Chem. Phys. 2017, 198, 374–379. [Google Scholar] [CrossRef]
- Hu, L.; Huang, Y.; Zhang, F.; Chen, Q. CuO/Cu2O composite hollow polyhedrons fabricated from metal-organic framework templates for lithium-ion battery anodes with a long cycling life. Nanoscale 2013, 5, 4186–4190. [Google Scholar] [CrossRef]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and characterization of antibacterial carboxymethylcellulose/CuO bio-nanocomposite hydrogels. Int. J. Biol. Macromol. 2015, 73, 109–114. [Google Scholar] [CrossRef]
- Maji, S.K.; Mukherjee, N.; Mondal, A.; Adhikary, B.; Karmakar, B. Chemical synthesis of mesoporous CuO from a single precursor: Structural, optical and electrical properties. J. Solid State. Chem. 2010, 183, 1900–1904. [Google Scholar] [CrossRef]
- Kalaivani, S.; Singh, R.K.; Ganesanb, V.; Kannan, S. Effect of copper (Cu2+) inclusion on the bioactivity and antibacterial behavior of calcium silicate coatings on titanium metal. J. Mater. Chem. B 2014, 2, 846–858. [Google Scholar] [CrossRef]
- Liu, S.Y.; Ru, J.L.; Liu, F.Z. NiP/CuO composites: Electroless plating synthesis, antibiotic photodegradation and antibacterial properties. Chemosphere 2021, 267, 129220–129229. [Google Scholar] [CrossRef]
- Ewald, A.; Kappel, C.; Vorndran, E.; Moseke, C.; Gelinsky, M.; Gbureck, U. The effect of Cu(II)-loaded brushite scaffolds on growth and activity of osteoblastic cells. J. Biomed. Mater. Res. Part A 2012, 100, 2392–2400. [Google Scholar] [CrossRef]
- Ghosh, D.; Godeshala, S.; Nitiyanandan, R.; Islam, M.S.; Yaron, J.R.; DiCaudo, D.; Kilbourne, J.; Rege, K. Copper-eluting fibers for enhanced tissue sealing and repair. ACS Appl. Mater. Interfaces 2020, 12, 27951–27960. [Google Scholar] [CrossRef]
- Karekar, N.; Karan, A.; Khezerlou, E.; Prajapati, N.; Pernici, C.D.; Murray, T.A.; DeCoster, M.A. Self-assembled metal–organic biohybrids (MOBs) using copper and silver for cell studies. Nanomaterials 2019, 9, 1282–1294. [Google Scholar] [CrossRef]
- Prajapati, N.; Karan, A.; Khezerlou, E.; DeCoster, M.A. The immunomodulatory potential of copper and silver based self-assembled metal organic biohybrids nanomaterials in cancer theranostics. Front. Chem. 2021, 8, 629835–629846. [Google Scholar] [CrossRef]
- Leng, X.; She, M.Y.; Jin, X.L.; Chen, J.; Ma, X.H.; Chen, F.L.; Li, J.L.; Yang, B.Q. A highly sensitive and selective fluorescein-based Cu2+ probe and its bioimaging in cell. Front. Nutr. 2022, 9, 932826–932833. [Google Scholar] [CrossRef]
- Mageshwari, K.; Sathyamoorthy, R. Flower-shaped CuO nanostructures: Synthesis, characterization and antimicrobial activity. J. Mater. Sci. Technol. 2013, 29, 909–914. [Google Scholar] [CrossRef]
- Bouazizi, N.; Vieillard, J.; Thebault, P.; Desriac, F.; Clamens, T.; Bargougui, R.; Couvrat, N.; Thoumire, O.; Brun, N.; Ladam, G.; et al. Silver nanoparticle embedded copper oxide as an efficient core–shell for the catalytic reduction of 4-nitrophenol and antibacterial activity improvement. Dalton Trans. 2018, 47, 9143–9155. [Google Scholar] [CrossRef]
- Poola, S.; Shaik, M.S.; Sudileti, M.; Yakkate, S.; Nalluri, V.; Chippada, A.; Cirandur, S.R. Nano CuO-Ag-catalyzed synthesis of some novel pyrano[2,3-d] pyrimidine derivatives and evaluation of their bioactivity. J. Chin. Chem. Sci. 2019, 67, 805–820. [Google Scholar] [CrossRef]
- Asamoah, R.B.; Annan, E.; Mensah, B.; Nbelayim, P.; Apalangya, V.; Onwona-Agyeman, B.; Yaya, A. A comparative study of antibacterial activity of CuO/Ag and ZnO/Ag nanocomposites. Adv. Mater. Sci. Eng. 2020, 4, 7814324. [Google Scholar] [CrossRef]
- Yan, J.L.; Xia, D.D.; Zhou, W.H.; Li, Y.Y.; Xiong, P.; Li, Q.Y.; Wang, P.; Li, M.; Zheng, Y.F.; Cheng, Y. pH-responsive silk fibroin-based CuO/Ag micro/nano coating endows polyetheretherketone with synergistic antibacterial ability, osteogenesis, and angiogenesis. Acta Biomater. 2020, 115, 220–234. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; Salem, J.; Kodeha, F.S.; Elmanamab, A.; Anbar, R. CuO-NPs, CuO-Ag nanocomposite and Cu(II)-curcumin complex coated cotton/starched cotton antimicrobial materials. Mater. Chem. Phys. 2022, 285, 126099–126105. [Google Scholar] [CrossRef]
- Ni, Z.H.; Gu, X.X.; He, Y.L.; Wang, Z.H.; Zou, X.Y.; Zhao, Y.B.; Sun, L. Synthesis of silver nanoparticle-decorated hydroxyapatite (HA@Ag) poriferous nanocomposites and the study of their antibacterial activities. RSC Adv. 2018, 8, 41722–41730. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.H.; Wang, Z.H.; Sun, L.; Li, B.J.; Zhao, Y.B. Synthesis of poly acrylic acid modified silver nanoparticles and their antimicrobial activities. Mater. Sci. Eng. C 2014, 41, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Francisco-Fernandez, M.; Quinto, E.J. A random effect multiplicative heteroscedastic model for bacterial growth. BMC Bioinform. 2010, 11, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.A.; An, I.B.; Lim, D.G.; Lim, J.Y.; Lee, S.Y.; Shim, W.S.; Kang, N.G.; Jeong, S.H. Effects of pH and buffer concentration on the thermal stability of etanercept using DSC and DLS. Biol. Pharm. Bull. 2014, 37, 808–816. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Gobinath, C.; Karpagam, K.; Hemamalini, V.; Premkumar, K.; Sivaramakrishnan, S. Phyto-synthesis of silver nanoscale particles using morinda citrifolia L. and its inhibitory activity against human pathogens. Colloids Surf. B Biointerfaces 2012, 95, 235–240. [Google Scholar] [CrossRef]
- Maribel, G.; Jean, D.; Stéphane, G. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef]

| MIC (μg/mL) | MBC (μg/mL) | |||||
|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | S. aureus | E. coli | P. aeruginosa | S. aureus | |
| Ag NPs | 125 | 250 | 125 | 250 | 500 | 250 |
| CuO@Ag | 13 | 6.5 | 3.2 | 26 | 26 | 13 |
| CuO nanosheet | 500 | 500 | 125 | 1000 | 1000 | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Z.; Wan, M.; Tang, G.; Sun, L. Synthesis of CuO and PAA-Regulated Silver-Carried CuO Nanosheet Composites and Their Antibacterial Properties. Polymers 2022, 14, 5422. https://doi.org/10.3390/polym14245422
Ni Z, Wan M, Tang G, Sun L. Synthesis of CuO and PAA-Regulated Silver-Carried CuO Nanosheet Composites and Their Antibacterial Properties. Polymers. 2022; 14(24):5422. https://doi.org/10.3390/polym14245422
Chicago/Turabian StyleNi, Zhihui, Menghui Wan, Gongming Tang, and Lei Sun. 2022. "Synthesis of CuO and PAA-Regulated Silver-Carried CuO Nanosheet Composites and Their Antibacterial Properties" Polymers 14, no. 24: 5422. https://doi.org/10.3390/polym14245422
APA StyleNi, Z., Wan, M., Tang, G., & Sun, L. (2022). Synthesis of CuO and PAA-Regulated Silver-Carried CuO Nanosheet Composites and Their Antibacterial Properties. Polymers, 14(24), 5422. https://doi.org/10.3390/polym14245422







