Abstract
To overcome the low water solubility and low bioavailability of curcumin (CUR), multiple delivery strategies have been proposed. Among these, cyclodextrin-based carriers have been widely used for the encapsulation and delivery of CUR. Cyclodextrins (CDs), as natural oligosaccharides, have been well known for their biodegradability, biocompatibility, non-toxicity, and internal hydrophobic and external hydrophilic structural features. This paper summarizes the recently reported CD-based carriers for encapsulating CUR. Particularly, the polymerization properties of CD self-assembly to enhance the encapsulation of CUR are discussed. In addition, the current progress on stimuli-responsive CD carriers for controlled release of CUR is described, which laid an important foundation for the development of CUR-based precision therapy in clinical practice. In conclusion, this review may provide ideas for the future development of a CD-based encapsulant for CUR.
1. Introduction
Curcumin (CUR) is a natural yellow polyphenolic compound extracted mainly from the roots of some plants in Zingiberaceae (commonly known as the ginger family) and Araceae [1]. CUR has received wide attention because of its various biological activities, including antioxidant, anti-inflammatory, anti-bacterial, anti-viral, anti-cancer, anti-diabetic, and neuroprotective [2,3,4,5,6]. Currently, many studies have shown that it has good effects in the treatment of cancer, cardiovascular disease, inflammation, diabetes, and neurological disorders [7,8,9,10,11]. Furthermore, the safety of CUR has been confirmed by pharmacological and toxicological studies; even at doses of 8 g/day to 12 g/day, it does not cause significant toxic side effects to humans [12]. Therefore, the US Food and Drug Administration approved curcumin as “Generally Recognized as Safe” (GRAS) [13]. However, poor water solubility and stability are the main reasons for the failure of most phytochemicals [14]. CUR also suffers from these defects, being highly lipophilic and having very low intrinsic water solubility, with a solubility of 11 μg/mL in water [15]. In addition, CUR is also susceptible to degradation during storage due to environmental factors such as light, heat, and oxygen, which greatly limits its application in pharmaceuticals, food, and other related fields [16]. Furthermore, CUR is poorly absorbed in the intestinal tract and is rapidly metabolized in the liver, preventing it from being effective in the body [17]. Therefore, there is an urgent need to develop methods that not only improve its water solubility and stability but also regulate its biodistribution after administration.
Currently, the application of cyclodextrins for curcumin encapsulation and delivery is a promising strategy to overcome the aforementioned limitations. Cyclodextrins (CDs), obtained by enzymatic digestion of starch using cyclodextrin glycosyltransferase, are made from D-glucopyranose units connected by α-1,4-glycosidic bonds, usually containing six, seven, or eight glucose units, called α, β, and γ-CD, respectively [18]. Due to the internal hydrophobic and external hydrophilic properties, CDs can be loaded with various hydrophobic drugs by forming inclusion complexes, thus improving the water solubility of these drugs [19]. In addition, CDs are safe for humans, so they are considered to be ideal carriers for various drugs [20]. Furthermore, the latest studies further demonstrate that CD could be used to develop intelligent, stimulus-responsive drug carriers, which can be stimulated by factors including changes in pH, light, and enzymes to release the encapsulated drugs [21]. This strategy could achieve targeted and on-demand drug delivery at specific pathological sites and decrease the undesirable effects on the sensitive normal tissues, which has drawn great attention in the field of cancer therapy due to the differences between the microenvironment of normal and tumor cells [22]. Indeed, the stimulus-responsive CDs-based nanoparticles have also been used successfully to enhance the therapeutic potential of CUR [21]. Therefore, this review will summarize the literature related to CD-based carriers for encapsulating CUR as well as discuss the latest progress on the stimulus-responsive CD carriers for the precise release of CUR.
2. Curcumin–Cyclodextrin Supramolecular System
Supramolecular systems are based on molecular recognition, in which two or more molecules are bound by intermolecular non-covalent bonding forces to form complex and ordered entities or aggregates with specific functions and properties [23]. CD can be used to encapsulate CUR due to its cylinder structure, forming a relatively simple host–guest supramolecular system, which can improve the water solubility and bioavailability of CUR [24,25,26]. Notably, instead of being limited to a single host–guest form, CDs monomers could also be constructed into functional polymers by chemical modification or physical aggregation [27,28]. This allows the cyclodextrin polymer to achieve enhanced loading capacity and solubility for CUR [29]. Furthermore, introducing other functional molecule(s) into the curcumin–cyclodextrin supramolecular system was also proven to be a promising strategy for its delivery properties. Herein, different supramolecular systems of curcumin cyclodextrins are presented.
2.1. Curcumin–Cyclodextrin Supramolecular System with Cyclodextrin as the Carrier
2.1.1. Natural Cyclodextrins
The first wave of studies has focused on the interaction of CUR with natural CDs. The researchers used solvent evaporation, freeze-drying, kneading, and other methods to prepare curcumin–cyclodextrin inclusion complexes [19]. When inclusion complexes are formed, the crystallinity, solubility, and optical properties of CUR molecules are changed, and the properties are subsequently measured using thermal analysis, spectroscopy, or chromatography to verify the formation of the inclusion complex [30]. The relevant articles on cyclodextrin-encapsulated curcumin are summarized in Table 1.
Solvent evaporation is the most commonly used method for the preparation of curcumin–cyclodextrin complexes due to its simplicity (Figure 1A). For instance, this method was used by Yallapu et al. to prepare CUR/β-CD as a light-yellow fluffy powder with excellent aqueous solubility (Figure 1B). Unlike pure curcumin, which readily precipitates in an aqueous solution, curcumin encapsulated by β-CD showed an increased solubility up to 1.84 mg/mL in water (Figure 1C) [31]. In another study, López-Tobar et al. used β-CD and γ-CD as the carriers to encapsulate curcumin and analyzed the stability of the inclusion complexes using Raman spectroscopy. The results illustrated that H-bonds play an important role in the encapsulation process of curcumin, prompting changes in the structure of curcumin from the planar keto-enol tautomer to the non-planar diketone tautomer. These changes may cause an increase in the bioavailability, bioactivity, and chemical stability of curcumin. It is also interesting to note that the authors mention that γ-CD affords better encapsulation than β-CD, which may be due to the fact that the size matching between curcumin and gamma CD cavity is better [32]. Similarly, Alizadeh et al. also demonstrated that hydrogen bonds play a key role in enhancing the physiological activity of CUR [33]. Their study compared the antioxidant activity of free CUR with CUR/β- or γ-CD. The results indicated that CUR/γ-CD had superior antioxidant activity to that of CUR/β-CD or free CUR. This was attributed to the formation of one or more intermolecular hydrogen bonds upon the complexation of CUR by the CDs, which affected the intramolecular hydrogen bonds of CUR, thus enhancing the hydrogen-donating ability (enhanced antioxidant activity) of CUR molecules. In addition, Jahed et al. further investigated the interaction forces between CUR and CD using NMR spectroscopy [34]. The 1H NMR and 2D ROESY spectra confirmed that the chemical shifts of the internal protons of β-CD (H-3 and H-5) were shifted after encapsulation, and there was a cross-peak between the H-3 proton of β-CD and the aromatic rings group of CUR. These studies show that the driving forces involved in the CD encapsulation of CUR include hydrophobic interactions between host and guest, hydrogen bonding, van der Waals forces, and other non-covalent bonding forces. These driving forces sometimes act individually, but in most cases, multiple forces act synergistically to promote the formation of supramolecular systems.
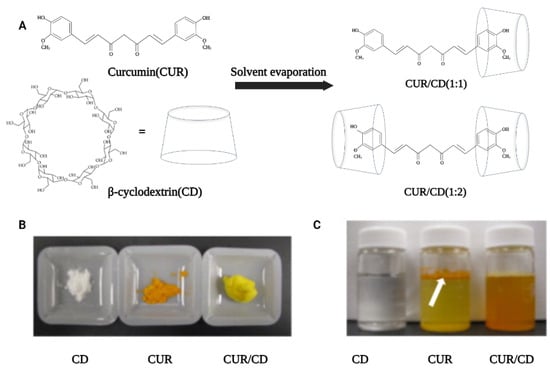
Figure 1.
Preparation process and solid-liquid form of CUR/β-CD. (A) Schematic diagram of the preparation of curcumin/β-cyclodextrin supramolecules by solvent evaporation. (B) Solid powder samples of β-cyclodextrin, curcumin and curcumin/β–cyclodextrin inclusion complex (CUR/CD). (C) Aqueous solutions of CD, CUR and CUR/CD inclusion complex (5 mg/mL) [31].
2.1.2. Cyclodextrin Derivatives
Natural CDs themselves have many shortcomings, such as the small pore size of α-CD cavities and relatively low water solubility of β-CD, which hinder the further application of CDs. For this reason, researchers have introduced modified groups to obtain cyclodextrin derivatives with different properties or functions, while keeping the basic skeleton of CD macrocycles unchanged. These derivatives are classified as hydrophilic, hydrophobic, ionic, and amphiphilic; and these modified CDs have also been widely investigated in the application of curcumin encapsulation.
Since the main objective of curcumin–cyclodextrin complexation is to obtain an inclusion complex with high water solubility, therefore, hydrophilic cyclodextrin derivatives are the primary choice for encapsulating CUR. For example, hydroxypropyl β-CD (HP-β-CD) is an alkylation product of β-CD. Alkylating the -OH groups on the periphery of β-CD with hydroxypropyl groups breaks the series of hydrogen bonds that these -OH groups make. This improves the solubility of the resulting HP-β-CD [35]. Li et al. used HP-β-CD as a carrier to improve the solubility and oral bioavailability of the poorly soluble drug CUR [36]. In rats, CUR/HP-β-CD and free CUR had similar pharmacokinetic behaviors after intravenous administration, and both had similar antitumor efficacy. Moreover, the oral bioavailability of CUR was enhanced 2.77-fold by HP-β-CD encapsulation. Additionally, Shityakov et al. demonstrated that the concentration of CUR in distilled water was about 60-fold higher when HP-γ-CD was used as compared to γ-CD due to the better hydrophilicity of HP-γ-CD [37]. These articles showed that CUR encapsulated in hydroxypropyl-modified CD resulted in a complex with superior water solubility. Articles that describe CUR encapsulated in other cyclodextrin derivatives are listed in Table 1.
Interestingly, Mai et al. prepared solid dispersions of CUR/HP-β-CD by grinding, freeze-drying, and common solvent evaporation methods [38]. The solubility of the inclusion complexes was increased 299, 180, and 489-fold, respectively, as compared with CUR crystals. Surprisingly, this solid dispersion did not consist of pure inclusion complexes but was rather a mixed system. The system consisted of free CUR molecules, inclusion complexes, CUR molecules not in inclusion complexes, and empty HP-β-CD molecules (Figure 2B). One or both of the aromatic rings of curcumin entered into the HP-β-CD cavity to form a 1:1 or 2:1 host-to-guest ratio inclusion complex (Figure 2A). In addition, unlike the free CUR molecules, the CUR molecules that were not in the inclusion complex may have been trapped in the cavities of a three-dimensional network structure formed by the polymerization of multiple cyclodextrin monomers. These results indicate that the actual process of curcumin inclusion in cyclodextrin does not present an ideal state in which the components are independent of each other, but rather is a complex system.
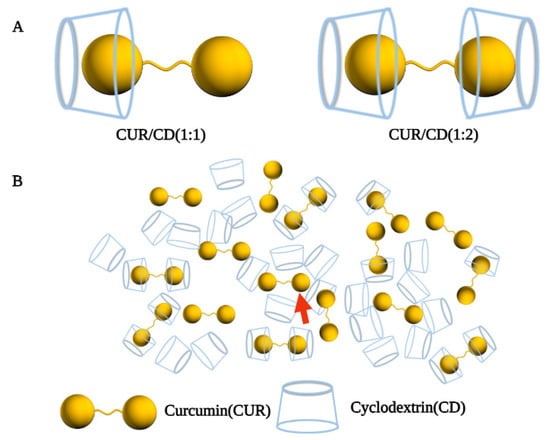


Figure 2.
A mixed system. (A) 1:1 CUR/ HP-β-CD complex; 1:2 CUR/ HP-β-CD complex; (B) Mixed system of curcumin complexed cyclodextrin and the red arrow marks the CUR that was not in the inclusion complex [38].

Table 1.
Introduction to various curcumin–cyclodextrin supramolecular systems, with emphasis on cyclodextrin types, preparation techniques and structural characterization techniques.
Table 1.
Introduction to various curcumin–cyclodextrin supramolecular systems, with emphasis on cyclodextrin types, preparation techniques and structural characterization techniques.
| CD Type | Preparation Method | Host to Guest Ratio | Characterization Techniques | Key Findings | Reference |
|---|---|---|---|---|---|
| HP-α-CD, HP-β-CD, HP-γ-CD | Co-evaporation, Freeze-drying | 2:1, 1:1 | FTIR, Raman spectra, XRD, UV-Vis and DSC | Raman spectroscopy can be used as an effective means of verifying the formation of inclusion compounds. | [39] |
| β-CD | Freeze-drying | - | FTIR, 1H NMR, DSC, TGA, XRD, SEM and TEM | CD enhanced the delivery of CUR in prostate cancer cells and improved its therapeutic efficacy compared to free CUR. | [31] |
| β-CD | Co-precipitation, Freeze-drying, Solvent evaporation | 2:1 | FTIR, Raman spectroscopy and XRD | The application of CUR/CD complex in vanilla ice creams intensified the color of the products and produced a great sensorial acceptance. | [40] |
| β-CD, γ-CD, HPβCD, 2-O-methyl-β-CD, HP-γ-CD | - | - | 1H NMR, 13C NMR, FTIR and DSC | All five CUR/CD inclusions showed improved hydrolytic stability compared to CUR, but all had reduced antioxidant potential. | [41] |
| β-CD | Solvent evaporation, Freeze-drying | 1:1 | NMR, | NMR spectroscopy elucidates the mechanism by which β-CD enhances the water solubility of CUR. | [34] |
| β-CD, γ-CD | Solvent evaporation, Freeze-drying | 1:1, 2:1, 4:1, 8:1 | Raman spectroscopy and UV-Vis | Raman spectroscopy elucidates the mechanism of interaction between CUR and CD. | [32] |
| β-CD | Kneading, Co-precipitation | 2:1 | FTIR, SEM, XRD and UV-Vis | β-CD proven to be an excellent sustained release carrier for CUR. | [42] |
| β-CD | Saturated aqueous solution | - | FTIR and UV-Vis | CD may be used as a carrier to improve the release and therapeutic efficacy of CUR in lung cancer. | [43] |
| β-CD, γ-CD | The soluble method | 1:1 | UV-Vis, FTIR and 1H NMR | The CUR/CD inclusion has more antioxidant activity than free CUR. | [33] |
| β-CD | Kneading | 1:1 | NMR, FTIR, XRD, TGA and SEM | β-CD as a carrier enhances the anti-proliferative effect of CUR during the complexation process. | [44] |
| β-CD | Coprecipitation, Kneading, Simple mixing | 2:1 | DSC, TGA and 1H NMR | CUR/β-CD has greater color development than pure colorants and the use of the complexes in dairy products can produce a great sensorial acceptance. | [45] |
| HP-β-CD, Sulfobutylether-β-CD(SBE-β-CD) | Solvent evaporation, Freeze-drying, Autoclaving | - | 1H NMR, Raman spectroscopy, DSC and XRD | The autoclaving method for complex formation was found to be the most efficient in terms of processing time and CUR encapsulation efficiency. | [46] |
| HP-β-CD | Solvent evaporation, Freeze-drying, PH shift | 1:1 | DSC and FTIR | Among the three methods of inclusion preparation, solvent evaporation is the most suitable method for preparation of CUR/HP-β-CD inclusion. | [47] |
| HP-β-CD | - | - | DSC | The PH value plays an important role in the formation of inclusion compounds. | [48] |
| HP-β-CD | Co-precipitation | - | FTIR, XRD and SEM | CUR/HP-β-CD inclusions have better potential than CUR nanoparticles for application in Alzheimer’s disease. | [49] |
| HP-β-CD | The grinding method | 1:1, 2:1, 3:1 | FTIR and DSC | CUR/ HP-β-CD in situ hydrogel are a promising formulation for melanoma treatment. | [50] |
| HP-β-CD | Kneading | 1:1 | SEM, DSC and FTIR | HP-β-CD complexation improves intestinal absorption of CUR. | [51] |
| HP-β-CD | Cosolvent-lyophilization | 3:1 | FTIR, XRD and DSC | The oral bioavailability of CUR was enhanced to 2.77-fold by the HP-β-CD. | [36] |
| HP-β-CD | Co-evaporation | 1.35:1 | UV-Vis, FTIR, NMR, XRD, DSC, TGA and SEM | A supramolecular system for the complexation of the modified CUR with HP-β-CD was established. | [52] |
| HP-β-CD | Grinding, Freeze-Drying, Common solvent evaporation | - | XRD, FTIR and DSC | The solid dispersion system consisting of CUR and HPβCD significantly increased the solubility of the drug compared to the inclusion complex. | [38] |
| SBE-β-CD | Freeze-drying, Kneading, Co-evaporation | 1:1 | 1H NMR, FTIR, DSC and SEM | The CUR/SBE-β-CD complex has potential in the treatment of lung cancer. | [53] |
| Methyl-β-CD (M-β-CD) | Solvent evaporation | - | SEM | The CUR/M-β-CD inclusion complex showed higher antimicrobial potency than CUR nanoparticles. | [54] |
| Randomly methylated-β-CD (RM-β-CD) | Saturated aqueous solution | - | UV-Vis and FTIR | CUR forms a 1:1 inclusion complex with RM-β-CD. | [55] |
| Succinic acid-β-CD | - | - | - | Succinic acid-β-cyclodextrin affects the biological accessibility of curcumin in the circulation by modulating the binding of curcumin to bovine serum proteins. | [56] |
| γ-CD, HP-γ-CD | - | - | UV-Vis | HP-γ -CD has a better solubilizing effect on CUR than γ-CD. | [37] |
2.2. Curcumin–Cyclodextrin Supramolecular System with Cyclodextrin Polymer as the Carrier
The structure of CD allows the formation of polymers with different structural characteristics, either covalently or non-covalently bonded [57]. These polymers have both the inclusion properties of CD and the favorable properties of polymers and are often used to form complexes with other molecules [58]. Indeed, several types of CD polymers have been reported to successfully encapsulate curcumin, which are summarized in Table 2.
2.2.1. Cyclodextrin Self-Assembled Supramolecular Networks with Curcumin Encapsulated
Self-assembly can be defined as the process by which molecules or other assembled substrates spontaneously form ordered structural bodies through weak interactions [59]. CDs form amorphous, micelle-like, high-molecular-weight polymers by self-assembly to form a supramolecular system with the guest [27]. In this system, changes to molecular structure translate to differences in supramolecular forms, including superlattice crystals, micelles, vesicles, and liquid crystals. Each of these has unique structural features and new physicochemical properties completely different from those of the original constituent molecules [60].
Supramolecular vesicles are hollow spheres with hydrophobic membranes and hydrophilic interiors, and the strategy of loading CUR in CD self-assembled supramolecular vesicles may be a good solution [61]. In a study by Ma et al., CD molecules encapsulated CUR through host–guest recognition to form a supramolecular amphiphile, which further self-assembled into vesicles due to hydrophobic interactions (Figure 3A). The resulting CUR/CD vesicles were hollow spheres with diameters in the range of 70–130 nm based on TEM and SEM observations, which increased the water solubility of CUR by 7000-fold (solubility up to 2 × 10−4 mol/L) [62]. In another study by Bai et al., the β-CD trimer(β-CD3) could form micelles in the presence of CUR as a guest unit by host–guest inclusion interaction and hydrophilic-hydrophobic interactions when the formed supramolecular self-assembly’s concentration was above the critical aggregation concentration. Furthermore, adjusting the ratio of β-CD3 to CUR, the transformation of the supramolecular self-assembled structure from spherical micelles (β-CD3: CUR at 2:3) to multi-compartment vesicles (β-CD3: CUR at 6:3) could be achieved (Figure 3B) [63]. Furthermore, in basal cell experiments, spindle-like complex micelles (β-CD3: CUR at 4:3) and multi-compartmental vesicles (β-CD3: CUR at 6:3) exhibited greater cytotoxicity, uptake capacity, and apoptosis rates than spherical complex micelles (β-CD3: CUR at 2:3), suggesting that altered self-assembly morphology somewhat influences the biological performance of the assemblies.
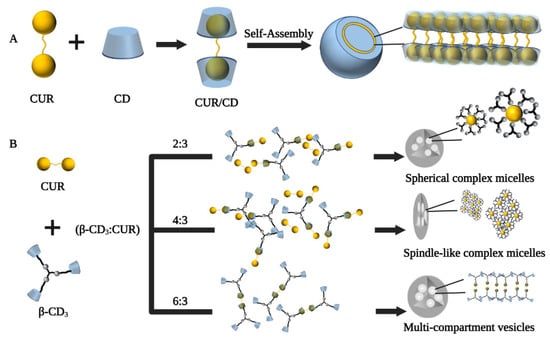
Figure 3.
Preparation process of cyclodextrin self-assembled supramolecular networks. (A) Schematic diagram of the proposed mechanism of vesicle formation from CD and CUR [62]. (B) Schematic diagram of supramolecular self-assembly of three different shapes of curcumin/β-cyclodextrin trimers [63].
2.2.2. Cross-Linked Cyclodextrin Polymer-Encapsulated Curcumin
Cross-linked CD polymers are formed by the covalent bonding of individual cyclodextrin monomers by cross-linking agents to form a cross-linked network structure, which is different from self-assembled non-covalently bonded polymerization [64]. The most common and widely reported method is the cross-linking of CD with epichlorohydrin, which was used by Chen et al. to prepare CD polymers for the encapsulation of CUR [65]. The resulting curcumin–cyclodextrin polymers exhibited higher anti-proliferative activity against A375 cells, compared to free CUR. In another study by Haimhoffer et al., polyethylene glycol was used as a cross-linking agent for CDs to form ternary complexes with CUR [66]. The resulting CD polymer effectively delivered the complexed CUR to the cell membrane, which improved the CUR permeability significantly more than the CD polymer cross-linked with encapsulation. Interestingly, the reaction of CDs with cross-linking agents such as diphenyl carbonate, diisocyanate, phthalic anhydride, and carbonyl compounds can yield cyclodextrin nano-sponges, cross-linked CD polymers with nano-sized, porous structures. Compared to common cyclodextrins, they form inclusion and non-inclusion complexes with drugs, which can improve drug delivery capacity as well as prolong the release of drug molecules. Mashaqbeh et al. prepared CD-based nano-sponges with diphenyl carbonate as a cross-linking agent, which enhanced the stability and solubility of CUR. The solubility of CUR was enhanced in the CD-based nano-sponges compared to the CUR/CD inclusion complex. In addition, the three-dimensional structure of the nano-sponges imparted higher stability to the complex [67]. In another study by Pushpalatha et al., CD-based nano-sponges (CDNS) prepared with two different cross-linking agents—diphenyl carbonate (DPC) and pyromellitic dianhydride (PMDA)—were compared for the delivery of CUR [68]. Compared to pure CUR, CUR-DPC-CDNS showed a 5-fold increase in solubility, while CUR-PMDA-CDNS showed a 16-fold increase in solubility. In cytotoxicity assays in MCF-7 cells, CUR-PMDA-CDNS exhibited higher cytotoxicity than CUR-DPC-CDNS. PMDA cross-linking may be a better method to obtain nano-sponges. In a similar study, Rafati et al. used this method to prepare CD nano-sponges, which were complexed with CUR to extend the drug release time, which was sustained over 42 h. The porous structure of the nano-sponges allowed CUR to bind to CD on the surface of the carrier and inside the cavity, exhibiting biphasic drug-release kinetics. The CUR molecules located on the surface of the nano-sponge were first released rapidly, followed by the slow release of CUR molecules located inside the cavity [69].
2.2.3. Diamine-Linked CD Dimer-Encapsulated Curcumin
In contrast to the high molecular weight possessed by cross-linked CDs, γ-CD oligomers could be used as carriers of CUR for the treatment of prostate cancer cells by Harada et al. [70]. In this drug-delivery system, two γ-CDs were substituted for each CD glucopyranose unit C6A site by succinamide or urea to form a diamine-linked γ-CD dimer, which then encapsulated the CUR by hydrogen bonding. During drug delivery, the diamine linker can be hydrolyzed by intracellular enzymes, resulting in the intracellular release of the drug CUR [71,72].


Table 2.
Summary of various cyclodextrin polymer-coated curcumin studies.
Table 2.
Summary of various cyclodextrin polymer-coated curcumin studies.
| CD Type | Material Classification | Preparation Method | Characterization Techniques | Key Findings | Reference |
|---|---|---|---|---|---|
| α-CD | Self-assembled supramolecular network | - | XRD, FTIR, 1H NMR | The slow release of CUR is achieved by complexing with α-CD and further forming a hydrogel. | [73] |
| β-CD | Self-assembled supramolecular network | - | TEM, AFM, DLS, 1H NMR and 2D NOESY NMR | Tunable CD supramolecular self-assembled carriers were successfully constructed for the controlled release of drugs. | [63] |
| β-CD | Self-assembled supramolecular network | - | SEM, AFM, FTIR, XRD, UV-Vis and NMR | Amphiphilic vesicle molecules of CUR/CD were prepared for the controlled release of CUR. | [62] |
| β-CD | Crosslinked CD polymer | - | XRD, FTIR, DSC and UV-Vis | CUR/β-CD polymer has higher anti-proliferative activity against A375 cells compared to free CUR. | [65] |
| β-CD | Crosslinked CD polymer | Freeze-drying | UV-Vis, FTIR, 1H NMR | Epichlorohydrin and citric acid cross-linked β-CD polymers were prepared for the encapsulation of CUR. | [74] |
| β-CD | Crosslinked CD polymer | - | - | Elucidating the molecular mechanisms by which CUR/β-CD polymers inhibit the growth of HepG2 cells. | [75] |
| β-CD | Crosslinked CD polymer | Freeze-drying | DLS, 1H NMR and 2D NOESY NMR | A water-soluble ‘two-in-one’ polymer containing covalently bonded polyethylene glycol and βCD groups has been prepared for the encapsulation of CUR. | [66] |
| β-CD | Crosslinked CD polymer | - | SEM, Raman spectroscopy and DLS | Encapsulation in CDNS greatly extends the long-term photostability and anti-cancer activity of curcumin. | [20] |
| β-CD | Crosslinked CD polymer | - | - | CUR/CD polymers have potential in the prevention of liver injury. | [76] |
| β-CD | Crosslinked CD polymer | Kneading | FTIR, 1H NMR, TGA and UV-Vis | CUR-β-CD polymers effectively inhibited the growth of HepG2 cells, while having little effect on non-tumor cells. | [77] |
| β-CD | Crosslinked CD polymer (NS) | Freeze-drying | DLS, FTIR, XRD and DSC | CUR/β-CDNS prepared with dimethyl carbonate crosslinker for the encapsulation of CUR. | [78] |
| β-CD | Crosslinked CD polymer (NS) | Co-evaporation | DSC, TGA, FTIR, XRD, NMR. SEM and AFM | PMDA cross-linking may be a better method to obtain nano-sponges. | [68] |
| β-CD | Crosslinked CD polymer (NS) | Co-evaporation, Freeze-drying | XRD, FTIR, TGA, DSC and UV-Vis | The ratio of crosslinker can influence the performance of CDNS and CDNS with a proper cross-linker ratio as a promising nanocarrier. | [79] |
| β-CD | Crosslinked CD polymer (NS) | Co-evaporation | SEM and UV-Vis | CUR/CDNS has a stronger in vitro release than free CUR. | [80] |
| β-CD | Crosslinked CD polymer (NS) | Freeze-drying | FTIR, TGA, XRD, DSC and SEM | CUR/CDNS prepared with phthalic anhydride as a cross-linking agent can be used in cancer therapy. | [69] |
| β-CD | Crosslinked CD polymer (NS) | Freeze-drying | XRD, DSC, FTIR and SEM | Compared to the CUR-β-CD complex, CUR in cross-linked β-CDNS resulted in a more significant enhancement in drug solubility and increased the complexation stability. | [67] |
| β-CD | Crosslinked CD triazine polymer | Freeze-drying | FTIR and 1H NMR | CD polymer-coated CUR is more cytotoxic to cancer cells than free CUR. | [29] |
| γ-CD | Crosslinked CD polymer | Co-evaporation, Freeze-drying | IR, UV-Vis and 1H NMR | γ-CD polymer complexation is a promising method for improving the water solubility of CUR. | [81] |
| γ-CD | Diamine linked CD dimers | - | UV-Vis and 1H NMR | Diamine-linked γ-CD dimers can be used as novel carriers for encapsulating CUR. | [70] |
2.3. Other Novel Cyclodextrin Nano-Supramolecular Systems with Curcumin
2.3.1. Chitosan-Based Nano-Systems
Chitosan (CS) is a linear polysaccharide produced by the deacetylation of chitin and is often used to develop nanomaterials as carriers [82]. In the delivery system of CUR and CD, the negative charge of CUR/CD limits its cellular delivery properties and its therapeutic efficacy. CS, as a cationic natural polysaccharide, can form more stable inclusion complexes through ionic interactions to facilitate intracellular drug transport [83,84]. Popat et al. prepared CUR/CD-CS nanoparticles with a particle size in the range of 180–200 nm, spherical shape, and zeta potential of +15 mv for the treatment of human skin cancer cells (SCC25) [85]. Highly soluble CUR/CD hollow spheres were first prepared by a spray drying method, followed by the addition of tripolyphosphate (TPP), thus encapsulating the CUR/CD using hydrogen bonding and ionic gelation of CS with TPP (Figure 4A). The encapsulated nanoparticles were still positively charged and transported CUR into cancer cells via the enhanced permeation and enhanced permeation retention effect, exhibiting higher cytotoxicity against the SCC25 cell line compared to free CUR, CUR-CS, and CUR/CD. In a similar study by Alizadeh et al., CUR/β-CD-CS and CUR/γ-CD-CS exhibited excellent in vitro release properties and high cytotoxicity against human lung cancer cells [86]. Similarly, Karpkird et al. synthesized nanocarriers consisting of CD polymers cross-linked by citric acid (pbCD) and CS for the encapsulation of CUR [87]. In vitro studies showed that the release rate of CSpbCD-CUR was slower than that of free CUR, resulting in a lower cytotoxicity of CSpbCD-CUR than pbCD-CUR or free CUR.
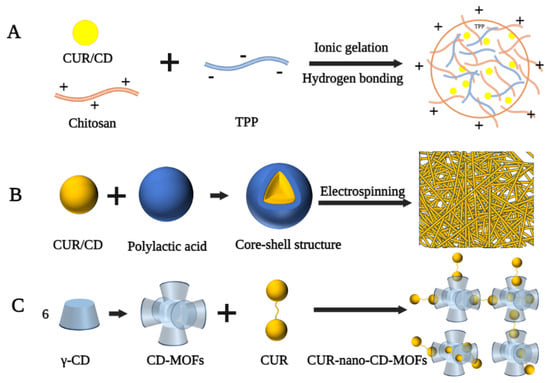
Figure 4.
Fabrication process of different types of curcumin-loaded CD-based nanocarriers. (A) Chitosan-based nanoparticles [85]. (B) Nanofiber [88]. (C) Cyclodextrin-based metal–organic framework nanoparticles [89].
2.3.2. Nanofibers
Electrospinning is a common method of preparing nanofibers by using an electrostatic force to stretch the electrospinning fluid [90]. Nanofibers made by this technique have many attractive properties, such as easily adjustable structure and size, large specific surface area, and diverse chemical composition, which make them suitable as transport systems for drug molecules [91]. Sun et al. prepared CUR/CD inclusion complex-loaded polyvinyl alcohol nanofibers via the electrospinning technique [92]. 1H NMR spectra suggested that the chemical integrity of CUR was not altered after electrostatic spinning. Therefore, the resulting nanofibers have the potential for development in drug delivery, wound healing, and cancer treatment. Rezaei et al. instead added almond gum to prepare CUR/CD inclusion complex-loaded almond gum/polyvinyl alcohol composite nanofibers [93]. The diameter of these nanofibers was in the range of 98–169 nm, and the inclusion complexes were present in a non-crystalline form. In addition, the solubility of the inclusion complex in the nanofibers was increased by 160-fold compared to that of pure CUR. In another study by Aytac et al., electrospinning was used to prepare core-shell nanofibers for the slow release of CUR [88]. In this formulation, CUR and HP-β-CD inclusion complexes were used as the core and polylactic acid (PLA) as the shell to form nanofibers with an average diameter of 695 nm (Figure 4B). In vitro release experiments showed that CUR/HP-β-CD-PLA nanofibers released CUR more slowly than CUR-PLA nanofibers during simulated gastric acid and intestinal fluid digestion due to the incorporation of a shell structure.
2.3.3. Cyclodextrin-Based Metal–Organic Framework Nanoparticle
CD-based metal–organic frameworks (CD-MOFs) are practical multifunctional materials with a porous structure and good biocompatibility, which can also be used as carriers to transport drugs [94]. Chen et al. used a modified solvothermal method and PEG to prepare a nano-CD-based organic backbone for the encapsulation of CUR [89]. In this nano-system, the CD-MOFs consisted of an extended body central framework of (γ-CD)6 cubic units, while CUR was present in the amorphous form in the hydrophobic cavity of (γ-CD)2 and the cycloidal cavity of (γ-CD)6 (Figure 4C). The resulting CUR-Nano-CD-MOFs still exhibited high antioxidant activity compared to free CUR after 120 min of continuous UV irradiation.
Up-to-date, several cyclodextrin-based nano-supramolecular systems were also designed to encapsulate and deliver curcumin, which are summarized in Table 3.

Table 3.
Summarized articles related to nano-systems containing cyclodextrins encapsulated with curcumin.
3. Application of Curcumin Release from Cyclodextrin Multi-Stimulatory Response Vehicle
Among the biological activities of CUR, its anticancer properties have been a primary focus of many studies [105]. CUR is known to inhibit signaling for cancer cell growth, thereby inhibiting tumor angiogenesis and inducing tumor cell apoptosis [106]. Furthermore, CUR could benefit from targeted therapy by precise delivery. The development of smart bio-stimulatory responsive drug carriers capable of achieving precise release of CUR according to the desired environment is one of the current approaches to improve the anti-cancer effects of CUR. The CDs are also considered to be one of the most useful building blocks for constructing stimuli-responsive drug carriers [107]. Due to the self-assembly properties of CDs and dynamic reversibility based on non-covalent interactions, they can be combined with other biocompatible materials to build supramolecular polymers or nano-systems to control the precise release of CUR [108].
3.1. Cyclodextrin-Based Polymer Vesicles Stimulate Response to Curcumin Delivery
CD-based polymeric vesicles serve as a promising drug delivery vehicle for controlled encapsulation and release of drugs, which can be designed for intelligent release in response to a range of stimuli (e.g., pH changes, enzymatic catalysis, temperature, magnetic fields, and light) [109]. Non-covalent interactions are critical in the construction of CD-based vesicles [61]. Bai et al. constructed three different morphologies of CD polymers by regulating the host–guest inclusion and hydrophilic interactions in the self-assembly system [63]. In addition, the amount of CUR released from the three different morphological carriers formed by the self-assembly at pH = 5.0 was significantly greater than that at pH = 7.4. This indicates that the release of CUR in this system can be controlled by pH and may be beneficial for cancer therapy under low pH conditions. To obtain better specificity and flexibility to adapt to the many influences changing in the organism, Ma et al. designed a CD-based multi-stimulus-responsive vesicle carrier that exhibited the ability to release CUR in response to three external stimuli [62]. Figure 5 shows the process of CUR release from sodium laurate-, α-amylase-, and copper ion-stimulated vesicles. Sodium laurate is a competing guest molecule, which replaced curcumin and bound to the CD cavity to form a new supramolecular system. Unlike this, α-amylase controlled the release of CUR by decomposing CD, i.e., disrupting the external hydrophilic layer of the vesicles, to induce the release of CUR from the vesicles. Alternatively, the addition of copper ions disrupted the hydrophobic backbone of the vesicles, and CUR formed complexes with the copper ions and detached from the vesicles.
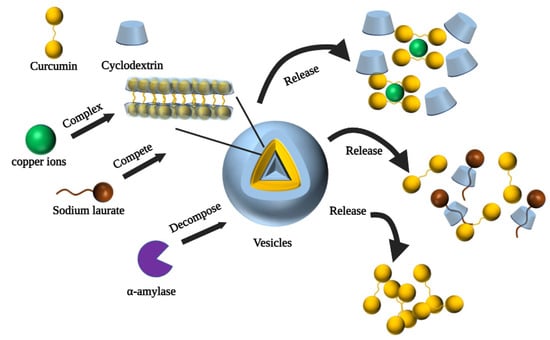
Figure 5.
Mechanism of curcumin release from vesicles triggered by sodium laurate, α-amylase and copper ions [62].
3.2. Cyclodextrin-Based Nano-Systems Stimulate Response to Curcumin Delivery
In recent years, CD-based nano-systems have been widely studied in the research and development of nanotechnology [110]. Complex nano-systems constructed with CD as the basic unit can rapidly respond to microenvironmental changes and achieve the on-demand release of drugs. When designing CD-based nanodrug carriers, the primary consideration is the distinctly different physiological characteristics between human tumor cells and normal cells. For example, the tumor microenvironment pH is acidic, as contrasted with normal tissue pH, which is neutral. Therefore, drug carriers with the ability to differentiate between tumor cells and normal cells could facilitate targeted and reliable drug delivery and release [27]. Indeed, a number of pH-sensitive CD-based nanocarriers have been successfully developed as CUR carriers in the treatment of cancer [111]. Wei et al. combined a pH-responsive penetrating peptide (R6H4) with Carboxymethyl-β-CD to synthesize pH-responsive CD derivatives with cell-membrane-penetrating abilities, based on which they further formed nanoparticles with CUR and studied their pH-responsive properties [103]. The results showed that nanoparticles had higher cytotoxicity at pH 6.4 compared to pH 7.4. In addition, similar findings were obtained in cell uptake and apoptosis studies in HepG2 cells. Based on the superior physiological activity of the nanoparticles in a mildly acidic (pH = 6.4) environment, these nanoparticles demonstrated desirable anti-cancer effects in tumor-bearing mice. In another study, Aytac et al. prepared core-shell nanofibers with CUR and CD inclusion complexes as the core and polylactic acid as the shell, which exhibited pH-dependent release in 0.1 M HCl (pH 1, simulated gastric fluid). Therefore, this pH-responsive drug delivery system of core-shell nanofibers may be a promising drug carrier for targeting gastric cancer [88]. Interestingly, Wen et al. prepared γ-CD-BSA nanoparticles for the slow release of CUR by grafting γ-CD onto bovine serum albumin (BSA) using epichlorohydrin as a cross-linking agent [104]. The results showed that CUR was released in PBS (pH 7.2) for 4 h with a release rate of 57% ± 1% and in HCl (pH 1.2) for 2 h with a release rate of 15.2% ± 0.2. Therefore, in contrast to the nanoparticles obtained by Aytac et al., γ-CD-BSA nanoparticles have the potential to protect CUR in the stomach (pH 2.0) and release CUR in the intestine (pH 7.0).
In addition, certain changes in temperature and enzyme activities at the lesion site may occur. For example, because tumor cells have unlimited proliferation and high metabolism, which increases their temperature (40–42 °C) above that of normal cells (37 °C), CD-based drug carriers can achieve drug release at the lesion site through temperature changes during the targeted drug delivery phase [112]. Sedghi et al. designed a novel intelligent thermoresponsive-magnetic molecularly imprinted polymer nanocomposite for the controlled and slow release of CUR, with the ability to respond to temperature stimuli [99]. In this response system, the release of the drug could be controlled by changing the temperature because of the phase transition behavior resulting from the inclusion of N-isopropylacrylamide monomers. The drug release experiments showed that approximately 62% of the CUR was released when the temperature was at 25 °C, but about 86% of the CUR was released when the temperature was increased to 38 °C. Although this drug carrier has thermally responsive properties, it needs further improvement to be applied to human anti-cancer treatment.
In addition to pH and temperature stimulation of CD-based drug carriers for CUR release, CD-based drug carriers based on other stimuli, such as enzymes, light, and magnetic fields, are also promising for CUR release. Due to the elevated levels of enzymes such as amylase and lipase at the lesion site, different CD-based drug carriers can be designed according to the target of enzymatic degradation during the construction of the enzyme-stimulated reaction system [27]. Park et al. designed CD-coated porous silica nanoparticles, which exhibited enzyme-responsive characteristics [113]. The CD on the surface of the silica nanoparticles was hydrolyzed by α-amylase to release the drug from the porous reservoir. The ester linkage in the CD stalk was also cleaved by lipase, resulting in the release of drug molecules from the channel. Namgung et al. formed ester linkages between paclitaxel and a CD polymer using maleic anhydride [114]. Due to the presence of high levels of esterase at the tumor site, the ester linkages to paclitaxel were degraded when the drug carrier reached the tumor site, thereby achieving precise release. The constructed nano-drug carriers showed significant anti-tumor activity in a mouse tumor model. In addition, photo-stimulated host–guest interactions can be used to develop CD-based carriers for controlled drug release, which consist of guest molecules, an azo compound, and CD. Azobenzene, a compound that undergoes reversible cis-trans isomerization upon illumination with ultraviolet light, disrupted the interaction of the guest molecule with CD [115]. The negatively charged polyelectrolyte chains cannot continue to hold the drug, and therefore the drug is released [116]. However, the application of CUR as a photosensitizing active substance in photo-stimulatory reaction systems remains to be investigated. Likewise, applications regarding the multi-stimulus responsive CD-based carrier delivery of CUR remains to be developed.
4. Conclusions
CUR has received widespread attention due to its multiple biological activities. However, the hydrophobicity, low bioavailability, and poor chemical stability of CUR pose great challenges to its effective delivery. Combining CUR with CD to construct a supramolecular system is one of the effective strategies to improve the therapeutic potential of CUR. Many studies have demonstrated the effectiveness of CD monomers in improving the aqueous solubility of CUR. However, in the last few years, the construction of new supramolecular systems has been used to improve their capabilities in other aspects. In this review, different supramolecular systems of CUR/CD are summarized. Hydrophobic interactions between the host and guest as well as non-covalent bonding forces such as hydrogen bonding and van der Waals forces are the main driving forces in the construction of supramolecular structures of CD monomers and CUR. Moreover, the non-covalent self-assembly and the covalent polymerization of cross-linkers give the CD polymer and CUR supramolecular systems different structural features and new physicochemical properties different from those of the original constituent molecules. More importantly, some of the complex nano-systems constructed with CD as the basic unit not only improve the solubility of CUR but also release it slowly, which greatly expands the application scope of CUR.
In the field of medical research, precise drug distribution allows the drug to achieve the best biological efficacy at the lowest dose to minimize side effects. Smart stimulus-responsive drug carriers enable the release of drugs at the right time and site for precise treatment. CD-based stimulus-responsive drug carriers can drive the development of precision medicine to a certain extent. To this end, we hope that the anti-cancer activity of CUR can be maximized. We summarize and discuss different stimulus-responsive CD-based carriers for the delivery of CUR. When factors such as temperature and pH change, they cause changes in the structure of some CD-based drug carriers. It is particularly exciting to induce the release of drug CUR from these smart drug carriers and to act precisely on different cells to achieve higher therapeutic indices. Although good in vitro results have been achieved with these stimulus-responsive CD-based drug delivery carriers, their design is often complex and most of them are still in the conceptualization and validation stages. There are still many issues to be faced in the precision delivery of CUR, such as the biocompatibility and biodegradability of the system, in addition to the complex environmental changes in the human body. Overall, the application of CD-based stimulation-responsive drug carriers has advanced the development of CUR in cancer therapy, but more research is still needed to support its use in clinical treatment.
Author Contributions
Conceptualization, J.H. and D.L.; Writing—original draft preparation, J.L. and F.X.; Writing—review and editing, N.S.; Visualization, Y.D., J.Z. and Y.S.; Supervision, J.H. Funding Acquisition, N.S. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32272450), Natural Science Foundation of Fujian Province (2020I0012, 2020I0010) and Special Funds for Science and Technology Innovation of Fujian Agriculture and Forestry University 2022-82.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, Z.B.; Guo, S.; Chen, D.Q.; Liu, J.Y. Encapsulation of Curcumin into Β-Cyclodextrins Inclusion: A Review. E3S Web. Conf. 2019, 131, 01100. [Google Scholar]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, S.; Tan, B.K.; Hamzah, S.S.; Lin, Y.; Kong, Z.; Zhang, Y.; Zheng, B.; Zeng, S. Photodynamic inactivation of Burkholderia cepacia by curcumin in combination with EDTA. Food Res. Int. 2018, 111, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, C.; Shi, H.; Yang, M.; Liu, Y.; Ji, P.; Chen, H.; Tan, R.X.; Li, E. Curcumin is a biologically active copper chelator with antitumor activity. Phytomedicine 2016, 23, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Ying, X.; Xu, H.; Yan, H.; Li, X.; Tang, H. The functional curcumin liposomes induce apoptosis in C6 glioblastoma cells and C6 glioblastoma stem cells in vitro and in animals. Int. J. Nanomed. 2017, 12, 1369–1384. [Google Scholar] [CrossRef]
- Gong, C.; Deng, S.; Wu, Q.; Xiang, M.; Wei, X.; Li, L.; Gao, X.; Wang, B.; Sun, L.; Chen, Y.; et al. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials 2013, 34, 1413–1432. [Google Scholar] [CrossRef]
- Bhat, A.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Hediyal, T.A.; Manthiannem, E.; Padamati, J.; Chandra, R.; Chidambaram, S.B.; Sakharkar, M.K. Benefits of curcumin in brain disorders. BioFactors 2019, 45, 666–689. [Google Scholar] [CrossRef]
- Blanco-García, E.; Otero-Espinar, F.; Blanco-Méndez, J.; Leiro-Vidal, J.; Luzardo-Álvarez, A. Development and characterization of anti-inflammatory activity of curcumin-loaded biodegradable microspheres with potential use in intestinal inflammatory disorders. Int. J. Pharm. 2017, 518, 86–104. [Google Scholar] [CrossRef]
- Kasi, P.D.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.M.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H.; Nabavi, S.M. Molecular targets of curcumin for cancer therapy: An updated review. Tumor Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef]
- Maiti, P.; Dunbar, G.L. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, C.; Sun, S.; Li, R.; Shi, X.; Wang, S.; Zeng, X.; Kuang, N.; Liu, Y.; Shi, Q.; et al. Curcumin attenuates collagen-induced rat arthritis via anti-inflammatory and apoptotic effects. Int. Immunopharmacol. 2019, 72, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.; Zia, K.M.; Zuber, M.; Salman, M.; Anjum, M.N. Recent developments in curcumin and curcumin based polymeric materials for biomedical applications: A review. Int. J. Biol. Macromol. 2015, 81, 877–890. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Bore, B.; Bhawar, C.; Mallya, R. Complexation of phytochemicals with cyclodextrins and their derivatives—An update. Biomed. Pharmacother. 2022, 149, 112862. [Google Scholar] [CrossRef]
- Cui, Z.; Yao, L.; Ye, J.; Wang, Z.; Hu, Y. Solubility measurement and thermodynamic modelling of curcumin in twelve pure solvents and three binary solvents at different temperature (T = 278.15–323.15 K). J. Mol. Liq. 2021, 338, 116795. [Google Scholar] [CrossRef]
- Mehanny, M.; Hathout, R.M.; Geneidi, A.S.; Mansour, S. Exploring the use of nanocarrier systems to deliver the magical molecule; Curcumin and its derivatives. J. Control. Release 2016, 225, 1–30. [Google Scholar] [CrossRef]
- Wahlström, B.; Blennow, G. A Study on the Fate of Curcumin in the Rat. Acta Pharmacol. Toxicol. 1978, 43, 86–92. [Google Scholar] [CrossRef]
- Suvarna, V.; Gujar, P.; Murahari, M. Complexation of phytochemicals with cyclodextrin derivatives—An insight. Biomed. Pharmacother. 2017, 88, 1122–1144. [Google Scholar] [CrossRef]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Möller, K.; Macaulay, B.; Bein, T. Curcumin Encapsulated in Crosslinked Cyclodextrin Nanoparticles Enables Immediate Inhibition of Cell Growth and Efficient Killing of Cancer Cells. Nanomaterials 2021, 11, 489. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Smart stimuli-responsive drug delivery systems based on cyclodextrin: A review. Carbohydr. Polym. 2021, 251, 116871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, Y. Cyclodextrin-Based Multistimuli-Responsive Supramolecular Assemblies and Their Biological Functions. Adv. Mater. Res. 2020, 32, 1806158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Xu, J.; Chen, D.; Shi, L.; Zhou, Y.; Shen, Z. Polymeric Supramolecular Systems: Design, Assembly and Functions. Acta. Polymerica. Sinica. 2019, 50, 973–987. [Google Scholar]
- Tang, B.; Ma, L.; Wang, H.Y.; Zhang, G.Y. Study on the Supramolecular Interaction of Curcumin and Beta-Cyclodextrin by Spectrophotometry and Its Analytical Application. J. Agric. Food Chem. 2002, 50, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Másson, M.; Loftsson, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: Solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Baglole, K.N.; Boland, P.G.; Wagner, B.D. Fluorescence enhancement of curcumin upon inclusion into parent and modified cyclodextrins. J. Photochem. Photobiol. A Chem. 2005, 173, 230–237. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host–Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Boztas, A.O.; Karakuzu, O.; Galante, G.; Ugur, Z.; Kocabas, F.; Altuntas, C.Z.; Yazaydin, A.O. Synergistic Interaction of Paclitaxel and Curcumin with Cyclodextrin Polymer Complexation in Human Cancer Cells. Mol. Pharm. 2013, 10, 2676–2683. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Beta-Cyclodextrin-Curcumin Self-Assembly Enhances Curcumin Delivery in Prostate Cancer Cells. Colloids Surf. B. 2010, 79, 113–125. [Google Scholar] [CrossRef] [PubMed]
- López-Tobar, E.; Blanch, G.; del Castillo, M.R.; Sanchez-Cortes, S. Encapsulation and isomerization of curcumin with cyclodextrins characterized by electronic and vibrational spectroscopy. Vib. Spectrosc. 2012, 62, 292–298. [Google Scholar] [CrossRef]
- Alizadeh, N.; Malakzadeh, S. Changes in chemical stability and bioactivities of curcumin by forming inclusion complexes of beta- and Gama-cyclodextrins. J. Polym. Res. 2020, 27, 42. [Google Scholar] [CrossRef]
- Jahed, V.; Zarrabi, A.; Bordbar, A.K.; Hafezi, M.S. Nmr (1H, Roesy) Spectroscopic and Molecular Modelling Investigations of Supramolecular Complex of Beta-Cyclodextrin and Curcumin. Food Chem. 2014, 165, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Song, L.T.; Jiang, X.Y.; Tang, K.W.; Miao, J.B. Study on Inclusion Interaction of Ibuprofen with B-Cyclodextrin Derivatives. Lat. Am. Appl. Res. 2011, 41, 147–151. [Google Scholar]
- Li, N.; Wang, N.; Wu, T.; Qiu, C.; Wang, X.; Jiang, S.; Zhang, Z.; Liu, T.; Wei, C.; Wang, T. Preparation of Curcumin-Hydroxypropyl-Beta-Cyclodextrin Inclusion Complex by Cosolvency-Lyophilization Procedure to Enhance Oral Bioavailability of the Drug. Drug Dev. Ind. Pharm. 2018, 44, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Salmas, R.E.; Durdagi, S.; Roewer, N.; Förster, C.; Broscheit, J. Solubility Profiles, Hydration and Desolvation of Curcumin Complexed with Γ-Cyclodextrin and Hydroxypropyl-Γ-Cyclodextrin. J. Mol. Struct. 2017, 1134, 91–98. [Google Scholar] [CrossRef]
- Mai, N.N.S.; Nakai, R.; Kawano, Y.; Hanawa, T. Enhancing the Solubility of Curcumin Using a Solid Dispersion System with Hydroxypropyl-Beta-Cyclodextrin Prepared by Grinding, Freeze-Drying, and Common Solvent Evaporation Methods. Pharmacy 2020, 8, 203. [Google Scholar] [CrossRef]
- Mohan, P.K.; Sreelakshmi, G.; Muraleedharan, C.; Joseph, R. Water soluble complexes of curcumin with cyclodextrins: Characterization by FT-Raman spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin-Beta-Cyclodextrin Inclusion Complex: Stability, Solubility, Characterisation by Ft-Ir, Ft-Raman, X-Ray Diffraction and Photoacoustic Spectroscopy, and Food Application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef]
- Tomren, M.; Másson, M.; Loftsson, T.; Tønnesen, H.H. Studies on curcumin and curcuminoids: XXXI. Symmetric and asymmetric curcuminoids: Stability, activity and complexation with cyclodextrin. Int. J. Pharm. 2007, 338, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Arya, P.; Raghav, N. In-vitro studies of Curcumin-β-cyclodextrin inclusion complex as sustained release system. J. Mol. Struct. 2020, 1228, 129774. [Google Scholar] [CrossRef]
- Zhang, L.; Man, S.; Qiu, H.; Liu, Z.; Zhang, M.; Ma, L.; Gao, W. Curcumin-cyclodextrin complexes enhanced the anti-cancer effects of curcumin. Environ. Toxicol. Pharmacol. 2016, 48, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ja’far, M.H.; Nik Mohamed Kamal, N.N.S.; Hui, B.Y.; Kamaruzzaman, M.F.; Mohamad Zain, N.N.; Yahaya, N.; Raoov, M. Inclusion of Curcumin in Β-Cyclodextrins as Potential Drug Delivery System: Preparation, Characterization and Its Preliminary Cytotoxicity Approaches. Sains. Malaysiana. 2018, 47, 977–989. [Google Scholar] [CrossRef]
- Marcolino, V.A.; Zanin, G.M.; Durrant, L.R.; Benassi Mde, T.; Matioli, G. Interaction of Curcumin and Bixin with Beta-Cyclodextrin: Complexation Methods, Stability, and Applications in Food. J. Agric. Food Chem. 2011, 59, 3348–3357. [Google Scholar] [CrossRef]
- Hagbani, T.A.; Nazzal, S. Curcumin Complexation with Cyclodextrins by the Autoclave Process: Method Development and Characterization of Complex Formation. Int. J. Pharm. 2017, 520, 173–180. [Google Scholar] [CrossRef]
- Jantarat, C.; Sirathanarun, P.; Ratanapongsai, S.; Watcharakan, P.; Sunyapong, S.; Wadu, A. Curcumin-Hydroxypropyl-β-Cyclodextrin Inclusion Complex Preparation Methods: Effect of Common Solvent Evaporation, Freeze Drying, and Ph Shift on Solubility and Stability of Curcumin. Trop. J. Pharm. Res. 2014, 13, 1215–1223. [Google Scholar] [CrossRef]
- Kabirov, D.; Silvestri, T.; Niccoli, M.; Usacheva, T.; Mayol, L.; Biondi, M.; Giancola, C. Phase solubility and thermoanalytical studies of the inclusion complex formation between curcumin and hydroxypropyl-β-cyclodextrin in hydroalcoholic solutions. J. Therm. Anal. 2020, 147, 347–353. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, S.; Wong, L.R.; Xie, H.; Ho, P.C. In Vitro and in Vivo Comparison of Curcumin-Encapsulated Chitosan-Coated Poly(Lactic-Co-Glycolic Acid) Nanoparticles and Curcumin/Hydroxypropyl-Beta-Cyclodextrin Inclusion Complexes Administered Intranasally as Therapeutic Strategies for Alzheimer’s Disease. Mol. Pharm. 2020, 17, 4256–4269. [Google Scholar]
- Sun, Y.; Du, L.; Liu, Y.; Li, X.; Li, M.; Jin, Y.; Qian, X. Transdermal Delivery of the in Situ Hydrogels of Curcumin and Its Inclusion Complexes of Hydroxypropyl-Beta-Cyclodextrin for Melanoma Treatment. Int. J. Pharm. 2014, 469, 31–39. [Google Scholar] [CrossRef]
- Wang, H.; Luo, J.; Zhang, Y.; He, D.; Jiang, R.; Xie, X.; Yang, Q.; Li, K.; Xie, J.; Zhang, J. Phospholipid/Hydroxypropyl-Beta-Cyclodextrin Supramolecular Complexes Are Promising Candidates for Efficient Oral Delivery of Curcuminoids. Int. J. Pharm. 2020, 582, 119301. [Google Scholar] [CrossRef] [PubMed]
- Khatun, B.; Baishya, P.; Ramteke, A.; Maji, T.K. Study of the complexation of structurally modified curcumin with hydroxypropyl beta cyclodextrin and its effect on anticancer activity. New J. Chem. 2020, 44, 4887–4897. [Google Scholar] [CrossRef]
- Cutrignelli, A.; Lopedota, A.; Denora, N.; Iacobazzi, R.M.; Fanizza, E.; Laquintana, V.; Perrone, M.; Maggi, V.; Franco, M. A New Complex of Curcumin with Sulfobutylether-Beta-Cyclodextrin: Characterization Studies and in Vitro Evaluation of Cytotoxic and Antioxidant Activity on Hepg-2 Cells. J. Pharm. Sci. 2014, 103, 3932–3940. [Google Scholar] [CrossRef] [PubMed]
- Shlar, I.; Droby, S.; Choudhary, R.; Rodov, V. The mode of antimicrobial action of curcumin depends on the delivery system: Monolithic nanoparticles vs. supramolecular inclusion complex. RSC Adv. 2017, 7, 42559–42569. [Google Scholar] [CrossRef]
- Kuang, C.T.; Li, X.Z.; Wang, Y.; He, Y.C.; Guo, Y.L. Study on the Supramolecular Interaction of Curcumin and RM-β-CD-m. Adv. Mater. Res. 2010, 152–153, 1377–1381. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, C.; Lin, Q.; Hu, J.; Li, X.; Sang, S.; McClements, D.J.; Long, J.; Jin, Z.; Wang, J.; et al. Complexation of curcumin with cyclodextrins adjusts its binding to plasma proteins. Food Funct. 2022, 13, 8920–8929. [Google Scholar] [CrossRef]
- Yao, X.; Huang, P.; Nie, Z. Cyclodextrin-based polymer materials: From controlled synthesis to applications. Prog. Polym. Sci. 2019, 93, 1–35. [Google Scholar] [CrossRef]
- Matencio, A.; Pedrazzo, A.R.; Difalco, A.; Navarro-Orcajada, S.; Monfared, Y.K.; Conesa, I.; Rezayat, A.; López-Nicolás, J.M.; Trotta, F. Advances and Classification of Cyclodextrin-Based Polymers for Food-Related Issues. Polymers 2021, 13, 4226. [Google Scholar] [CrossRef]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef]
- Wei, P.; Yan, X.; Huang, F. Supramolecular polymers constructed by orthogonal self-assembly based on host–guest and metal–ligand interactions. Chem. Soc. Rev. 2014, 44, 815–832. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, B.; Chen, S.; Du, J. Polymer vesicles: Mechanism, preparation, application, and responsive behavior. Prog. Polym. Sci. 2017, 64, 1–22. [Google Scholar] [CrossRef]
- Ma, M.; Sun, T.; Xing, P.; Li, Z.; Li, S.; Su, J.; Chu, X.; Hao, A. A supramolecular curcumin vesicle and its application in controlling curcumin release. Colloids Surf. A Physicochem. Eng. Asp. 2014, 459, 157–165. [Google Scholar] [CrossRef]
- Bai, Y.; An, N.; Chen, D.; Liu, Y.Z.; Liu, C.P.; Yao, H.; Wang, C.; Song, X.; Tian, W. Facile Construction of Shape-Regulated Beta-Cyclodextrin-Based Supramolecular Self-Assemblies for Drug Delivery. Carbohydr. Polym. 2020, 231, 115714. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z.-G. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of Curcumin/Cyclodextrin Polymer Inclusion Complex and Investigation on Its Antioxidant and Antiproliferative Activities. Molecules 2018, 23, 1179. [Google Scholar] [CrossRef] [PubMed]
- Haimhoffer, A.; Dossi, E.; Beresova, M.; Bacskay, I.; Varadi, J.; Afsar, A.; Rusznyak, A.; Vasvari, G.; Fenyvesi, F. Preformulation Studies and Bioavailability Enhancement of Curcumin with a ‘Two in One’ Peg-Beta-Cyclodextrin Polymer. Pharmaceutics 2021, 13, 1710. [Google Scholar] [CrossRef] [PubMed]
- Mashaqbeh, H.; Obaidat, R.; Al-Shar’i, N. Evaluation and Characterization of Curcumin-Beta-Cyclodextrin and Cyclodextrin-Based Nanosponge Inclusion Complexation. Polymers 2021, 13, 4073. [Google Scholar] [CrossRef] [PubMed]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cross-linked, cyclodextrin-based nanosponges for curcumin delivery—Physicochemical characterization, drug release, stability and cytotoxicity. J. Drug Deliv. Sci. Technol. 2018, 45, 45–53. [Google Scholar] [CrossRef]
- Rafati, N.; Zarrabi, A.; Caldera, F.; Trotta, F.; Ghias, N. Pyromellitic dianhydride crosslinked cyclodextrin nanosponges for curcumin controlled release; formulation, physicochemical characterization and cytotoxicity investigations. J. Microencapsul. 2019, 36, 715–727. [Google Scholar] [CrossRef]
- Harada, T.; Giorgio, L.; Harris, T.J.; Pham, D.T.; Ngo, H.T.; Need, E.F.; Coventry, B.J.; Lincoln, S.F.; Easton, C.J.; Buchanan, G.; et al. Diamide Linked Gamma-Cyclodextrin Dimers as Molecular-Scale Delivery Systems for the Medicinal Pigment Curcumin to Prostate Cancer Cells. Mol. Pharm. 2013, 10, 4481–4490. [Google Scholar] [CrossRef]
- Harada, T.; McTernan, H.L.; Pham, D.T.; Lincoln, S.F.; Kee, T.W. Femtosecond Transient Absorption Spectroscopy of the Medicinal Agent Curcumin in Diamide Linked Gamma-Cyclodextrin Dimers. J. Phys. Chem. B 2015, 119, 2425–2433. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Pham, D.T.; Leung, M.H.; Ngo, H.T.; Lincoln, S.F.; Easton, C.J.; Kee, T.W. Cooperative Binding and Stabilization of the Medicinal Pigment Curcumin by Diamide Linked Gamma-Cyclodextrin Dimers: A Spectroscopic Characterization. J. Phys. Chem. B 2011, 115, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Torchio, A.; Cassino, C.; Lavella, M.; Gallina, A.; Stefani, A.; Boffito, M.; Ciardelli, G. Injectable Supramolecular Hydrogels Based on Custom-Made Poly(Ether Urethane)S and Alpha-Cyclodextrins as Efficient Delivery Vehicles of Curcumin. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112194. [Google Scholar] [CrossRef] [PubMed]
- Karpkird, T.; Khunsakorn, R.; Noptheeranuphap, C.; Midpanon, S. Inclusion complexes and photostability of UV filters and curcumin with beta-cyclodextrin polymers: Effect on cross-linkers. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 37–45. [Google Scholar] [CrossRef]
- Chen, J.; Cao, X.; Qin, X.; Liu, H.; Chen, S.; Zhong, S.; Li, Y. Proteomic Analysis of the Molecular Mechanism of Curcumin/Beta-Cyclodextrin Polymer Inclusion Complex Inhibiting Hepg2 Cells Growth. J. Food Biochem. 2020, 44, e13119. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, J.; Fan, T.; Zhong, S.; Qin, X.; Li, R.; Gao, J.; Liang, Y. Protective effects of curcumin/cyclodextrin polymer inclusion complex against hydrogen peroxide-induced LO2 cells damage. Food Sci. Nutr. 2022, 10, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, P.; Lin, L.; Guo, F.; Wang, Q.; Piao, Y.; Diao, G. Study of a water-soluble supramolecular complex of curcumin and β-cyclodextrin polymer with electrochemical property and potential anti-cancer activity. Chin. Chem. Lett. 2021, 33, 4043–4047. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Cyclodextrin-based nanosponges of curcumin: Formulation and physicochemical characterization. J. Incl. Phenom. Macrocycl. Chem. 2012, 75, 315–322. [Google Scholar] [CrossRef]
- Gholibegloo, E.; Mortezazadeh, T.; Salehian, F.; Ramazani, A.; Amanlou, M.; Khoobi, M. Improved Curcumin Loading, Release, Solubility and Toxicity by Tuning the Molar Ratio of Cross-Linker to Beta-Cyclodextrin. Carbohydr. Polym. 2019, 213, 70–78. [Google Scholar] [CrossRef]
- Pushpalatha, R.; Selvamuthukumar, S.; Kilimozhi, D. Cyclodextrin nanosponge based hydrogel for the transdermal co-delivery of curcumin and resveratrol: Development, optimization, in vitro and ex vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 52, 55–64. [Google Scholar] [CrossRef]
- Karpkird, T.; Khunsakorn, R.; Noptheeranuphap, C.; Jettanasen, J. Photostability of water-soluble inclusion complexes of UV-filters and curcumin with gamma-cyclodextrin polymer. J. Incl. Phenom. Macrocycl. Chem. 2015, 84, 121–128. [Google Scholar] [CrossRef]
- Elgadir, M.; Uddin, M.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Negm, N.A.; Hefni, H.H.; Abd-Elaal, A.A.; Badr, E.A.; Kana, M.T.A. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Popat, A.; Karmakar, S.; Jambhrunkar, S.; Xu, C.; Yu, C. Curcumin-cyclodextrin encapsulated chitosan nanoconjugates with enhanced solubility and cell cytotoxicity. Colloids Surf. B 2014, 117, 520–527. [Google Scholar] [CrossRef]
- Alizadeh, N.; Malakzadeh, S. Antioxidant, Antibacterial and Anti-Cancer Activities of Beta-and Gamma-Cds/Curcumin Loaded in Chitosan Nanoparticles. Int. J. Biol. Macromol. 2020, 147, 778–791. [Google Scholar] [CrossRef]
- Karpkird, T.; Manaprasertsak, A.; Penkitti, A.; Sinthuvanich, C.; Singchuwong, T.; Leepasert, T. A novel chitosan-citric acid crosslinked beta-cyclodextrin nanocarriers for insoluble drug delivery. Carbohydr. Res. 2020, 498, 108184. [Google Scholar] [CrossRef]
- Aytac, Z.; Uyar, T. Core-shell nanofibers of curcumin/cyclodextrin inclusion complex and polylactic acid: Enhanced water solubility and slow release of curcumin. Int. J. Pharm. 2017, 518, 177–184. [Google Scholar] [CrossRef]
- Chen, Y.; Su, J.; Dong, W.; Xu, D.; Cheng, L.; Mao, L.; Gao, Y.; Yuan, F. Cyclodextrin-Based Metal-Organic Framework Nanoparticles as Superior Carriers for Curcumin: Study of Encapsulation Mechanism, Solubility, Release Kinetics, and Antioxidative Stability. Food Chem. 2022, 383, 132605. [Google Scholar] [CrossRef]
- Nie, G.; Li, S.; Lu, X.; Wang, C. Progress on Applications of Inorganic Nanofibers Synthesized by Electrospinning Technique. Chem. J. Chin. Univ.-Chin. 2013, 34, 15–29. [Google Scholar]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Z.; Williams, G.R.; Hou, X.-X.; Zhu, L.-M. Electrospun curcumin-loaded fibers with potential biomedical applications. Carbohydr. Polym. 2013, 94, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Nasirpour, A. Encapsulation of curcumin using electrospun almond gum nanofibers: Fabrication and characterization. Int. J. Food Prop. 2018, 21, 1608–1618. [Google Scholar] [CrossRef]
- Roy, I.; Stoddart, J.F. Cyclodextrin Metal–Organic Frameworks and Their Applications. Accounts Chem. Res. 2021, 54, 1440–1453. [Google Scholar] [CrossRef]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular Inclusion Complex of Curcumin-Beta-Cyclodextrin Nanoparticle to Enhance Curcumin Skin Permeability from Hydrophilic Matrix Gel. AAPS. Pharm. Sci. Tech. 2013, 14, 1303–1312. [Google Scholar] [CrossRef]
- Liu, C.H.; Lee, G.W.; Wu, W.C.; Wang, C.C. Encapsulating Curcumin in Ethylene Diamine-Beta-Cyclodextrin Nanoparticle Improves Topical Cornea Delivery. Colloids Surf. B 2020, 186, 110726. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Satapathy, B.K. Fabrication of optimally controlled electrosprayed polymer-free nano-particles of curcumin/β-cyclodextrin inclusion complex. Colloids Surf. A 2021, 618, 126504. [Google Scholar] [CrossRef]
- Aadinath, W.; Bhushani, A.; Anandharamakrishnan, C. Synergistic Radical Scavenging Potency of Curcumin-in-Beta-Cyclodextrin-in-Nanomagnetoliposomes. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 293–302. [Google Scholar] [CrossRef]
- Sedghi, R.; Yassari, M.; Heidari, B. Thermo-responsive molecularly imprinted polymer containing magnetic nanoparticles: Synthesis, characterization and adsorption properties for curcumin. Colloids Surf. B 2018, 162, 154–162. [Google Scholar] [CrossRef]
- Omrani, Z.; Tehrani, A.D. New cyclodextrin-based supramolecular nanocapsule for codelivery of curcumin and gallic acid. Polym. Bull. 2019, 77, 2003–2019. [Google Scholar] [CrossRef]
- Serri, C.; Argiro, M.; Piras, L.; Mita, D.G.; Saija, A.; Mita, L.; Forte, M.; Giarra, S.; Biondi, M.; Crispi, S.; et al. Nano-Precipitated Curcumin Loaded Particles: Effect of Carrier Size and Drug Complexation with (2-Hydroxypropyl)-Beta-Cyclodextrin on Their Biological Performances. Int. J. Pharm. 2017, 520, 21–28. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Fast-Dissolving antioxidant curcumin/cyclodextrin inclusion complex electrospun nanofibrous webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, Y.; Lei, M.; Qin, Y.; Wang, Z.; Chen, Z.; Zhang, L.; Zhu, Y. Development of Oral Curcumin Based on Ph-Responsive Transmembrane Peptide-Cyclodextrin Derivative Nanoparticles for Hepatoma. Carbohydr. Polym. 2022, 277, 118892. [Google Scholar] [CrossRef] [PubMed]
- Hedi, W.; Jingbo, L.; Yiding, Y.; Yuxi, S.; Jiyun, L.; Qinqin, D.; Yan, C.; Boqun, L.; Ting, Z. γ-Cyclodextrin-BSA for nano-encapsulation of hydrophobic substance. Food Biosci. 2021, 41, 101009. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Harsha, C.; Banik, K.; Gupta, S.C.; Aggarwal, B.B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin. Sci. 2017, 131, 1781–1799. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Yao, X.; Mu, J.; Zeng, L.; Lin, J.; Nie, Z.; Jiang, X.; Huang, P. Stimuli-responsive cyclodextrin-based nanoplatforms for cancer treatment and theranostics. Mater. Horizons 2019, 6, 846–870. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.-M.; Liu, Y. Multidimensional Nanoarchitectures Based on Cyclodextrins. Chem. Commun. 2010, 46, 5622–5633. [Google Scholar] [CrossRef]
- Menezes, P.D.P.; Andrade, T.D.A.; Frank, L.A.; de Souza, E.P.B.S.S.; Trindade, G.D.G.G.; Trindade, I.A.S.; Serafini, M.R.; Guterres, S.S.; Araújo, A.A.D.S. Advances of nanosystems containing cyclodextrins and their applications in pharmaceuticals. Int. J. Pharm. 2019, 559, 312–328. [Google Scholar] [CrossRef]
- Dan, Z.; Cao, H.; He, X.; Zeng, L.; Zou, L.; Shen, Q.; Zhang, Z. Biological stimuli-responsive cyclodextrin-based host–guest nanosystems for cancer therapy. Int. J. Pharm. 2015, 483, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.J.T.; Nabid, M.R.; Niknejad, H.; Entezami, A.A. Folate-decorated thermoresponsive micelles based on star-shaped amphiphilic block copolymers for efficient intracellular release of anticancer drugs. Int. J. Pharm. 2012, 437, 70–79. [Google Scholar] [CrossRef]
- Park, C.; Kim, H.; Kim, S.; Kim, C. Enzyme Responsive Nanocontainers with Cyclodextrin Gatekeepers and Synergistic Effects in Release of Guests. J. Am. Chem. Soc. 2009, 131, 16614–16615. [Google Scholar] [CrossRef] [PubMed]
- Namgung, R.; Lee, Y.M.; Kim, J.; Jang, Y.; Lee, B.-H.; Kim, I.-S.; Sokkar, P.; Rhee, Y.; Hoffman, A.S.; Kim, W.J. Poly-cyclodextrin and poly-paclitaxel nano-assembly for anticancer therapy. Nat. Commun. 2014, 5, 3702. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liu, X.; Shi, W.; Cheng, C.; He, C.; Zhao, C. Light-Triggered Switching of Reversible and Alterable Biofunctionality via β-Cyclodextrin/Azobenzene-Based Host–Guest Interaction. ACS Macro Lett. 2014, 3, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Wajs, E.; Nielsen, T.T.; Larsen, K.L.; Fragoso, A. Preparation of stimuli-responsive nano-sized capsules based on cyclodextrin polymers with redox or light switching properties. Nano Res. 2016, 9, 2070–2078. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).