Coaxial Electrospun Nanofibrous Membranes for Enhanced Water Recovery by Direct Contact Membrane Distillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
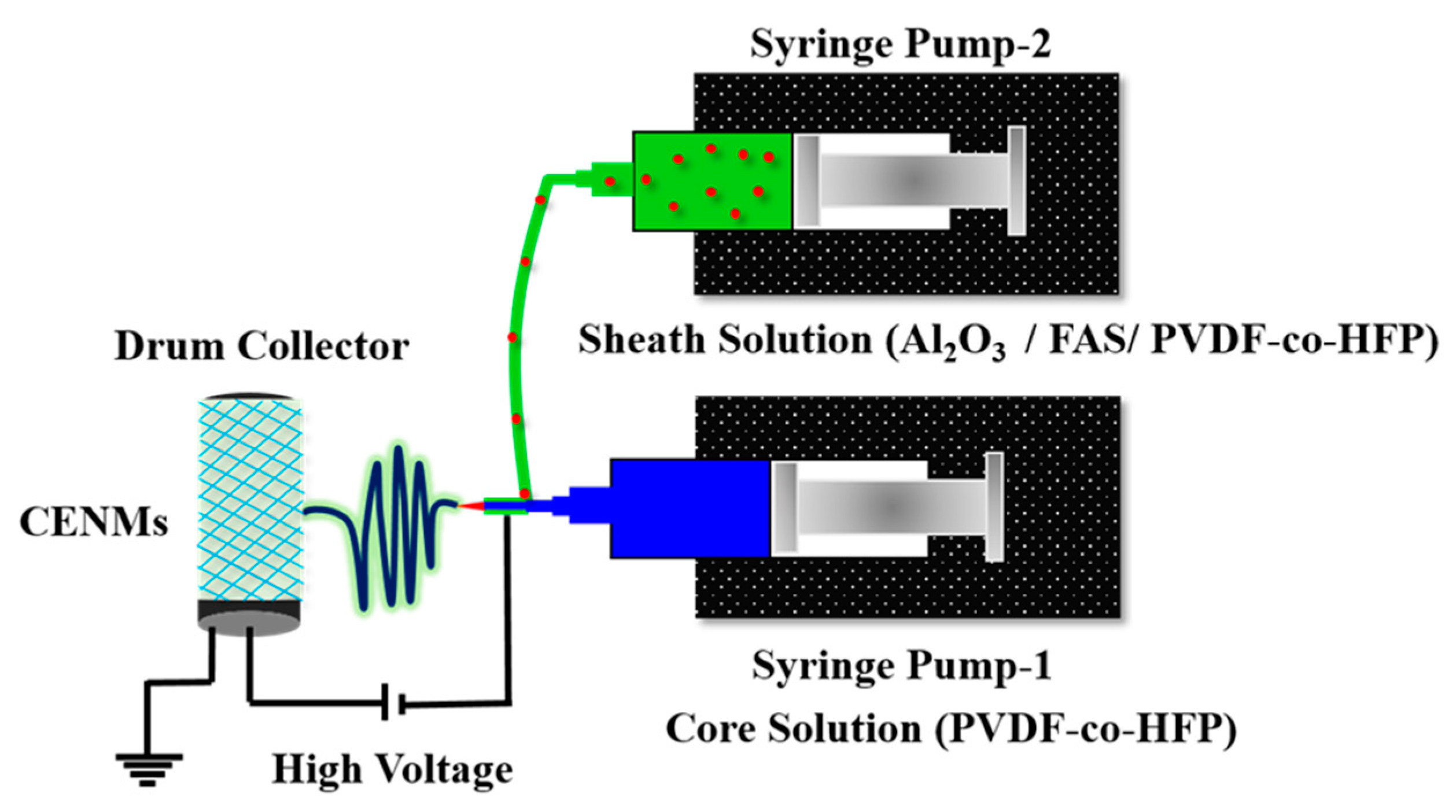
2.2. Preparation of Coaxial Electrospun Nanofibrous Membranes
2.3. Characterization of Nanoparticles and Membranes
2.4. Evaluation of CENMs for Direct Contact Membrane Distillation
3. Results
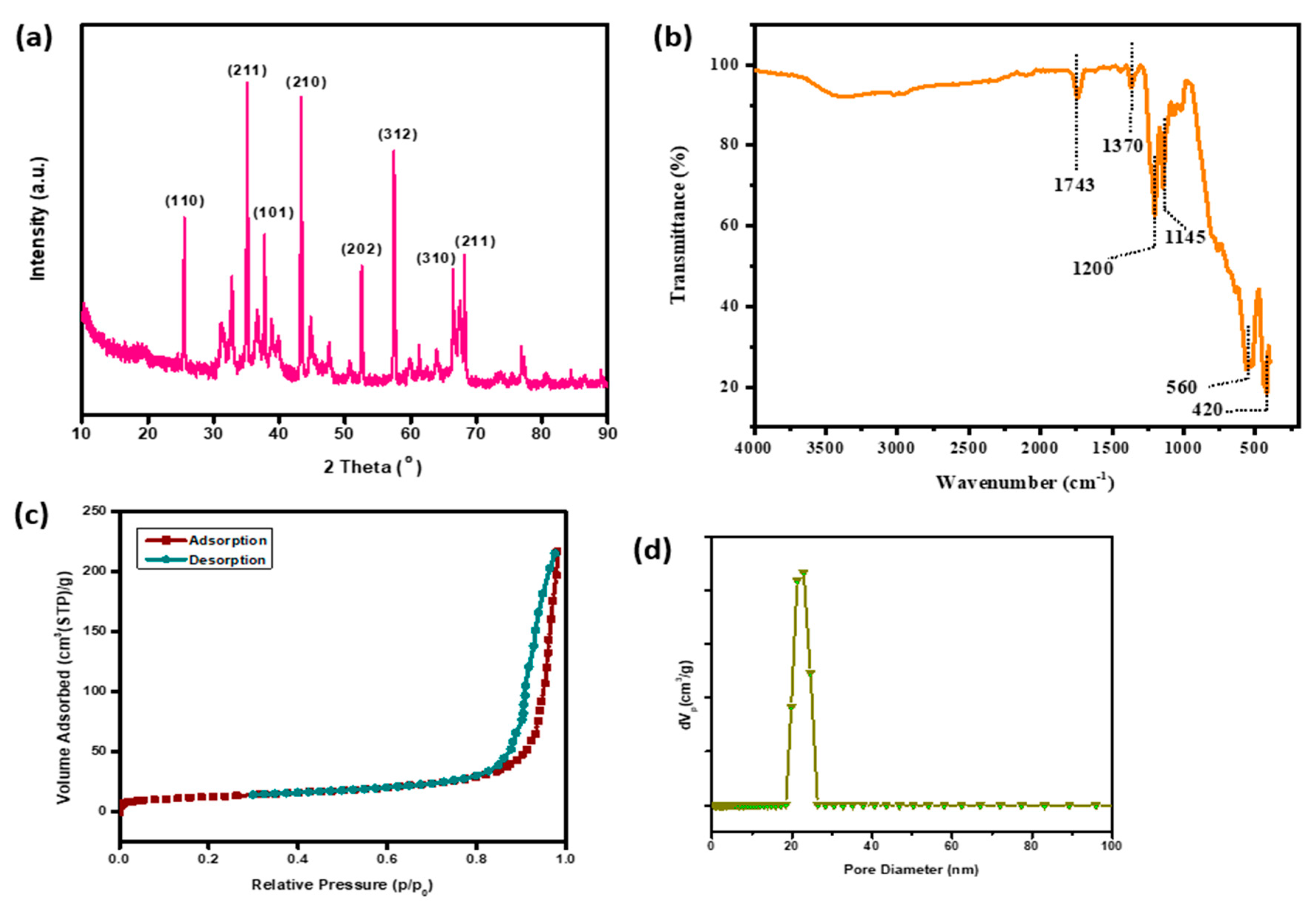
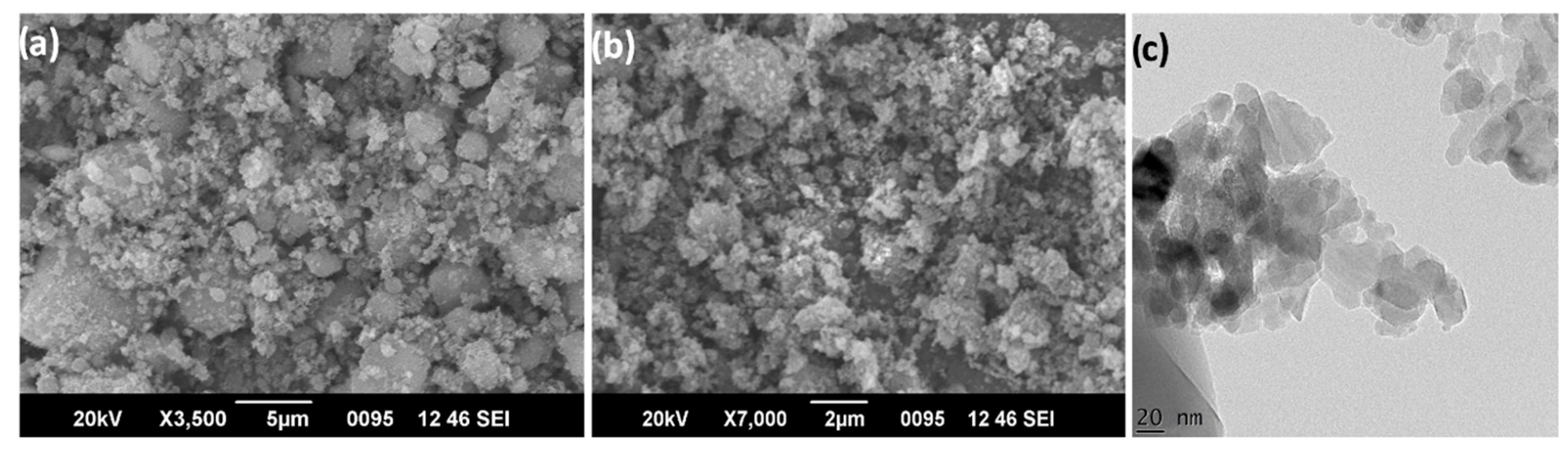
3.1. Characterization of Alumina Nanoparticles
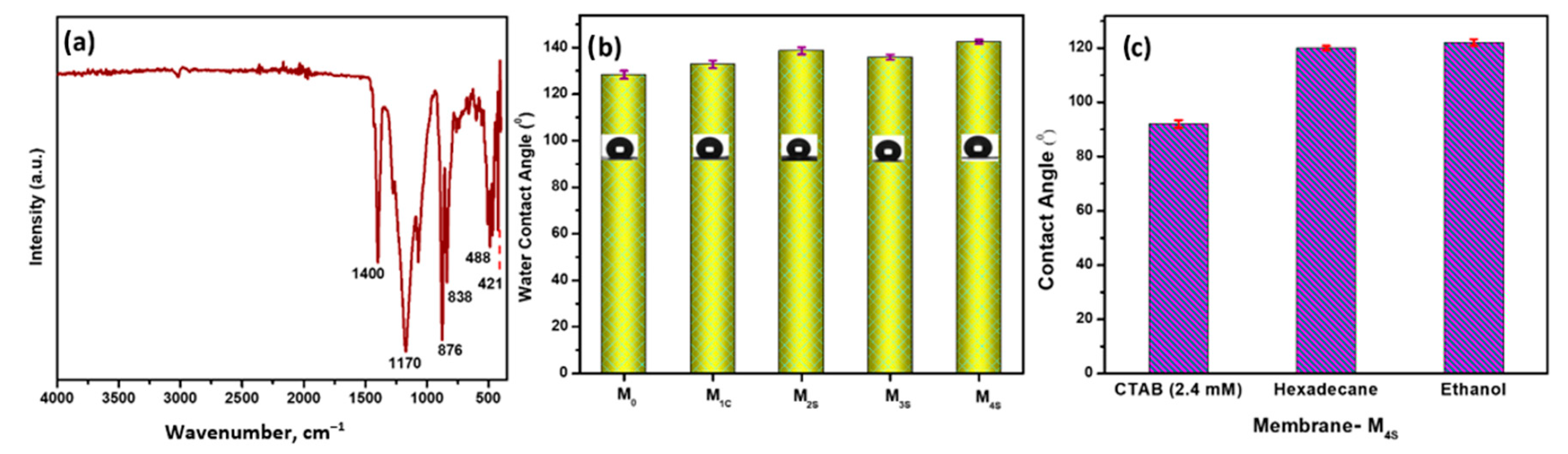
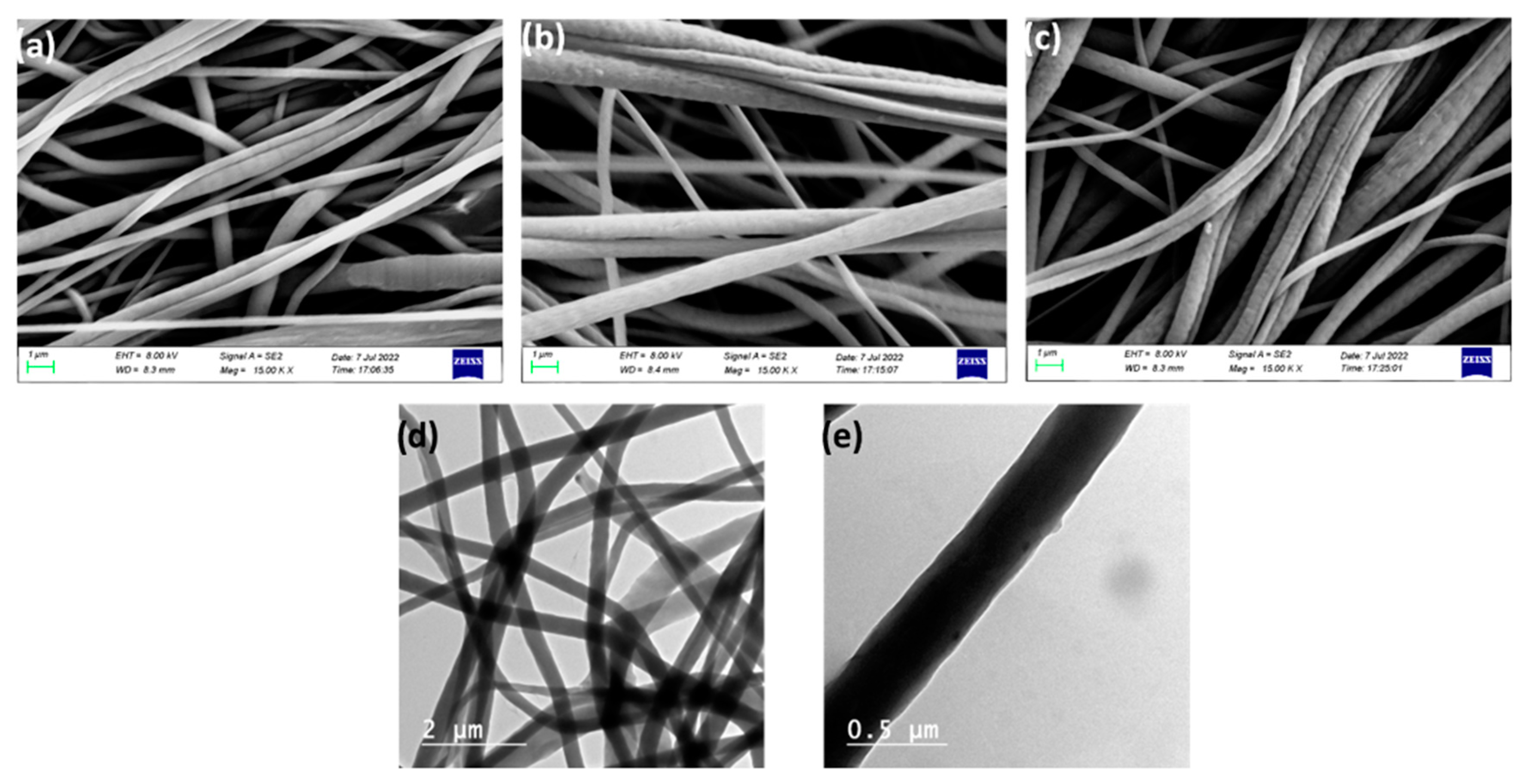
3.2. Characterization of CENMs
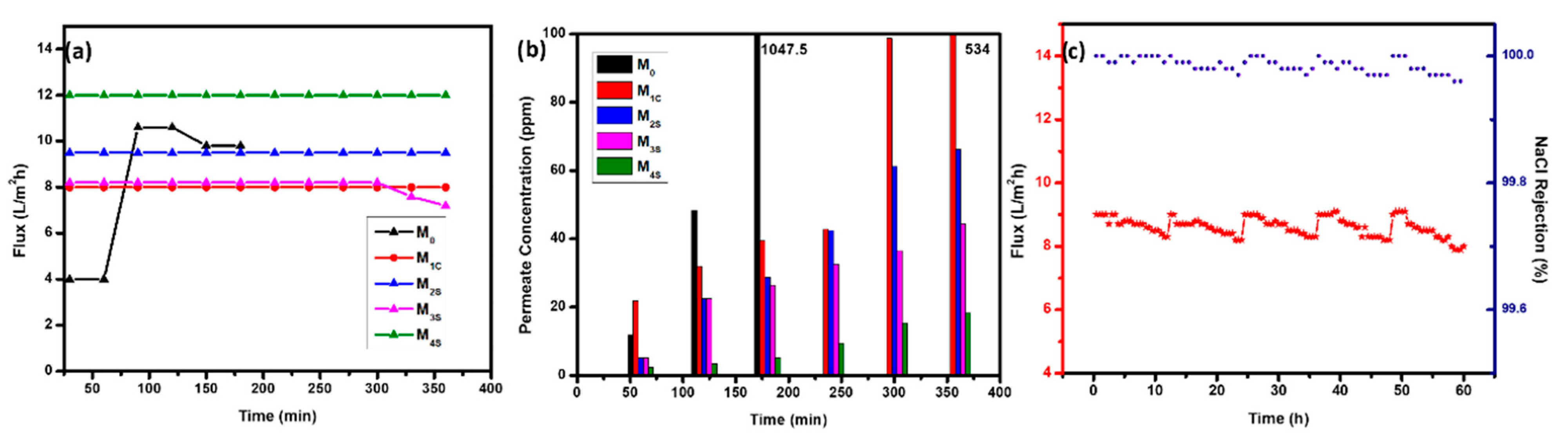
3.3. DCMD Performance of CENMs—Water Recovery from Hypersaline Wastewater
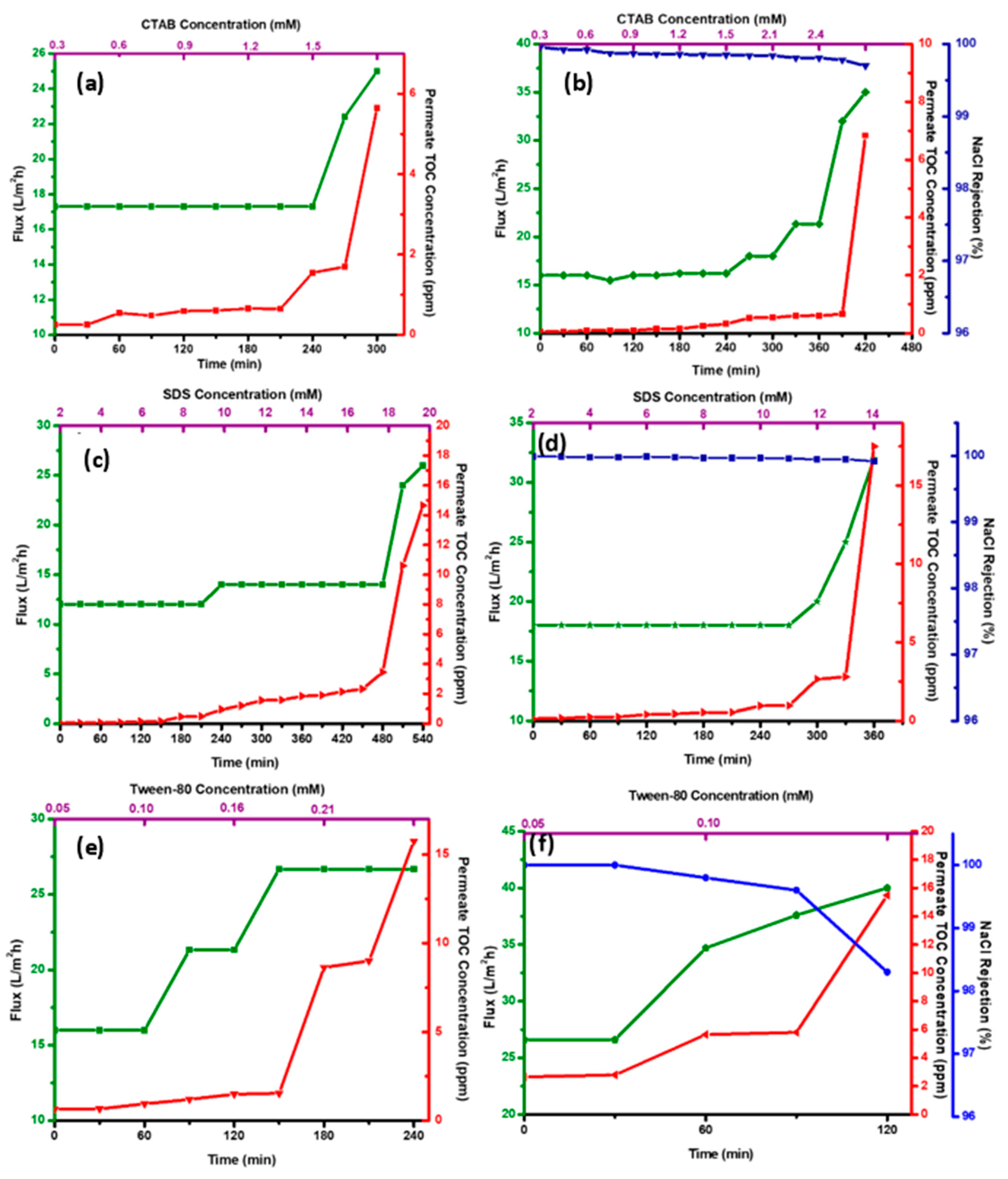
3.4. DCMD Performance of CENMs—Effect of Surfactants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Curto, D.; Franzitta, V.; Guercio, A. A Review of the Water Desalination Technologies. Appl. Sci. 2021, 11, 670. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]
- Guo, J.; Deka, B.J.; Wong, P.W.; Sun, J.; An, A.K. Fabrication of robust green superhydrophobic hybrid nanofiber-nanosphere membrane for membrane distillation. Desalination 2021, 520, 115314. [Google Scholar] [CrossRef]
- Shah, K.M.; Billinge, I.H.; Chen, X.; Fan, H.; Huang, Y.; Winton, R.K.; Yip, N.Y. Drivers, challenges, and emerging technologies for desalination of high-salinity brines: A critical review. Desalination 2022, 538, 115827. [Google Scholar] [CrossRef]
- Guan, G.; Yao, C.; Lu, S.; Jiang, Y.; Yu, H.; Yang, X. Sustainable operation of membrane distillation for hypersaline applications: Roles of brine salinity, membrane permeability and hydrodynamics. Desalination 2018, 445, 123–137. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Velioglu, S.; Han, L.; Joseph, B.D.; Unnithan, L.G.; Chew, J.W. Effect of surfactant hydrophobicity and charge type on membrane distillation performance. J. Membr. Sci. 2019, 587, 117168. [Google Scholar] [CrossRef]
- Chang, J.; Chang, H.; Meng, Y.; Zhao, H.; Lu, M.; Liang, Y.; Yan, Z.; Liang, H. Effects of surfactant types on membrane wetting and membrane hydrophobicity recovery in direct contact membrane distillation. Sep. Purif. Technol. 2022, 301, 122029. [Google Scholar] [CrossRef]
- Teoh, G.H.; Chin, J.Y.; Ooi, B.S.; Jawad, Z.A.; Leow, H.T.L.; Low, S.C. Superhydrophobic membrane with hierarchically 3D-microtexture to treat saline water by deploying membrane distillation. J. Water Process. Eng. 2020, 37, 101528. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhong, L.; Horseman, T.; Liu, Z.; Zeng, G.; Li, Z.; Lin, S.; Wang, W. Superhydrophobic-omniphobic membrane with anti-deformable pores for membrane distillation with excellent wetting resistance. J. Membr. Sci. 2021, 620, 118768. [Google Scholar] [CrossRef]
- Horseman, T.; Yin, Y.; Christie, K.S.; Wang, Z.; Tong, T.; Lin, S. Wetting, Scaling, and Fouling in Membrane Distillation: State-of-the-Art Insights on Fundamental Mechanisms and Mitigation Strategies. ACS ES&T Eng. 2021, 1, 117–140. [Google Scholar] [CrossRef]
- Yuan, Z.; Yu, Y.; Wei, L.; Sui, X.; She, Q.; Chen, Y. Pressure-retarded membrane distillation for simultaneous hypersaline brine desalination and low-grade heat harvesting. J. Membr. Sci. 2020, 597, 117765. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, Z.; Li, J. Recent Progress in Electrospun Nanofibers for the Membrane Distillation of Hypersaline Wastewaters. Adv. Fiber Mater. 2022, 1–18. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Lin, S.H.; Lin, C.M.; Leu, H.G. Operating characteristics and kinetic studies of surfactant wastewater treatment by Fenton oxidation. Water Res. 1999, 33, 1735–1741. [Google Scholar] [CrossRef]
- Li, J.; Guo, S.; Xu, Z.; Li, J.; Pan, Z.; Du, Z.; Cheng, F. Preparation of omniphobic PVDF membranes with silica nanoparticles for treating coking wastewater using direct contact membrane distillation: Electrostatic adsorption vs. chemical bonding. J. Membr. Sci. 2019, 574, 349–357. [Google Scholar] [CrossRef]
- Lin, P.-J.; Yang, M.-C.; Li, Y.-L.; Chen, J.-H. Prevention of surfactant wetting with agarose hydrogel layer for direct contact membrane distillation used in dyeing wastewater treatment. J. Membr. Sci. 2015, 475, 511–520. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Low, A.; Rabat, N.E. A review on recent developments in the adsorption of surfactants from wastewater. J. Environ. Manag. 2020, 254, 109797. [Google Scholar] [CrossRef]
- Badmus, S.O.; Amusa, H.K.; Oyehan, T.A.; Saleh, T. Environmental risks and toxicity of surfactants: Overview of analysis, assessment, and remediation techniques. Environ. Sci. Pollut. Res. 2021, 28, 62085–62104. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Z.; Liu, Y.; Li, X.; Yin, H.; Volkov, A.; He, T. Understanding the fouling/scaling resistance of superhydrophobic/omniphobic membranes in membrane distillation. Desalination 2021, 499, 114864. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Wang, Z.; Hou, D.; Lin, S. Coaxially electrospun super-amphiphobic silica-based membrane for anti-surfactant-wetting membrane distillation. J. Membr. Sci. 2017, 531, 122–128. [Google Scholar] [CrossRef]
- Chiao, Y.-H.; Cao, Y.; Ang, M.B.M.Y.; Sengupta, A.; Wickramasinghe, S.R. Application of superomniphobic electrospun membrane for treatment of real produced water through membrane distillation. Desalination 2022, 528, 115602. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Farid, M.U.; Kharraz, J.A.; Choi, J.; Tang, C.Y.; Nghiem, L.D.; Jang, A.; An, A.K. Removal of organic micropollutants using advanced membrane-based water and wastewater treatment: A review. J. Membr. Sci. 2020, 598, 117672. [Google Scholar] [CrossRef]
- Deka, B.J.; Guo, J.; An, A.K. Robust dual-layered omniphobic electrospun membrane with anti-wetting and anti-scaling functionalised for membrane distillation application. J. Membr. Sci. 2021, 624, 119089. [Google Scholar] [CrossRef]
- Hou, D.; Ding, C.; Fu, C.; Wang, D.; Zhao, C.; Wang, J. Electrospun nanofibrous omniphobic membrane for anti-surfactant-wetting membrane distillation desalination. Desalination 2019, 468, 114068. [Google Scholar] [CrossRef]
- Sangeetha, V.; Kaleekkal, N.J. Investigation of the performance of dual-layered omniphobic electrospun nanofibrous membranes for direct contact membrane distillation. J. Environ. Chem. Eng. 2022, 10, 108661. [Google Scholar] [CrossRef]
- Jia, W.; Kharraz, J.A.; Guo, J.; An, A.K. Superhydrophobic (polyvinylidene fluoride-co-hexafluoropropylene)/ (polystyrene) composite membrane via a novel hybrid electrospin-electrospray process. J. Membr. Sci. 2020, 611, 118360. [Google Scholar] [CrossRef]
- Robbins, C.A.; Yin, Y.; Hanson, A.J.; Blotevogel, J.; Borch, T.; Tong, T. Mitigating membrane wetting in the treatment of unconventional oil and gas wastewater by membrane distillation: A comparison of pretreatment with omniphobic membrane. J. Membr. Sci. 2022, 645, 120198. [Google Scholar] [CrossRef]
- Woo, Y.C.; Yao, M.; Shim, W.-G.; Kim, Y.; Tijing, L.D.; Jung, B.; Kim, S.-H.; Shon, H.K. Co-axially electrospun superhydrophobic nanofiber membranes with 3D-hierarchically structured surface for desalination by long-term membrane distillation. J. Membr. Sci. 2021, 623, 119028. [Google Scholar] [CrossRef]
- Attia, H.; Alexander, S.; Wright, C.J.; Hilal, N. Superhydrophobic electrospun membrane for heavy metals removal by air gap membrane distillation (AGMD). Desalination 2017, 420, 318–329. [Google Scholar] [CrossRef]
- Aghayan, M.; Voltsihhin, N.; Rodríguez, M.A.; Rubio-Marcos, F.; Dong, M.; Hussainova, I. Functionalization of gamma-alumina nanofibers by alpha-alumina via solution combustion synthesis. Ceram. Int. 2014, 40, 12603–12607. [Google Scholar] [CrossRef]
- Kathirvel, P.; Chandrasekaran, J.; Manoharan, D.; Kumar, S. Preparation and characterization of alpha alumina nanoparticles by in-flight oxidation of flame synthesis. J. Alloys Compd. 2014, 590, 341–345. [Google Scholar] [CrossRef]
- Prashanth, P.; Raveendra, R.; Krishna, R.H.; Ananda, S.; Bhagya, N.; Nagabhushana, B.; Lingaraju, K.; Naika, H.R. Synthesis, characterizations, antibacterial and photoluminescence studies of solution combustion-derived α-Al2O3 nanoparticles. J. Asian Ceram. Soc. 2015, 3, 345–351. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Dao, T.H.; Truong, T.T.; Nguyen, T.M.T.; Pham, T.D. Adsorption characteristic of ciprofloxacin antibiotic onto synthesized alpha alumina nanoparticles with surface modification by polyanion. J. Mol. Liq. 2020, 309, 113150. [Google Scholar] [CrossRef]
- Attia, H.; Johnson, D.J.; Wright, C.J.; Hilal, N. Robust superhydrophobic electrospun membrane fabricated by combination of electrospinning and electrospraying techniques for air gap membrane distillation. Desalination 2018, 446, 70–82. [Google Scholar] [CrossRef]
- Zheng, G.; Yao, L.; You, X.; Liao, Y.; Wang, R.; Huang, J.J. Effects of different secondary nano-scaled roughness on the properties of omniphobic membranes for brine treatment using membrane distillation. J. Membr. Sci. 2021, 620, 118918. [Google Scholar] [CrossRef]
- Chen, T.; Ma, W.; Lee, J.; Jassby, D.; Rahaman, S. Development of robust and superamphiphobic membranes using reduced graphene oxide (rGO)/PVDF-HFP nanocomposite mats for membrane distillation. Environ. Sci. Nano 2021, 8, 2883–2893. [Google Scholar] [CrossRef]
- Lee, E.-J.; An, A.K.; Hadi, P.; Lee, S.; Woo, Y.C.; Shon, H.K. Advanced multi-nozzle electrospun functionalized titanium dioxide/polyvinylidene fluoride-co-hexafluoropropylene (TiO2/PVDF-HFP) composite membranes for direct contact membrane distillation. J. Membr. Sci. 2017, 524, 712–720. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, K.J.; Chung, T.-S. An omniphobic slippery membrane with simultaneous anti-wetting and anti-scaling properties for robust membrane distillation. J. Membr. Sci. 2020, 595, 117572. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, W.; Zhang, Q.; Chen, X. Insight into the feed/permeate flow velocity on the trade-off of water flux and scaling resistance of superhydrophobic and welding-pore fibrous membrane in membrane distillation. J. Membr. Sci. 2021, 620, 118883. [Google Scholar] [CrossRef]
- Sallakhniknezhad, R.; Niknejad, A.S.; Barani, M.; Ranjbari, E.; Bazgir, S.; Kargari, A.; Rasouli, M.; Chae, S. Hypersaline drilling mud water treatment using pretreatment-free DCMD process. Desalination 2022, 539, 115938. [Google Scholar] [CrossRef]
- Li, J.; Ren, L.-F.; Huang, M.; Yang, J.; Shao, J.; He, Y. Facile preparation of omniphobic PDTS-ZnO-PVDF membrane with excellent anti-wetting property in direct contact membrane distillation (DCMD). J. Membr. Sci. 2022, 650, 120404. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Lienhard, J.H.L.; Samhaber, W.M. Wetting prevention in membrane distillation through superhydrophobicity and recharging an air layer on the membrane surface. J. Membr. Sci. 2017, 530, 42–52. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Zhang, F.; Lin, S. Significance of surface excess concentration in the kinetics of surfactant-induced pore wetting in membrane distillation. Desalination 2019, 450, 46–53. [Google Scholar] [CrossRef]
- Liao, X.; Wang, Y.; Liao, Y.; You, X.; Yao, L.; Razaqpur, A.G. Effects of different surfactant properties on anti-wetting behaviours of an omniphobic membrane in membrane distillation. J. Membr. Sci. 2021, 634, 119433. [Google Scholar] [CrossRef]
- Liang, Y.; Teng, X.; Chen, R.; Zhu, Y.; Jin, J.; Lin, S. Polyamide Nanofiltration Membranes from Emulsion-Mediated Interfacial Polymerization. ACS ES&T Eng. 2021, 1, 533–542. [Google Scholar] [CrossRef]
- Shao, S.; Shi, D.; Hu, J.; Qing, W.; Li, X.; Li, X.; Ji, B.; Yang, Z.; Guo, H.; Tang, C.Y. Unraveling the Kinetics and Mechanism of Surfactant-Induced Wetting in Membrane Distillation: An In Situ Observation with Optical Coherence Tomography. Environ. Sci. Technol. 2022, 56, 556–563. [Google Scholar] [CrossRef]
- Sayegh, A.; Prakash, N.S.; Horn, H.; Saravia, F. Membrane distillation as a second stage treatment of hydrothermal liquefaction wastewater after ultrafiltration. Sep. Purif. Technol. 2022, 285, 120379. [Google Scholar] [CrossRef]
- Ardeshiri, F.; Akbari, A.; Peyravi, M.; Jahanshahi, M. PDADMAC/PAA semi-IPN hydrogel-coated PVDF membrane for robust anti-wetting in membrane distillation. J. Ind. Eng. Chem. 2019, 74, 14–25. [Google Scholar] [CrossRef]


| Membrane Code | Core Solution | Sheath Solution | FAS | Type of FAS Modification | |
|---|---|---|---|---|---|
| Polymer Solution | Alumina | ||||
| M0 | 15% PVDF-HFP + 85% Solvent Mixture (DMF: Acetone: 7:3) | 9% PVDF-HFP + 85% Solvent Mixture (DMF: Acetone: 7:3) | 0.1% | 0% | No |
| M1C | 0.1% | 0.025% | Surface Coating | ||
| M2S | 0.1% | 0.025% | In Sheath solution | ||
| M3S | 0.2% | 0.025% | In Sheath solution | ||
| M4S | 0.2% | 0.05% | In Sheath solution | ||
| Feed Solution | Concentration | Properties |
|---|---|---|
| Synthetic Hypersaline solutions | 7 wt.% NaCl | Conductivity ≃ 98,450 µS/cm |
| 10.5 wt.% NaCl | Conductivity ≃ 98,450 µS/cm | |
| Synthetic Surfactant wastewater | CTAB Initial CTAB concentration: 0.3 mM | Conductivity ≃ 54,690 µS/cm Total Organic Carbon: 152.6 ppm |
| CTAB + 3.5 wt.% NaClInitial CTAB Concentration: 0.3 mM | ||
| SDS (Initial SDS Concentration: 2 mM) | Conductivity ≃ 54,690 µS/cm Total Organic Carbon:198.65 ppm | |
| SDS + 3.5 wt.% NaCl (Initial SDS Concentration: 2 mM) | ||
| Tween-80 (Initial Concentration: 0.106 mM) | Conductivity ≃ 54,690 µS/cm Total Organic Carbon: 164.5 ppm | |
| Tween-80 + 3.5 wt.% NaCl (Initial Concentration: 0.106 mM) |
| Surfactant Used | Feed Solution Concentration | Membrane Used | Performance of the Membrane | Reference |
|---|---|---|---|---|
| CTAB, SDS, Tween 20 | 50 mg/L of surfactant + 35 mg/L of NaCl | Commercial hydrophobic PVDF | Wetting time for
| [7] |
| CTAB, SDS, Tween 20, 2-EHS, SDBS (0.5 × CMC) (With and without NaCl) | (0.5 × CMC) + 35 g/L NaCl | Flat-sheet PVDF commercial membrane | Wetting time and flux decline
| [6] |
| SLS, SDS, CTAB, DTAB | SDS, SLS—0.0003 mM + 3.5% NaCl DTAB—0.0005 mM + 3.5% NaCl CTAB—0.0001 mM + 3.5% NaCl | PVDF membrane coated with fluorinated Silver nanoparticles |
| [44] |
| CTAB, SDS, Tween 80 | 10–50 mg/L + 0.5 g/L of gasoline + 30 g/L of NaCl | PDADMAC/PAA semi-IPN hydrogel-coated PVDF membrane |
| [48] |
| CTAB, SDS, Tween 20 (With and Without 3.5% NaCl) | Increasing surfactant concentration with time | Alumina incorporated coaxially electrospun PVDF-HFP amphiphobic membrane | Membrane wetting time and feed concentration
| This Study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangeetha, V.; Kaleekkal, N.J.; Vigneswaran, S. Coaxial Electrospun Nanofibrous Membranes for Enhanced Water Recovery by Direct Contact Membrane Distillation. Polymers 2022, 14, 5350. https://doi.org/10.3390/polym14245350
Sangeetha V, Kaleekkal NJ, Vigneswaran S. Coaxial Electrospun Nanofibrous Membranes for Enhanced Water Recovery by Direct Contact Membrane Distillation. Polymers. 2022; 14(24):5350. https://doi.org/10.3390/polym14245350
Chicago/Turabian StyleSangeetha, Vivekanandan, Noel Jacob Kaleekkal, and Saravanamuthu Vigneswaran. 2022. "Coaxial Electrospun Nanofibrous Membranes for Enhanced Water Recovery by Direct Contact Membrane Distillation" Polymers 14, no. 24: 5350. https://doi.org/10.3390/polym14245350
APA StyleSangeetha, V., Kaleekkal, N. J., & Vigneswaran, S. (2022). Coaxial Electrospun Nanofibrous Membranes for Enhanced Water Recovery by Direct Contact Membrane Distillation. Polymers, 14(24), 5350. https://doi.org/10.3390/polym14245350







