Biodegradable Interpolycomplexes for Anti-Erosion Stabilization of Soil and Sand
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
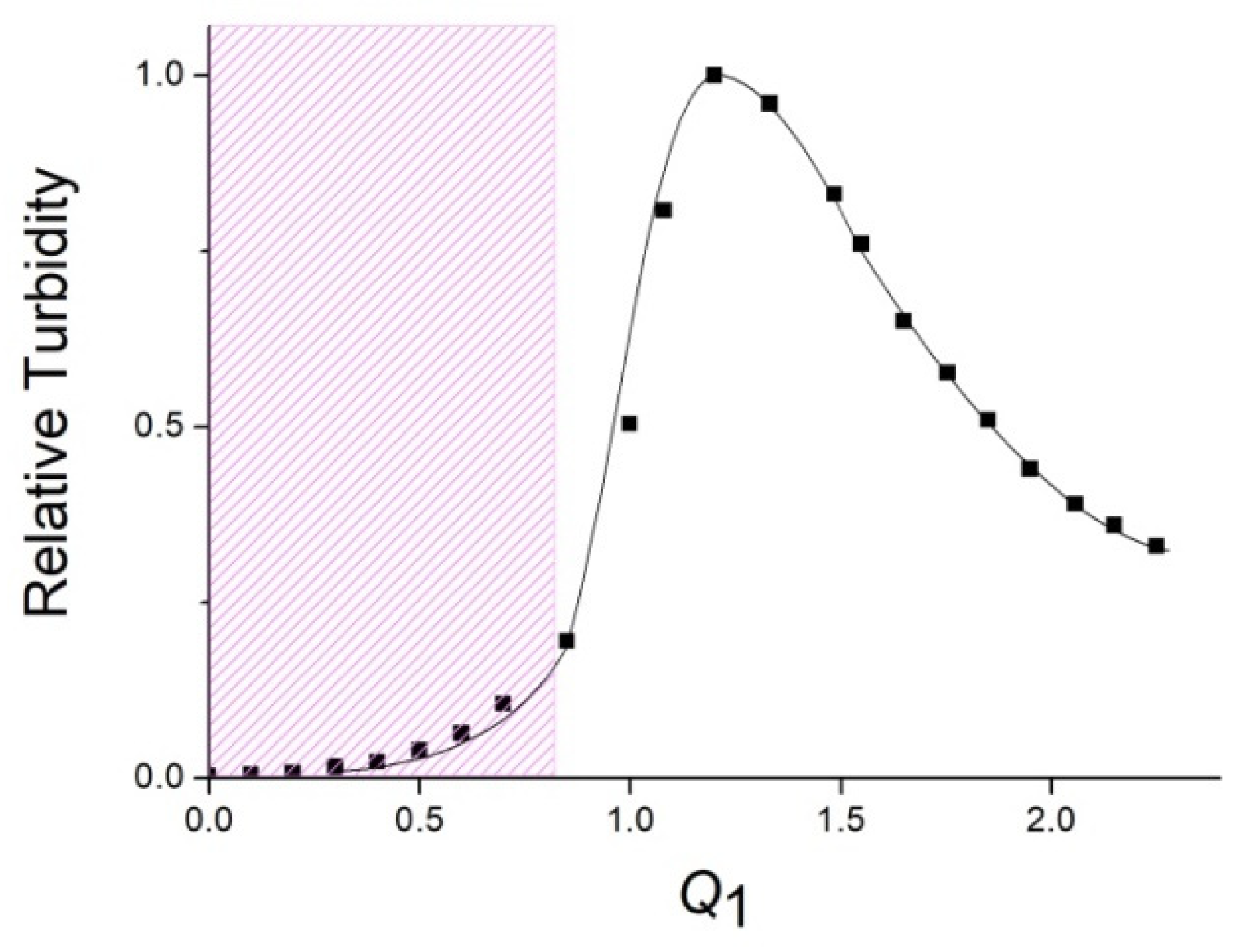
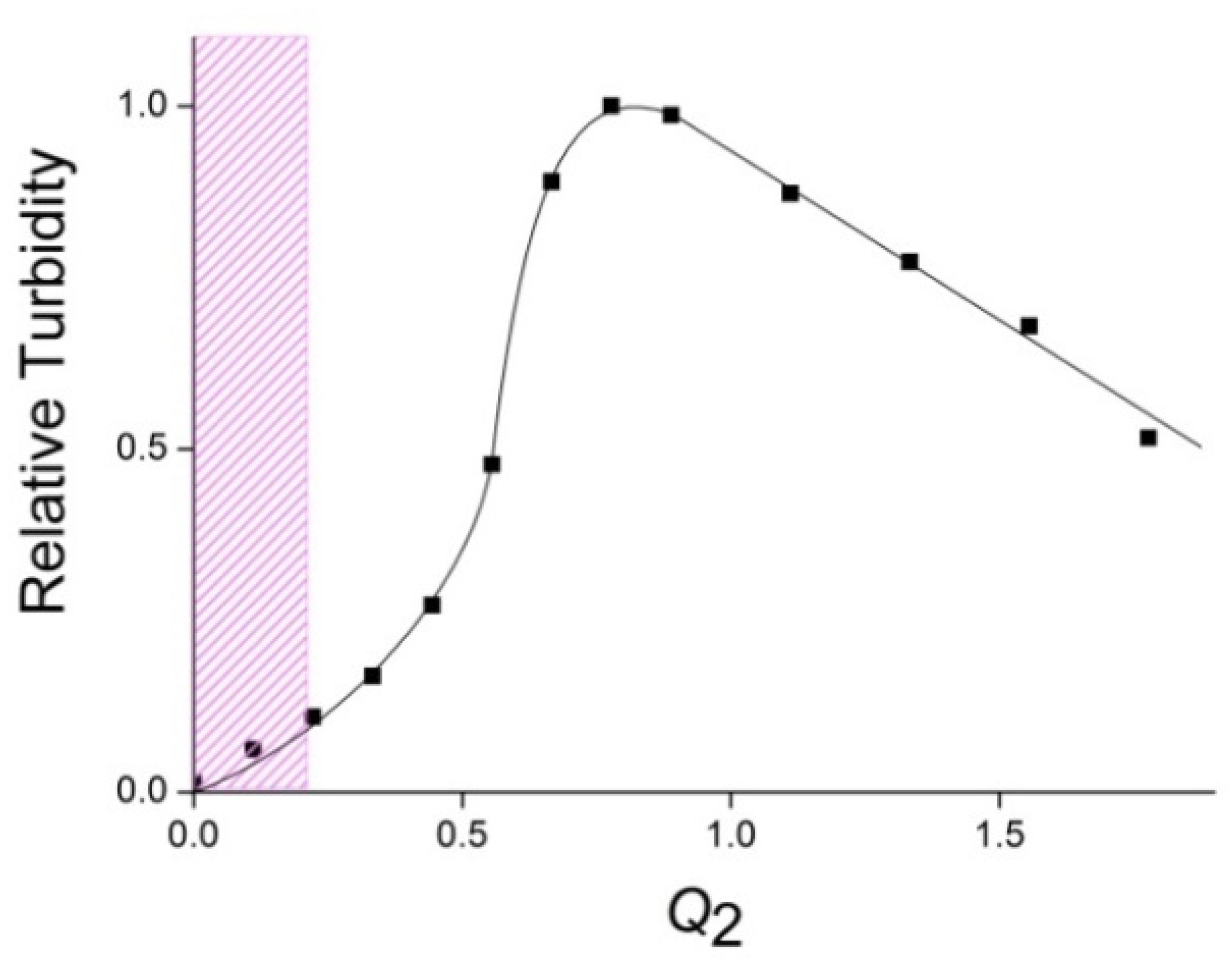
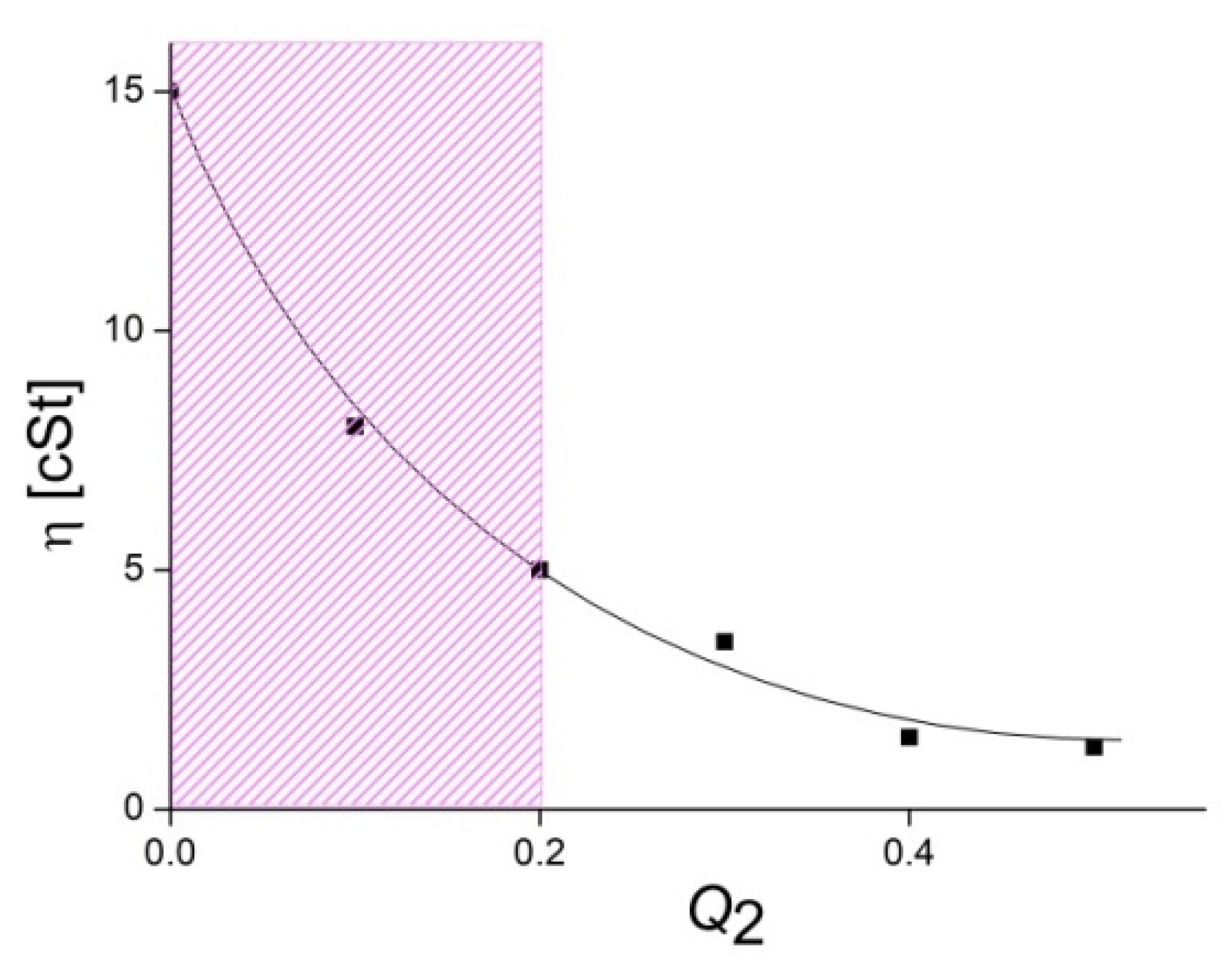
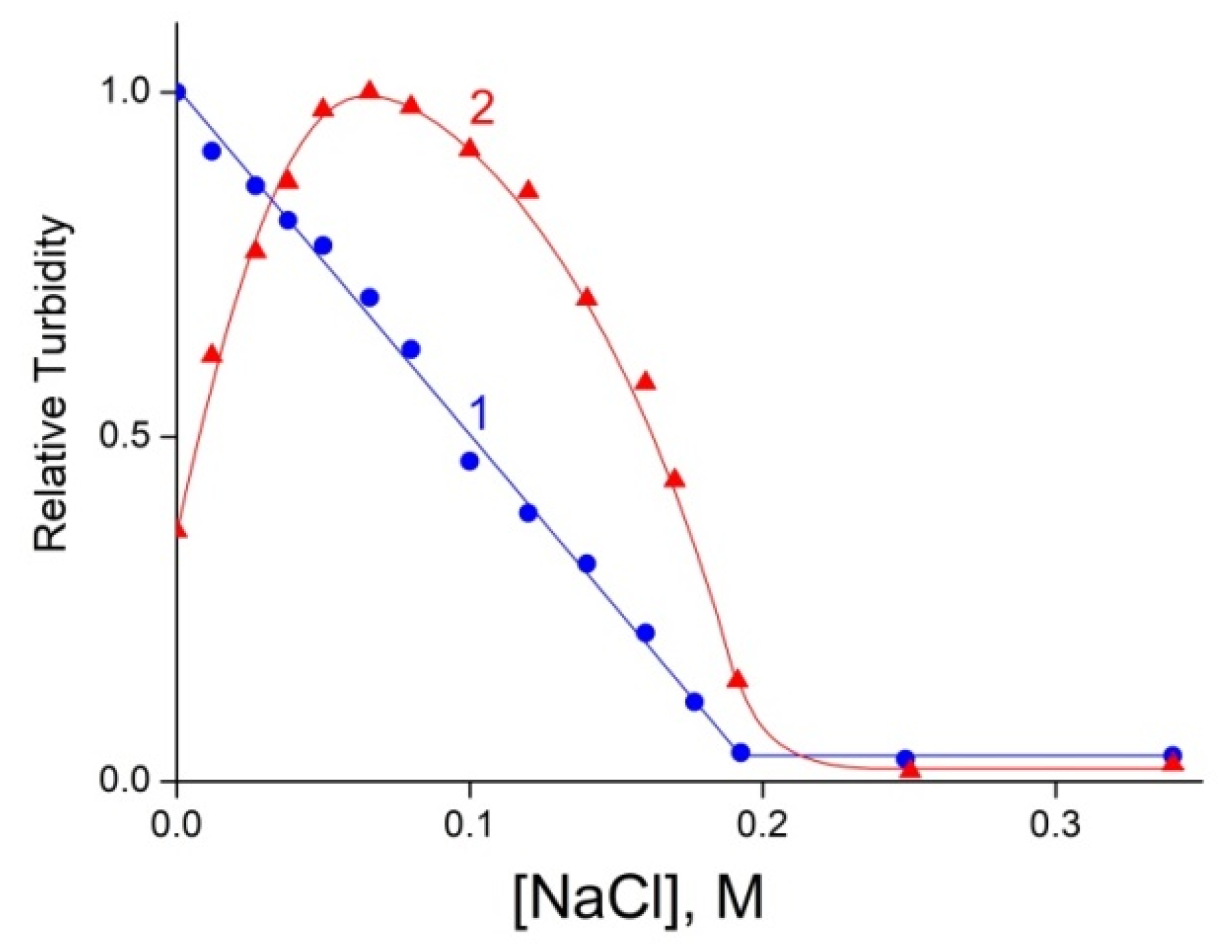
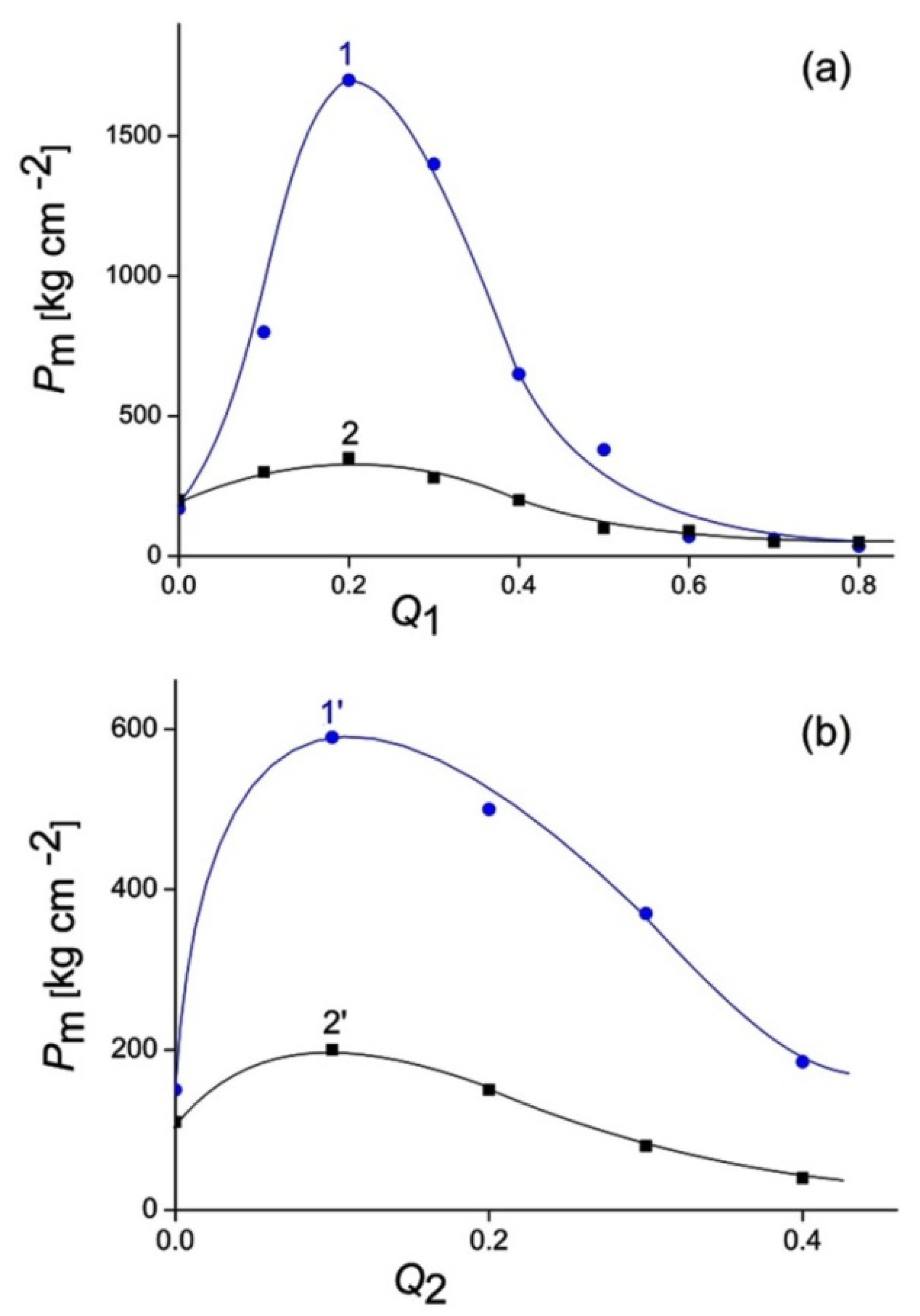
3.1. Formation and Properties of ALG–QHECE Polycomplexes
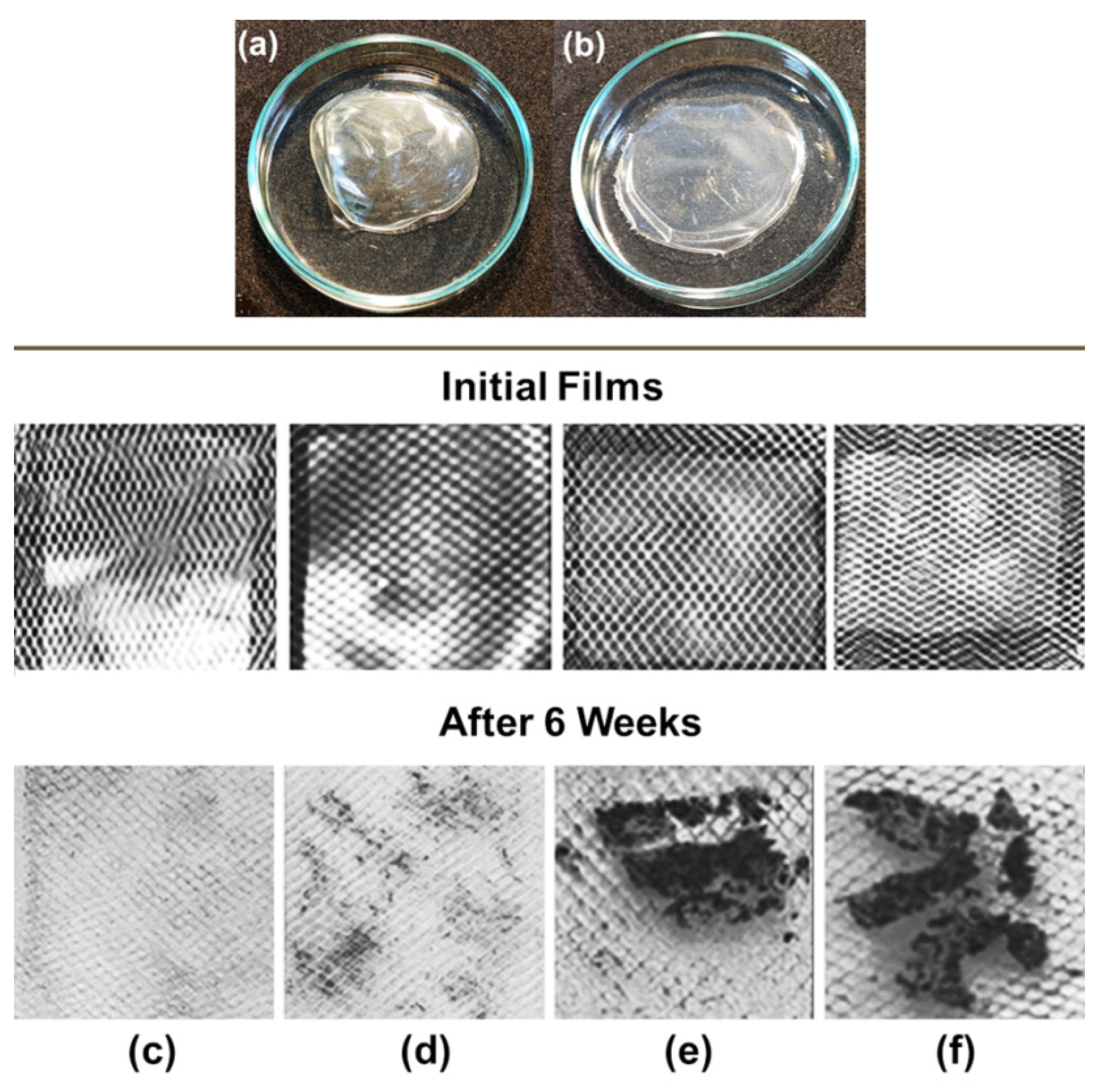
3.2. Polysaccharides and Polycomplexes for the Protection of Soil and Sand against Water Erosion
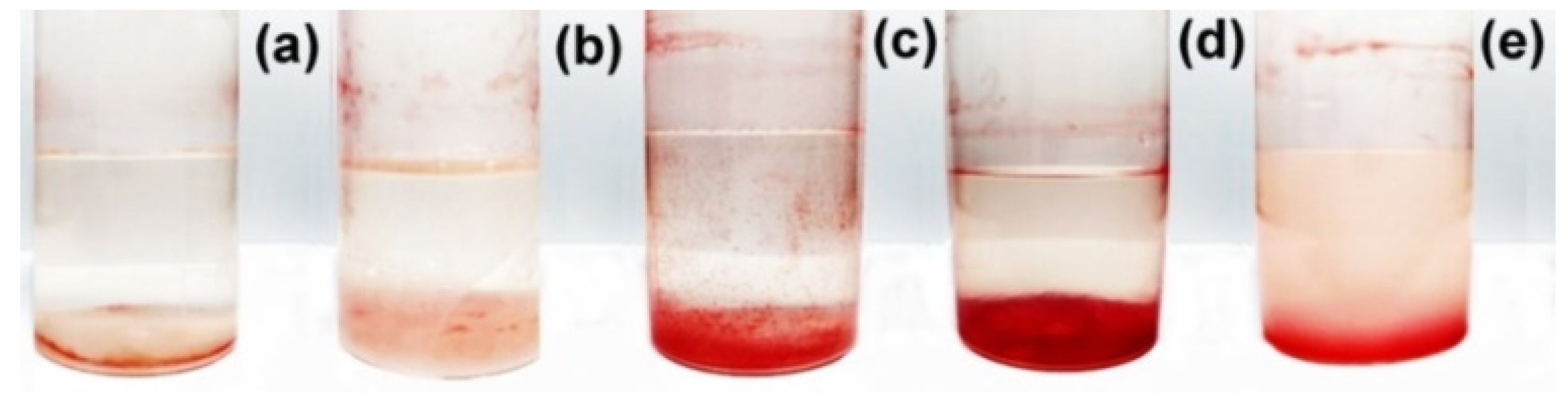
3.3. Biological Activity of Polysaccharide Formulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eisenhauer, N.; Buscot, F.; Heintz-Buschart, A.; Jurburg, S.D.; Küsel, K.; Sikorski, J.; Vogel, H.J.; Guerra, C.A. The multidimensionality of soil macroecology. Glob. Ecol. Biogeogr. 2021, 30, 4–10. [Google Scholar] [CrossRef]
- Guerra, C.A.; Rosa, I.; Valentini, E.; Wolf, F.; Filipponi, F.; Karger, D.N.; Xuan, A.N.; Mathieu, J.; Lavelle, P.; Eisenhauer, N. Global vulnerability of soil ecosystems to erosion. Landsc. Ecol. 2020, 35, 823–842. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Pateiro-Moure, M.; López-Periago, E.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C. Copper distribution and acid-base mobilization in vineyard soils and sediments from Galicia (NW Spain). Eur. J. Soil Sci. 2008, 59, 315–326. [Google Scholar] [CrossRef]
- Panagos, P.; Borrelli, P.; Poesen, J.; Ballabio, C.; Lugato, E.; Meusburger, K.; Montanarella, L.; Alewell, C. The new assessment of soil loss by water erosion in Europe. Environ. Sci. Policy 2015, 54, 438–447. [Google Scholar] [CrossRef]
- Shen, H.O.; Wang, D.L.; Wen, L.L.; Zhao, W.T.; Zhang, Y. Soil erosion and typical soil and water conservation measures on hillslopes in the Chinese Mollisol region. Eurasian Soil Sci. 2020, 53, 1509–1519. [Google Scholar] [CrossRef]
- Wang, X.; Oenema, O.; Hoogmoed, W.B.; Perdok, U.D.; Cai, D. Dust storm erosion and its impact on soil carbon and nitrogen losses in northern China. Catena 2006, 66, 221–227. [Google Scholar] [CrossRef]
- Tsymbarovich, P.; Kust, G.; Kumani, M.; Golosov, V.; Andreeva, O. Soil erosion: An important indicator for the assessment of land degradation neutrality in Russia. Int. Soil Water Conserv. Res. 2020, 8, 418–429. [Google Scholar] [CrossRef]
- Alexandrovskiy, A.L. Rates of soil-forming processes in three main models of pedogenesis. Rev. Mex. Cienc. Geol. 2007, 24, 283–292. [Google Scholar]
- Jafari, M.; Tahmoures, M.; Ehteram, M.; Ghorbani, M.; Panahi, F. Soil erosion: Factors, processes and effects. In Soil Erosion Control in Drylands; Springer: Cham, Switzerland, 2022; pp. 1–3. [Google Scholar] [CrossRef]
- Guerra, A.J.T.; Fullen, M.A.; Jorge, M.D.C.O.; Bezerra, J.F.R.; Shokr, M.S. Slope processes, mass movement and soil erosion: A review. Pedosphere 2017, 27, 27–41. [Google Scholar] [CrossRef]
- Wuepper, D.; Borrelli, P.; Finger, R. Countries and the global rate of soil erosion. Nat. Sustain. 2020, 3, 51–55. [Google Scholar] [CrossRef]
- Zang, Y.X.; Gong, W.; Xie, H.; Liu, B.L.; Chen, H.L. Chemical sand stabilization: A review of material, mechanism, and problems. Environ. Technol. Rev. 2015, 4, 119–132. [Google Scholar] [CrossRef]
- Zezin, A.B.; Mikheikin, S.V.; Rogacheva, V.B.; Zansokhova, M.F.; Sybachin, A.V.; Yaroslavov, A.A. Polymeric stabilizers for protection of soil and ground against wind and water erosion. Adv. Colloid Interface Sci. 2015, 226, 17–23. [Google Scholar] [CrossRef]
- Panova, I.G.; Ilyasov, L.O.; Yaroslavov, A.A. Polycomplex formulations for the protection of soils against degradation. Polym. Sci. Ser. C 2021, 63, 237–248. [Google Scholar] [CrossRef]
- Albalasmeh, A.A.; Hamdan, E.H.; Gharaibeh, M.A.; El Hanandeh, A. Improving aggregate stability and hydraulic properties of sandy loam soil by applying polyacrylamide polymer. Soil Tillage Res. 2021, 206, 104821. [Google Scholar] [CrossRef]
- Mamedov, A.I.; Tsunekawa, A.; Haregeweyn, N.; Tsubo, M.; Fujimaki, H.; Kawai, T.; Kebede, B.; Mulualem, T.; Abebe, G.; Wubet, A.; et al. Soil structure stability under different land uses in association with polyacrylamide effects. Sustainability 2021, 13, 1407. [Google Scholar] [CrossRef]
- Seybold, C.A. Polyacrylamide review: Soil conditioning and environmental fate. Commun. Soil Sci. Plant Anal. 2008, 25, 2171–2185. [Google Scholar] [CrossRef]
- Sojka, R.E.; Bjorneberg, D.L.; Entry, J.A.; Lentz, R.D.; Orts, W.J. Polyacrylamide in agriculture and environmental land management. Adv. Agron. 2007, 92, 75–162. [Google Scholar] [CrossRef]
- Izumrudov, V.A. Self-assembly and molecular ‘recognition’ phenomena in solutions of (bio)polyelectrolyte complexes. Russ. Chem. Rev. 2008, 77, 381–393. [Google Scholar] [CrossRef]
- Kabanov, V.A. Polyelectrolyte complexes in solution and in bulk. Russ. Chem. Rev. 2005, 74, 3–20. [Google Scholar] [CrossRef]
- Van der Gucht, J.; Spruijt, E.; Lemmers, M.; Stuart, M.A.C. Polyelectrolyte complexes: Bulk phases and colloidal systems. J. Colloid Interface Sci. 2011, 361, 407–422. [Google Scholar] [CrossRef]
- Novoskoltseva, O.A.; Loiko, N.G.; Nikolaev, Y.A.; Lisin, A.O.; Panova, I.G.; Yaroslavov, A.A. Interpolyelectrolyte complexes based on hydrolyzed polyacrylonitrile for anti-erosion stabilization of soils and ground. Polym. Int. 2022, 71, 697–705. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yanase, N.; Nagano, T.; Mitamura, H.; Naganawa, H. Decontamination and volume reduction of cesium-contaminated soil by combining soil solidification with interpolyelectrolyte complex and wet classification. J. Radioanal. Nucl. Chem. 2015, 305, 583–587. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Langford, S.J.; Ahmad, M.B.; Lim, Y.Y.; Hashim, K. Eco-friendly smart hydrogels for soil conditioning and sustain release fertilizer. Int. J. Environ. Sci. Technol. 2018, 15, 2059–2074. [Google Scholar] [CrossRef]
- Zinchenko, A.; Sakai, T.; Morikawa, K.; Nakano, M. Efficient stabilization of soil, sand, and clay by a polymer network of biomass-derived chitosan and carboxymethyl cellulose. J. Environ. Chem. Eng. 2022, 10, 107084. [Google Scholar] [CrossRef]
- Klivenko, A.; Orazzhanova, L.; Mussabayeva, B.; Yelemessova, G.; Kassymova, Z. Soil structuring using interpolyelectrolyte complexes of water-soluble polysaccharides. Polym. Adv. Technol. 2020, 31, 3292–3301. [Google Scholar] [CrossRef]
- Orazzhanova, L.K.; Kassymova, Z.S.; Mussabayeva, B.K.; Klivenko, A.N. Soils structuring in the presence of the chitosan–polyacrylic acid interpolymer complex. Eurasian Soil. Sci. 2020, 53, 1773–1781. [Google Scholar] [CrossRef]
- Kabanov, V.A.; Zezin, A.B.; Mustafaev, M.I.; Kasaikin, V.A. Soluble interpolymer complexes of polyamines and polyammonium salts. In Polymeric Amines and Ammonium Salts; Goethals, E.J., Ed.; Pergamon Press: Oxford, NY, USA, 1980; pp. 173–192. [Google Scholar]
- Novoskoltseva, O.A.; Ryabaya, O.O.; Pozdniakova, N.V.; Yaroslavov, A.A. Low-toxic multi-liposomal containers for encapsulation of bioactive compounds. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125282. [Google Scholar] [CrossRef]
- Khaidapova, D.D.; Pestonova, E.A. Strength of interparticle bonds in soil pastes and aggregates. Eurasian Soil Sci. 2007, 40, 1187–1192. [Google Scholar] [CrossRef]
- Panova, I.G.; Khaydapova, D.D.; Ilyasov, L.O.; Umarova, A.B.; Yaroslavov, A.A. Polyelectrolyte complexes based on natural macromolecules for chemical sand/soil stabilization. Colloids Surf. A Physicochem. Eng. Asp. 2020, 590, 124504. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Elbing, K.L.; Brent, R. Recipes and tools for culture of Escherichia coli. Curr. Protoc. Mol. Biol. 2019, 125, e83. [Google Scholar] [CrossRef] [PubMed]
- Loiko, N.; Danilova, Y.; Moiseenko, A.; Kovalenko, V.; Tereshkina, K.; Tutukina, M.; El-Registan, G.; Sokolova, O.; Krupyanskii, Y. Morphological peculiarities of the DNA-protein complexes in starved Escherichia coli cells. PLoS ONE 2020, 15, e0231562. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, P.G.; Jozala, A.F.; Novaes, L.C.D.L.; Moriel, P.; Penna, T.C.V. Minimal inhibitory concentration (MIC) determination of disinfectant and/or sterilizing agents. Braz. J. Pharm. Sci. 2009, 45, 241–248. [Google Scholar] [CrossRef]
- Gryta, A.; Frąc, M.; Oszust, K. The application of the Biolog EcoPlate approach in ecotoxicological evaluation of dairy sewage sludge. Appl. Biochem. Biotechnol. 2014, 174, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Tarafdar, J.C. 2, 3, 5-Triphenyltetrazolium chloride (TTC) as electron acceptor of culturable soil bacteria, fungi and actinomycetes. Biol. Fertil. Soils 2003, 38, 186–189. [Google Scholar] [CrossRef]
- Debandi, M.V.; Bernal, C.; Francois, N.J. Development of biodegradable films based on chitosan/glycerol blends suitable for biomedical applications. J. Tissue Sci. Eng. 2016, 7, 3. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation of three-layer laminate films based on gelatin under indoor soil conditions. Polym. Degrad. Stab. 2009, 94, 1307–1313. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Pothan, L.A.; Cordeiro, N.; Faria, M.; Thomas, S. Biodegradable nanocomposite films based on sodium alginate and cellulose nanofibrils. Materials 2016, 9, 50. [Google Scholar] [CrossRef]
- Xiao, L.; Salmi, J.; Laine, J.; Stenius, P. The effects of polyelectrolyte complexes on dewatering of cellulose suspension. Nord Pulp. Pap. Res. J. 2009, 24, 148–157. [Google Scholar] [CrossRef]
- Brand, F.; Dautzenberg, H. Structural analysis in interpolyelectrolyte complex formation of sodium poly(styrenesulfonate) and diallyldimethylammonium chloride—acrylamide copolymers by viscometry. Langmuir 1997, 13, 2905–2910. [Google Scholar] [CrossRef]
- Liu, R.C.; Morishima, Y.; Winnik, F.M. Rheological properties of mixtures of oppositely charged polyelectrolytes. A study of the interactions between a cationic cellulose ether and a hydrophobically modified poly [sodium 2-(acrylamido)-2-methylpropanesulfonate]. Polym. J. 2002, 34, 340–346. [Google Scholar] [CrossRef]
- Mende, M.; Petzold, G.; Buchhammer, H.M. Polyelectrolyte complex formation between poly(diallyldimethylammonium chloride) and copolymers of acrylamide and sodium-acrylate. Colloid. Polym. Sci. 2002, 280, 342–351. [Google Scholar] [CrossRef]
- Zhang, L.M.; Huang, S.J. Viscosity properties of homogeneous polyelectrolyte complex solutions from sodium carboxymethyl cellulose and poly(acrylamide-co-dimethyldiallylammonium chloride). Polym. Int. 2000, 49, 528–532. [Google Scholar] [CrossRef]
- Nurkeeva, Z.S.; Mun, G.A.; Khutoryanskiy, V.V. Interpolymer complexes of water-soluble nonionic polysaccharides with polycarboxylic acids and their applications. Macromol. Biosci. 2003, 3, 283–295. [Google Scholar] [CrossRef]
- Ilyasov, L.O.; Panova, I.G.; Kushchev, P.O.; Belov, A.A.; Maksimova, I.A.; Smagin, A.V.; Yaroslavov, A.A. Sparsely cross-linked hydrogel with starch fragments as a multifunctional soil conditioner. J. Compos. Sci. 2022, 6, 347. [Google Scholar] [CrossRef]
- Abrusci, C.; Marquina, D.; Del Amo, A.; Corrales, T.; Catalina, F. A viscometric study of the biodegradation of photographic gelatin by fungi isolated from cinematographic films. Int. Biodeterior. Biodegrad. 2006, 58, 142–149. [Google Scholar] [CrossRef]

| 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|
| Formulation | Loss of Soil, % | Crusts after Water Treatment | Loss of Sand, % | Crusts after Water Treatment |
| QHECE | <5 |  | 50 |  |
| Q1 = 0.4 | <1 |  | 50 |  |
| Q1 = 0.8 | <1 |  | 50 |  |
| ALG | <5 |  | 50 |  |
| Q2 = 0.2 | <1 |  | 40 |  |
| Q2 = 0.3 | <1 |  | 20 |  |
| Q2 = 0.4 | <1 |  | <10 |  |
| Formulation | Parameter | MIC and MBC, wt% | ||
|---|---|---|---|---|
| P. aeruginosa | S. aureus | Y. lipolytica | ||
| QHECE | MIC | >1 | >1 | >1 |
| MBC | >1 | >1 | >1 | |
| ALG | MIC | >1 | >1 | >1 |
| MBC | >1 | >1 | >1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novoskoltseva, O.A.; Belov, A.A.; Loiko, N.G.; Nikolaev, Y.A.; Panova, I.G.; Yaroslavov, A.A. Biodegradable Interpolycomplexes for Anti-Erosion Stabilization of Soil and Sand. Polymers 2022, 14, 5383. https://doi.org/10.3390/polym14245383
Novoskoltseva OA, Belov AA, Loiko NG, Nikolaev YA, Panova IG, Yaroslavov AA. Biodegradable Interpolycomplexes for Anti-Erosion Stabilization of Soil and Sand. Polymers. 2022; 14(24):5383. https://doi.org/10.3390/polym14245383
Chicago/Turabian StyleNovoskoltseva, Olga A., Andrey A. Belov, Nataliya G. Loiko, Yury A. Nikolaev, Irina G. Panova, and Alexander A. Yaroslavov. 2022. "Biodegradable Interpolycomplexes for Anti-Erosion Stabilization of Soil and Sand" Polymers 14, no. 24: 5383. https://doi.org/10.3390/polym14245383
APA StyleNovoskoltseva, O. A., Belov, A. A., Loiko, N. G., Nikolaev, Y. A., Panova, I. G., & Yaroslavov, A. A. (2022). Biodegradable Interpolycomplexes for Anti-Erosion Stabilization of Soil and Sand. Polymers, 14(24), 5383. https://doi.org/10.3390/polym14245383








