Thermal Aging Properties of 500 kV AC and DC XLPE Cable Insulation Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Methods
2.2.1. Calculation of the Crosslinking Degree
2.2.2. Mechanical Tests
2.2.3. Thermal Analysis
2.2.4. FTIR Method
2.2.5. XPS Method
2.2.6. Space Charge Measurement
3. Results
3.1. Crosslinking Properties
3.1.1. Crosslinking Degree
3.1.2. Thermal Elongation
3.2. Thermal Properties
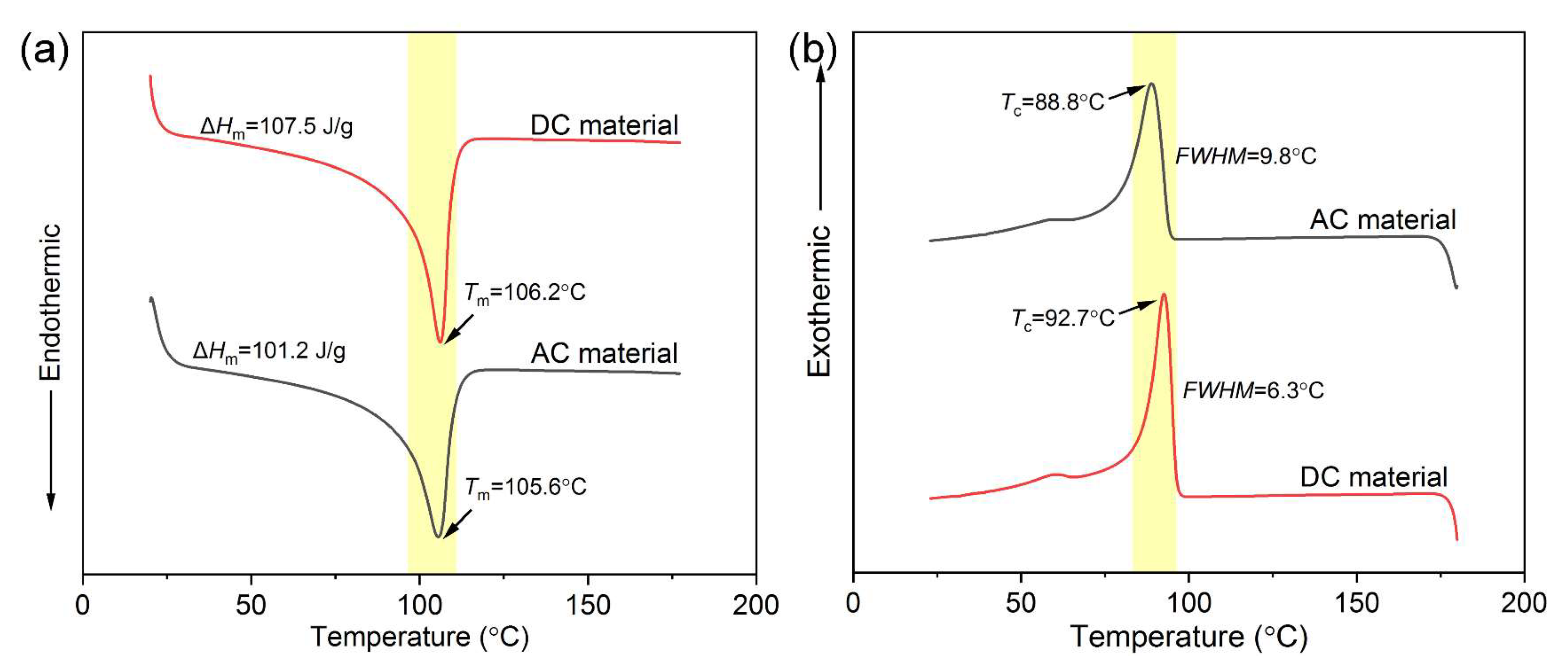
3.2.1. Melt and Crystallization Properties
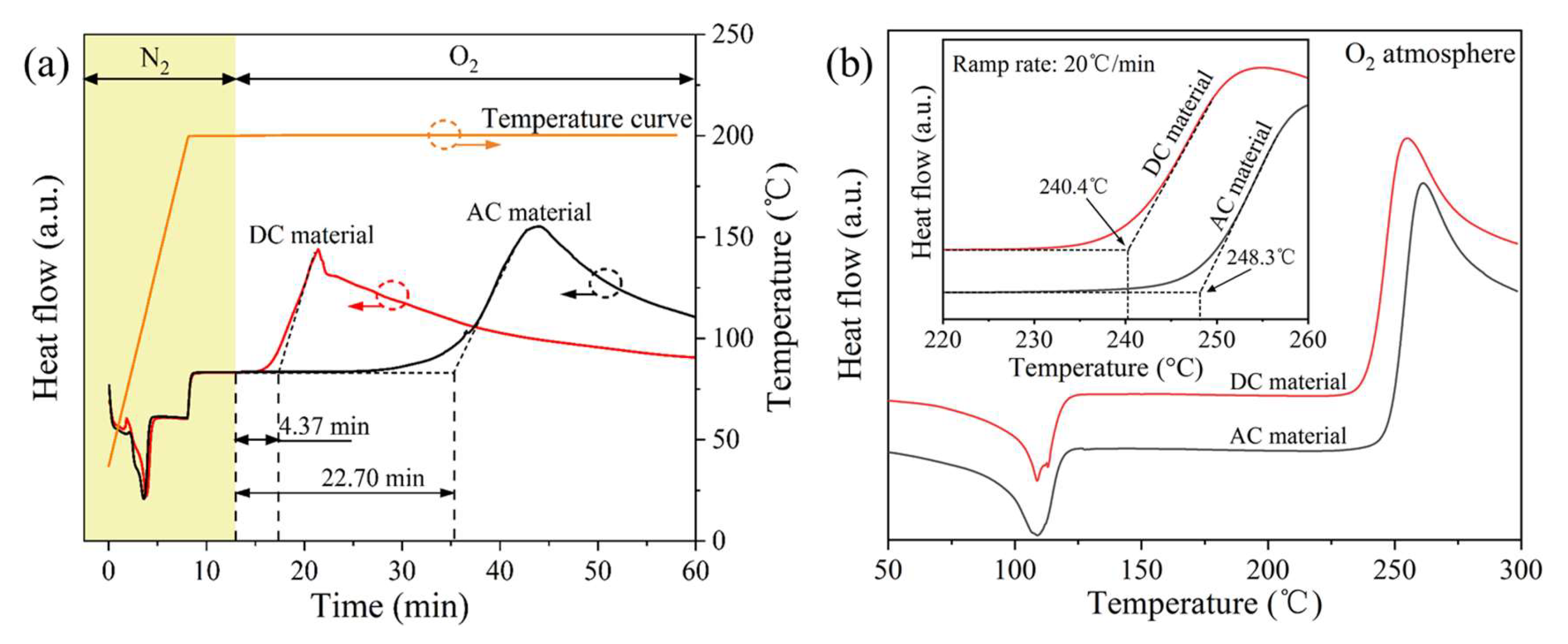
3.2.2. DSC OIT Properties
3.2.3. Thermal Oxygen Decomposition
3.3. Space Charge Properties
3.4. Thermal Aging Properties
3.4.1. Tensile Properties of Thermally Aged XLPE Samples
3.4.2. FTIR Spectra of Thermally Aged XLPE Samples
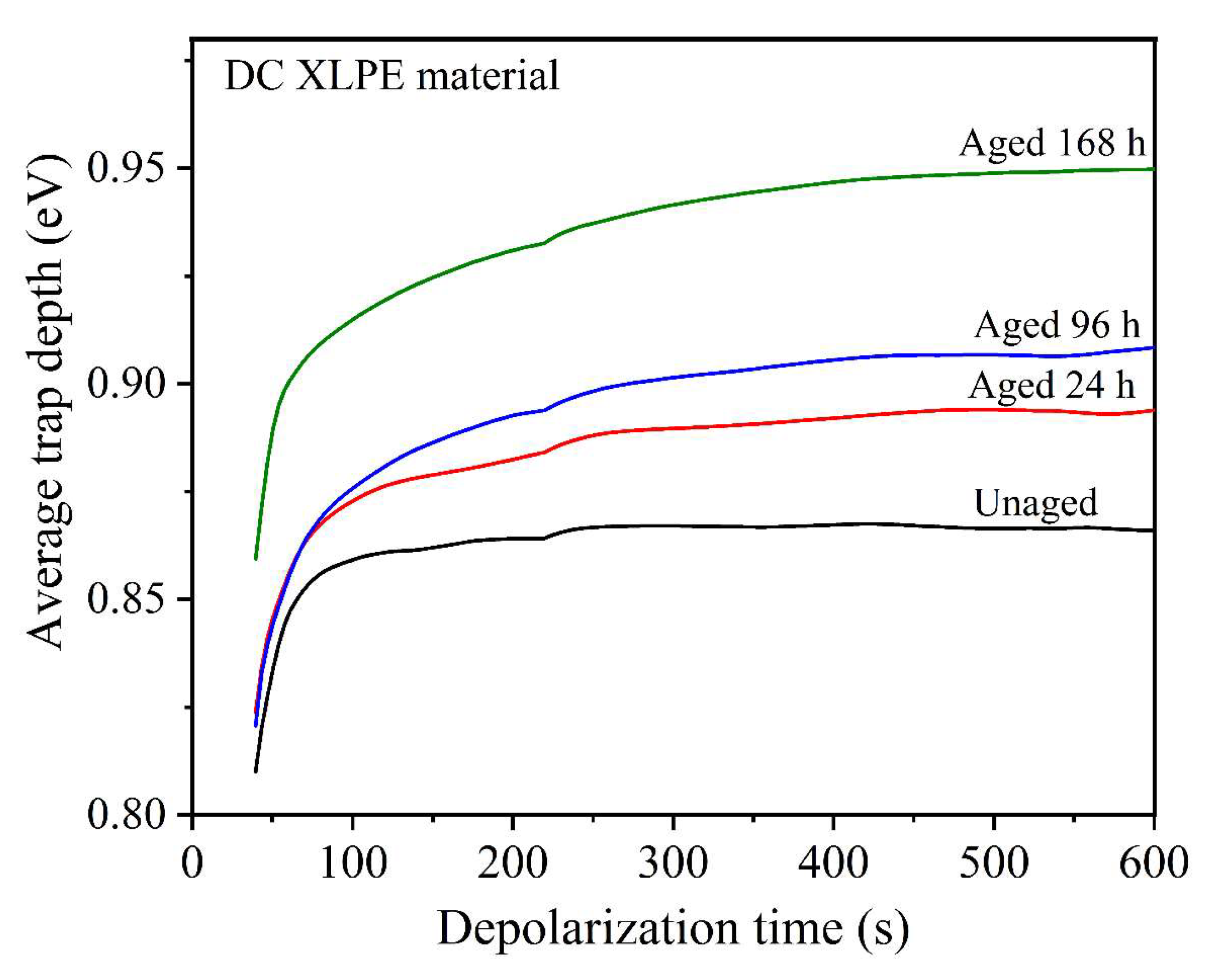
3.4.3. Space Charge of Thermally Aged XLPE Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.S.; Li, G.H.; Guo, J.B. Large-Scale Renewable Energy Transmission by HVDC: Challenges and Proposals. Engineering 2022, in press. [CrossRef]
- Zhuo, Z.Y.; Du, E.S.; Zhang, N.; Nielsen, C.P.; Lu, X.; Xiao, J.Y.; Wu, J.W.; Kang, C.Q. Cost increase in the electricity supply to achieve carbon neutrality in China. Nat. Commun. 2022, 13, 3172. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.X.; Zhu, Z.E.; Yang, L.M.; Jiang, Z.X.; Ma, Y.L. Research on EPDM for ±535 kV HVDC Cable Accessories. In Proceedings of the 7th International Conference on Condition Monitoring and Diagnosis, Perth, WA, Australia, 23–26 September 2018; p. 8535830. [Google Scholar]
- Wang, H.Y.; Sun, M.L.; Zhao, K.J.; Wang, X.W.; Xu, Q.L.; Wang, W.; Li, C.R. High-Voltage FDS of Thermally Aged XLPE Cable and Its Correlation with Physicochemical Properties. Polymers 2022, 14, 3519. [Google Scholar] [CrossRef]
- Zhao, X.D.; Zhao, H.; Sun, W.F. Significantly Improved Electrical Properties of Crosslinked Polyethylene Modified by UV-Initiated Grafting MAH. Polymers 2020, 12, 62. [Google Scholar] [CrossRef]
- Li, Y.; Krentz, T.M.; Wang, L.; Benicewicz, B.C.; Schadler, L.S. Ligand Engineering of Polymer Nanocomposites: From the Simple to the Complex. ACS Appl. Mater. Interfaces 2014, 6, 6005–6021. [Google Scholar] [CrossRef]
- Pleşa, I.; Noţingher, P.V.; Stancu, C.; Wiesbrock, F.; Schlögl, S. Polyethylene Nanocomposites for Power Cable Insulations. Polymers 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Chen, P.X.; Li, H.; Li, J.Y.; Chen, Z.Z. Improved DC performance of crosslinked polyethylene insulation depending on a higher purity. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1809–1817. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.Z.; Ye, X.Z.; Wu, K. Study on Storage Activity of Cross-Linkable Polyethylene Material Used for High-Voltage Cables. IEEE Trans. Dielectr. Electr. Insul. 2022, 2, 437–445. [Google Scholar] [CrossRef]
- Zhang, L.; Khani, M.; Krentz, T.M.; Huang, Y.H.; Zhou, Y.X.; Benicewicz, B.C.; Nelson, J.K. Suppression of space charge in crosslinked polyethylene filled with poly(stearyl methacrylate)-grafted SiO2 nanoparticles. Appl. Phys. Lett. 2017, 110, 132903. [Google Scholar] [CrossRef]
- Stamm, M.; Sommer, J.U. Polymer-Nanoparticle Films: Entropy and Enthalpy at Play. Nat. Mater. 2007, 6, 260–261. [Google Scholar] [CrossRef]
- Green, P.F. The Structure of Chain End-Grafted Nanoparticle/Homopolymer Nanocomposites. Soft Matter 2011, 7, 7914–7926. [Google Scholar] [CrossRef]
- Beer, S.; Teasdale, I.; Brueggemann, O. Immobilization of antioxidants via ADMET polymerization for enhanced long-term stabilization of polyolefins. Eur. Polym. J. 2013, 49, 4257–4264. [Google Scholar] [CrossRef]
- Englund, V.; Huuva, R.; Gubanski, S.M.; Hjertberg, T. Synthesis and efficiency of voltage stabilizers for XLPE cable insulation. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 1455–1461. [Google Scholar] [CrossRef]
- Youn, D.J.; Li, J.F.; Livazovic, S.; Sun, Y.B.; Sun, S.Y. Controlling Factors of Degassing in Crosslinked Polyethylene Insulated Cables. Polymers 2019, 11, 1439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Zhao, Y.T.; Yu, G.J.; Chen, L.; Wang, Y.F.; Zhang, L. Research of the key issues of ±535kV HVDC extruded cable development. In Proceedings of the 2020 4th International Conference on HVDC (HVDC), Xi’an, China, 6–9 November 2020; pp. 1139–1142. [Google Scholar]
- Meng, F.B.; Chen, X.R.; Xu, X.; Dai, C.; Paramane, A.; Tanaka, Y. Effect of degassing treatment durations on physico-chemical and electrical properties of 500 kV extra HVDC XLPE cable insulation. Polym. Degrad. Stab. 2021, 188, 109566. [Google Scholar] [CrossRef]
- Hanley, T.L.; Burford, R.P.; Fleming, R.J.; Barber, K.W. A general review of polymeric insulation for use in HVDC cables. IEEE Electr. Insul. Mag. 2003, 19, 13–24. [Google Scholar] [CrossRef]
- Tzimas, A.; Antoniou, D.; Rowland, S.M. Low voltage DC cable insulation challenges and opportunities. In Proceedings of the 2012 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Montreal, QC, Canada, 13 December 2012; pp. 14–17. [Google Scholar]
- Tanaka, Y.; Fujitomi, T.; Kato, T.; Miyake, H.; Mori, H.; Kikuchi, S.; Yagi, Y. Packet-like charge formation in cable insulating materials at polarity reversal. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1372–1379. [Google Scholar] [CrossRef]
- Li, X.F.; Yang, Y.M.; Wang, Q.; Gao, Y.; Zhang, W.; Liu, Z.D. Study on the water-tree ageing characteristics of polyethylene/organic montmorillonite and crosslinked polyethylene/organic montmorillonite nanocomposites. High Volt. 2022. [Google Scholar] [CrossRef]
- Ahmad, H.; Rodrigue, D. Crosslinked polyethylene: A review on the crosslinking techniques, manufacturing methods, applications, and recycling. Polym. Eng. Sci. 2022, 62, 2376–2401. [Google Scholar] [CrossRef]
- Khonakdar, H.A.; Jafari, S.H.; Haghighi-Asl, A.; Wagenknecht, U.; Haussler, L.; Reuter, U. Thermal and mechanical properties of uncrosslinked and chemically crosslinked polyethylene/ethylene vinyl acetate copolymer blends. J. Appl. Polym. Sci. 2007, 103, 3261–3270. [Google Scholar] [CrossRef]
- Kemari, Y.; Mekhaldi, A.; Teyssèdre, G.; Teguar, M. Correlations between structural changes and dielectric behavior of thermally aged XLPE. IEEE Trans. Dielect. Electr. Insul. 2019, 26, 1859–1866. [Google Scholar] [CrossRef]
- Ahmed, M.; Zhong, L.S.; Li, F.; Xu, N.; Gao, J.H. Improving the DC Dielectric Properties of XLPE with Appropriate Content of Dicumyl Peroxide for HVDC Cables Insulation. Materials 2022, 15, 5857. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Versey, S.W.; Fifield, L.S.; Bowler, N. Quantitative analysis of changes in antioxidant in crosslinked polyethylene (XLPE) cable insulation material exposed to heat and gamma radiation. Polym. Degrad. Stab. 2018, 156, 252–258. [Google Scholar] [CrossRef]
- Shimada, A.; Sugimoto, M.; Kudoh, H.; Tamura, K.; Seguchi, T. Degradation distribution in insulation materials of cables by accelerated thermal and radiation ageing. IEEE Trans. Dielect. Electr. Insul. 2013, 20, 2017–2116. [Google Scholar] [CrossRef]
- Qiao, Z.C.; Wu, W.S.; Wang, Z.W.; Zhang, L.; Zhou, Y.X. Space Charge Behavior of Thermally Aged Polyethylene Insulation of Track Cables. Polymers 2022, 14, 2162. [Google Scholar] [CrossRef] [PubMed]
- Tzimas, A.; Rowland, S.M.; Dissado, L.A. Effect of electrical and thermal stressing on charge traps in XLPE cable insulation. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 2145–2154. [Google Scholar] [CrossRef]
- Ho, Y.F.F.; Che, G.; Davies, A.E.; Swingler, S.G.; Sutton, S.J.; Hampton, R.N.; Hobdell, S. Measurement of space charge in XLPE insulation under 50 Hz ac electric stresses using the LIPP method. IEEE Trans. Dielectr. Electr. Insul. 2002, 9, 362–370. [Google Scholar] [CrossRef]
- Zhu, T.G.; Li, X.L.; Zhao, X.Y.; Zhang, X.; Lu, Y.L.; Zhang, L.Q. Stress-strain behavior and corresponding crystalline structures of four types of polyethylene under a wide range of strain rates. Polym. Test. 2022, 106, 107460. [Google Scholar] [CrossRef]
- Andjelkovic, D.; Rajakovic, N. Influence of accelerated aging on mechanical and structural properties of crosslinked polyethylene(XLPE) insulation. Electr. Eng. 2001, 83, 83–87. [Google Scholar] [CrossRef]
- Lv, H.K.; Lu, T.H.; Xiong, L.Q.; Zheng, X.G.; Huang, Y.Y.; Ying, M.L.; Cai, J.C.; Li, Z. Assessment of thermally aged XLPE insulation material under extreme operating temperatures. Polym. Test. 2020, 88, 106569. [Google Scholar] [CrossRef]
- Huzayyin, A.; Boggs, S.; Ramprasad, R. Density functional analysis of chemical impurities in dielectric polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 926–930. [Google Scholar] [CrossRef]
- Shi, X.M.; Wang, J.D.; Jiang, B.B.; Yang, Y.R. Hindered phenol grafted carbon nanotubes for enhanced thermal oxidative stability of polyethylene. Polymer 2013, 54, 1167–1176. [Google Scholar] [CrossRef]
- Montanari, G.C. The Electrical Degradation Threshold of Polyethylene Investigated by Space Chrge and Conduction Current Measurements. IEEE Trans. Dielectr. Electr. Insul. 2000, 7, 309–315. [Google Scholar] [CrossRef]











| AC Material | DC Material | |
|---|---|---|
| Melting point Tm (°C) | 105.6 | 106.2 |
| Melting enthalpy ΔHm (J/g) | 101.2 | 107.5 |
| Crystallinity Xc (%) | 35.2 | 37.4 |
| Crystallization point Tc (°C) | 88.8 | 92.7 |
| FWHM (°C) | 9.8 | 6.3 |
| Apparatus | Parameters | AC Material | DC Material |
|---|---|---|---|
| DSC | Isothermal OIT (min) | 22.70 | 4.37 |
| DSC | Dynamic OIT (°C) | 248.3 | 240.4 |
| TGA | Thermal-oxidative temperature (°C) | 242.2 | 240.2 |
| TGA | 5% weight loss temperature (°C) | 259.1 | 252.8 |
| Parameters | Thermal Aging Time | AC Material | DC Material |
|---|---|---|---|
| Carbonyl index | 6 h | 1.50 | 1.54 |
| 24 h | 1.47 | 1.34 | |
| 48 h | 1.46 | 1.39 | |
| 96 h | 1.78 | 1.65 | |
| 168 h | 1.90 | 6.13 | |
| Maximum internal field distortion (%) | 6 h | 49.6 | 28.0 |
| 24 h | 18.0 | 12.4 | |
| 48 h | 18.6 | 18.8 | |
| 96 h | 8.8 | 32.0 | |
| 168 h | 14.4 | 120.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, Z.; Tian, J.; Meng, S.; Zhou, Y. Thermal Aging Properties of 500 kV AC and DC XLPE Cable Insulation Materials. Polymers 2022, 14, 5400. https://doi.org/10.3390/polym14245400
Zhang L, Wang Z, Tian J, Meng S, Zhou Y. Thermal Aging Properties of 500 kV AC and DC XLPE Cable Insulation Materials. Polymers. 2022; 14(24):5400. https://doi.org/10.3390/polym14245400
Chicago/Turabian StyleZhang, Ling, Zhaowei Wang, Jihuan Tian, Shaoxin Meng, and Yuanxiang Zhou. 2022. "Thermal Aging Properties of 500 kV AC and DC XLPE Cable Insulation Materials" Polymers 14, no. 24: 5400. https://doi.org/10.3390/polym14245400
APA StyleZhang, L., Wang, Z., Tian, J., Meng, S., & Zhou, Y. (2022). Thermal Aging Properties of 500 kV AC and DC XLPE Cable Insulation Materials. Polymers, 14(24), 5400. https://doi.org/10.3390/polym14245400






