Preparation and Synergistic Effect of Biomimetic Poly(lactic acid)/Graphene Oxide Composite Scaffolds Loaded with Dual Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
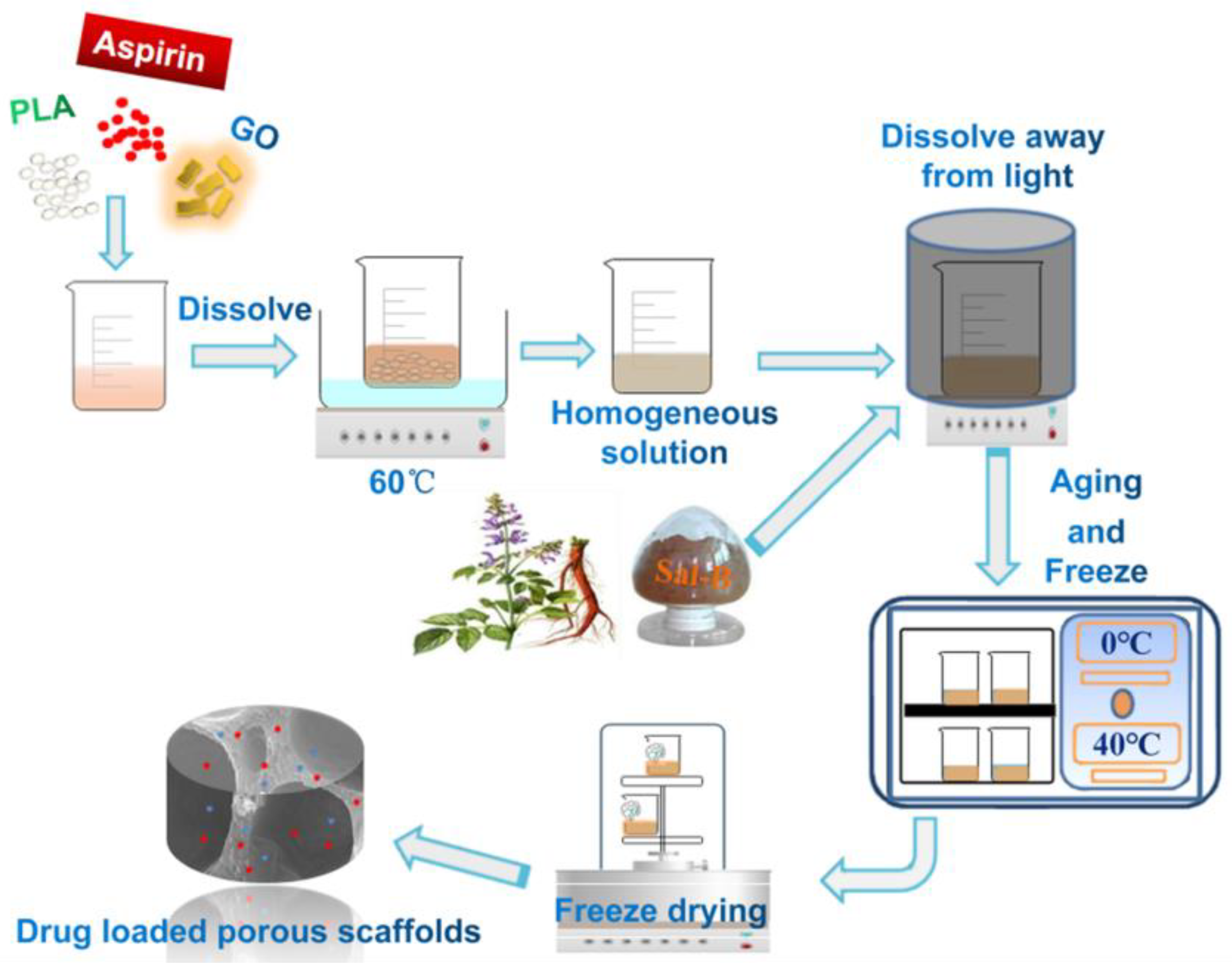
2.2. Fabrication of Dual Drug-Loaded Biomimetic Composite Scaffolds
2.3. Characterization of PLA/GO/ASA/Sal-B Dual Drug-Loaded Biomimetic Composite Scaffold
2.4. Water Absorption and Porosity of the PLA/GO/ASA/Sal-B Dual Drug-Loaded Biomimetic Composite Scaffold
2.5. Cell Culture
2.6. Cell Proliferation
2.7. Alkaline Phosphatase (ALP) Activity
2.8. In Vitro Release of ASA and Sal-B
2.9. Haemocompatibility
2.10. Statistical Analysis
3. Results and Discussion
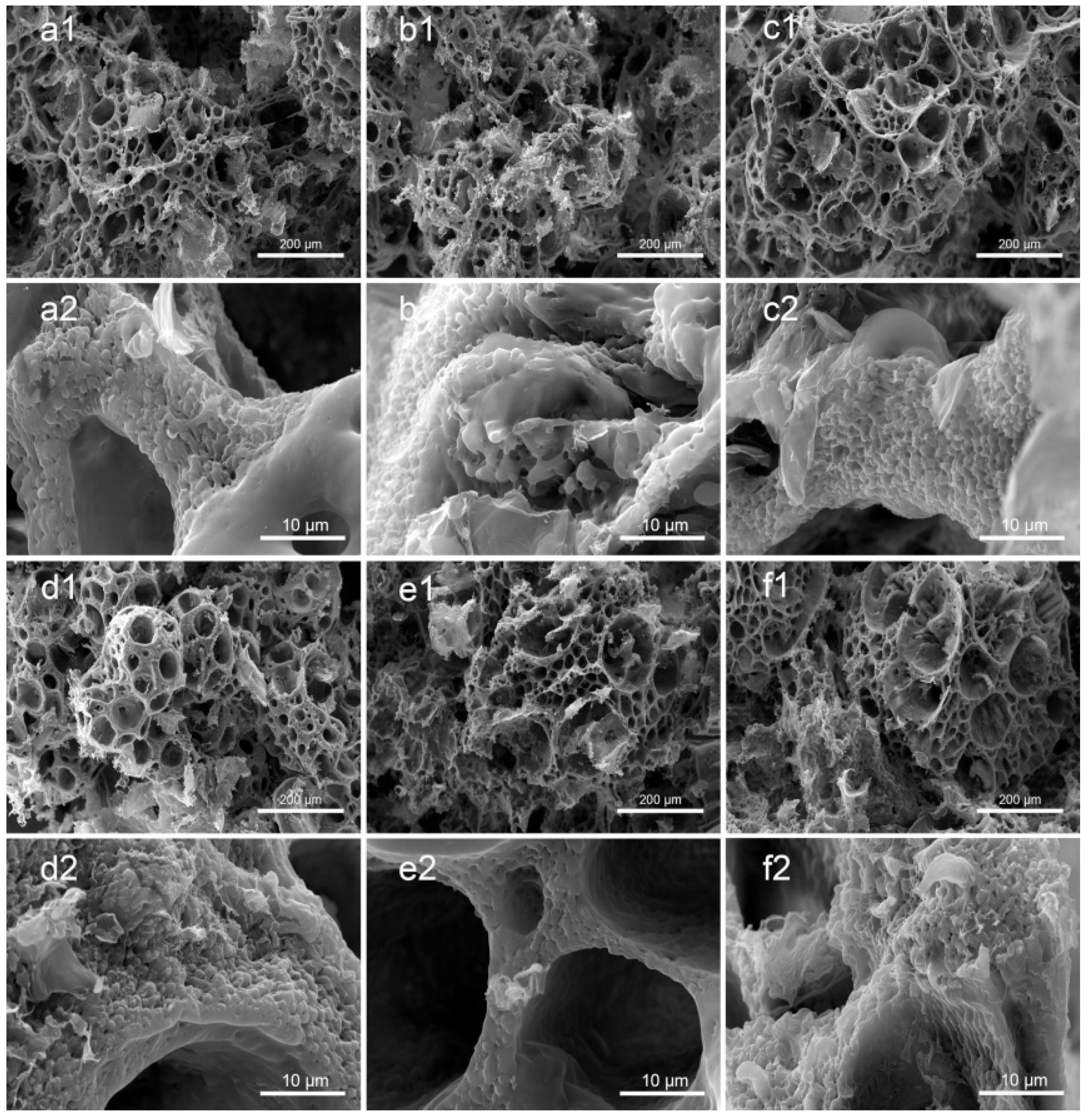
3.1. Morphology of PLA/GO/ASA/Sal-B Dual Drug-Loaded Biomimetic Composite Scaffolds
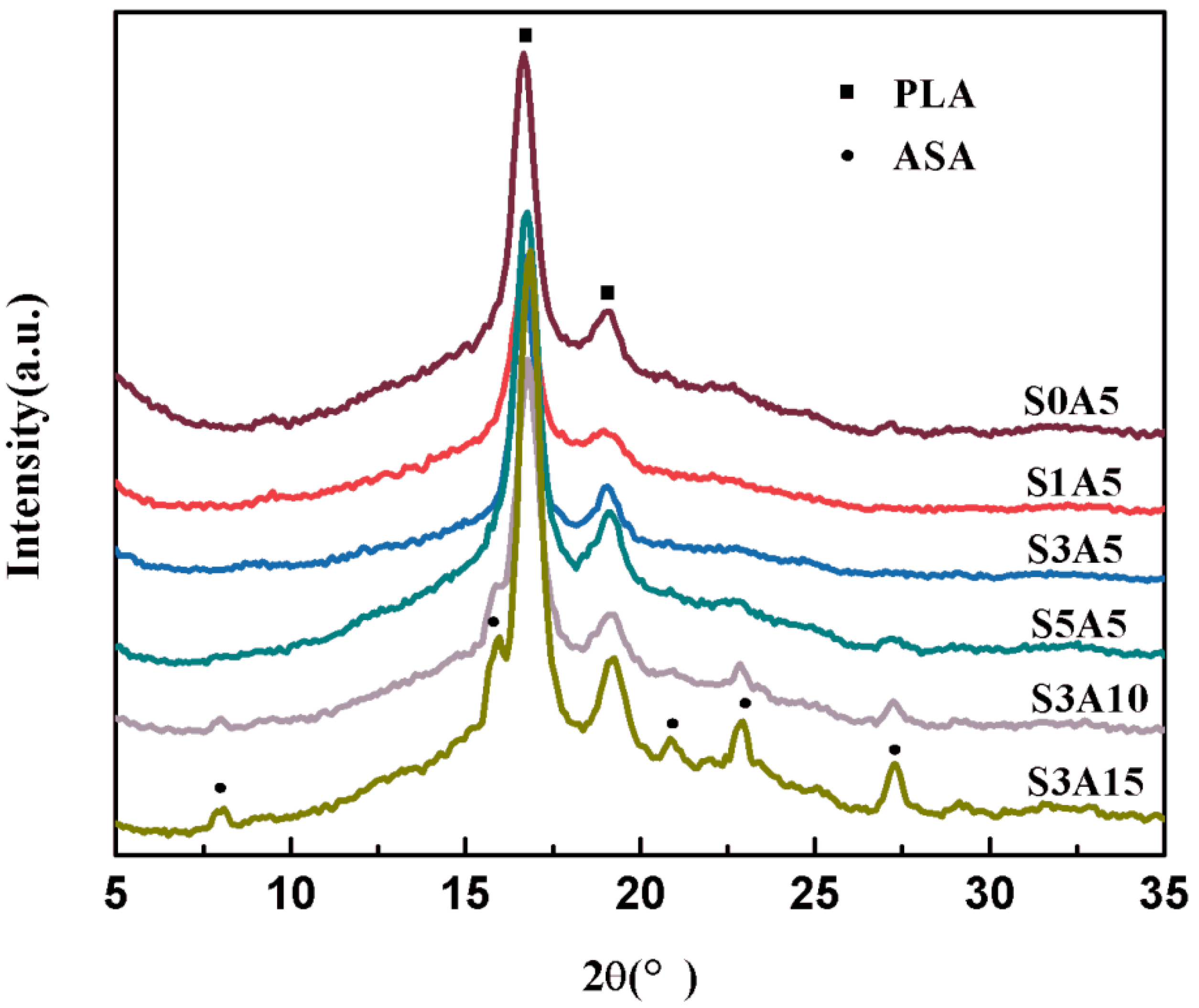
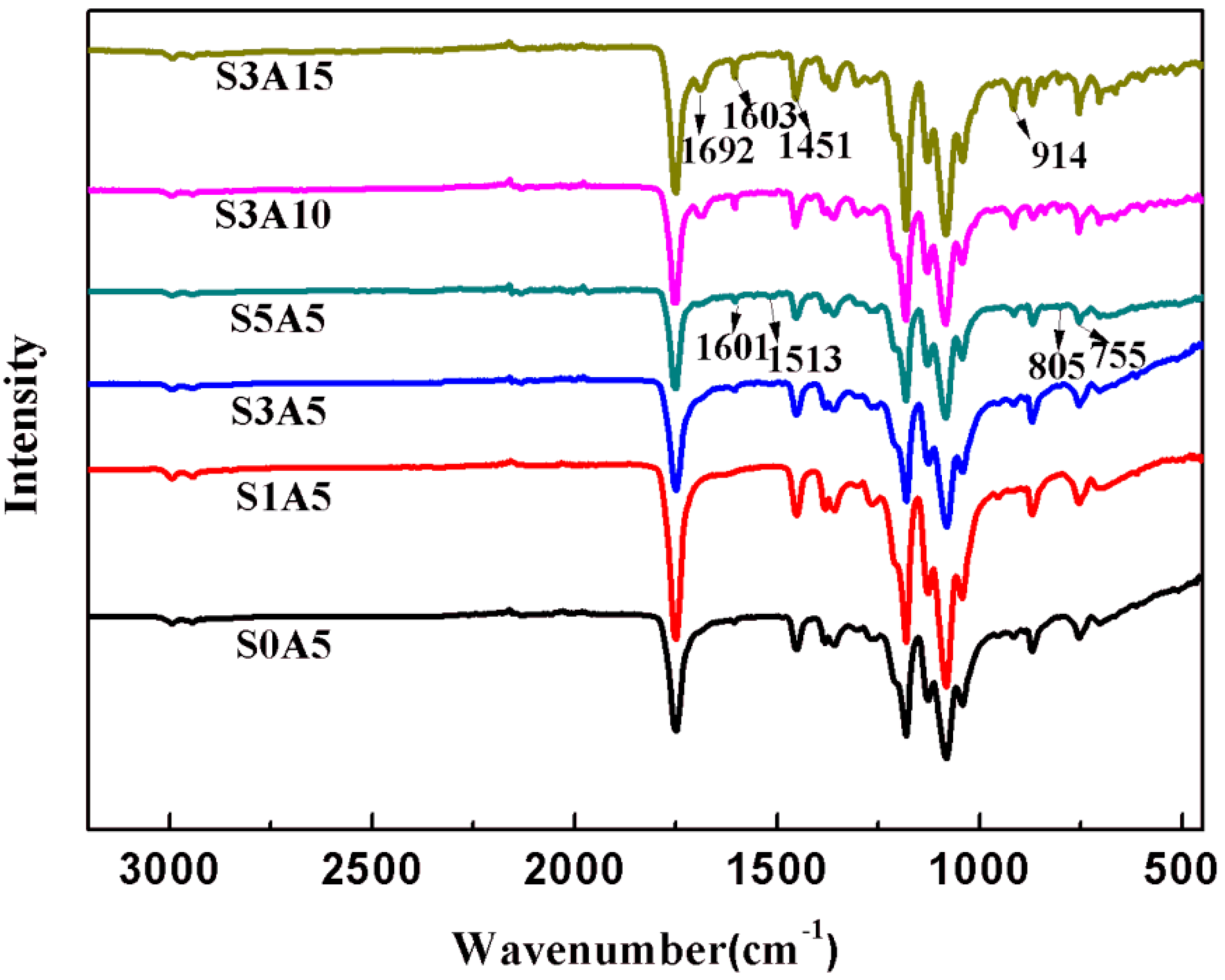
3.2. XRD and FTIR of PLA/GO/ASA/Sal-B Dual Drug-Loaded Biomimetic Composite Scaffolds
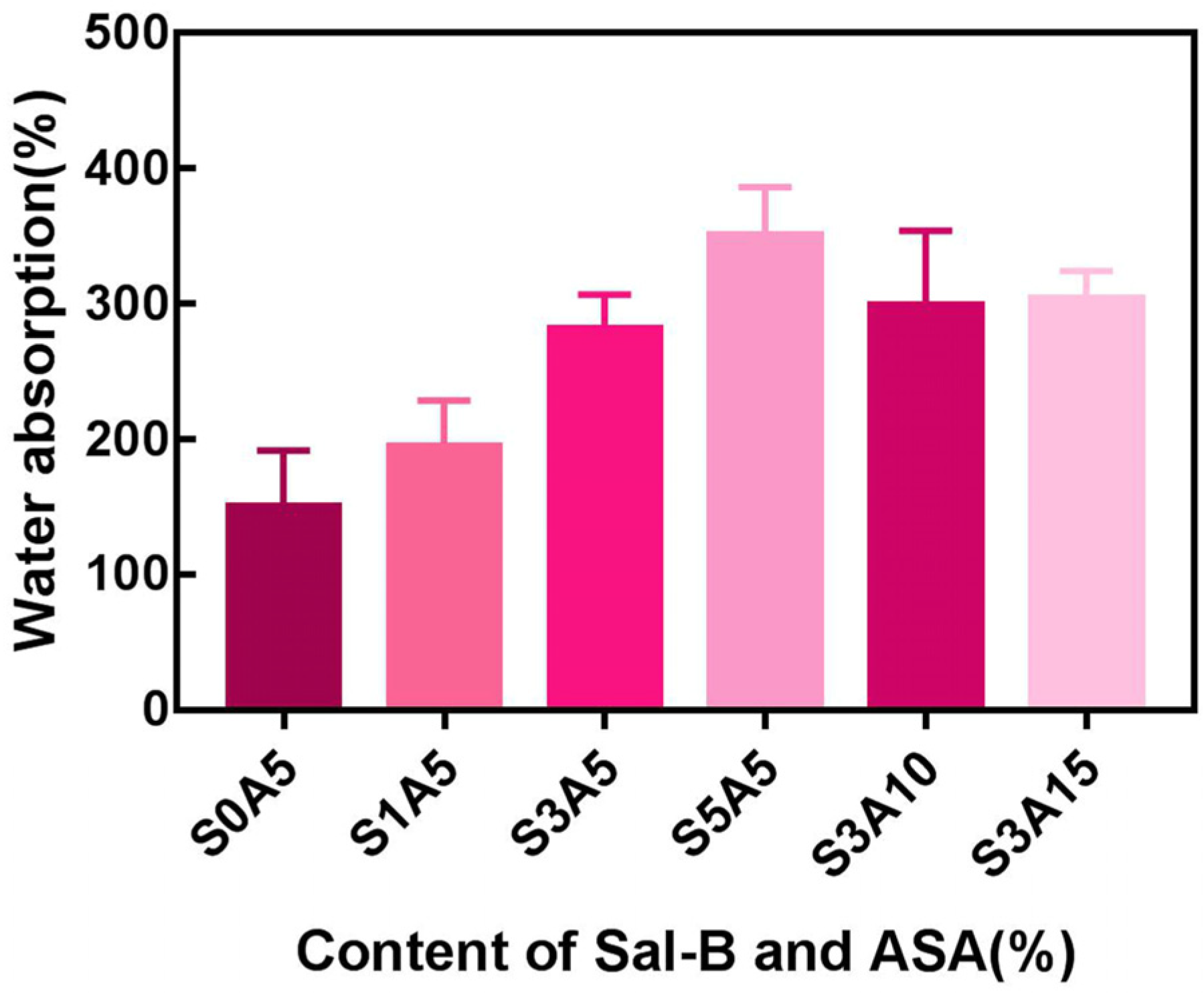
3.3. Water Absorption of PLA/GO/ASA/Sal-B Dual Drug-Loaded Biomimetic Composite Scaffolds
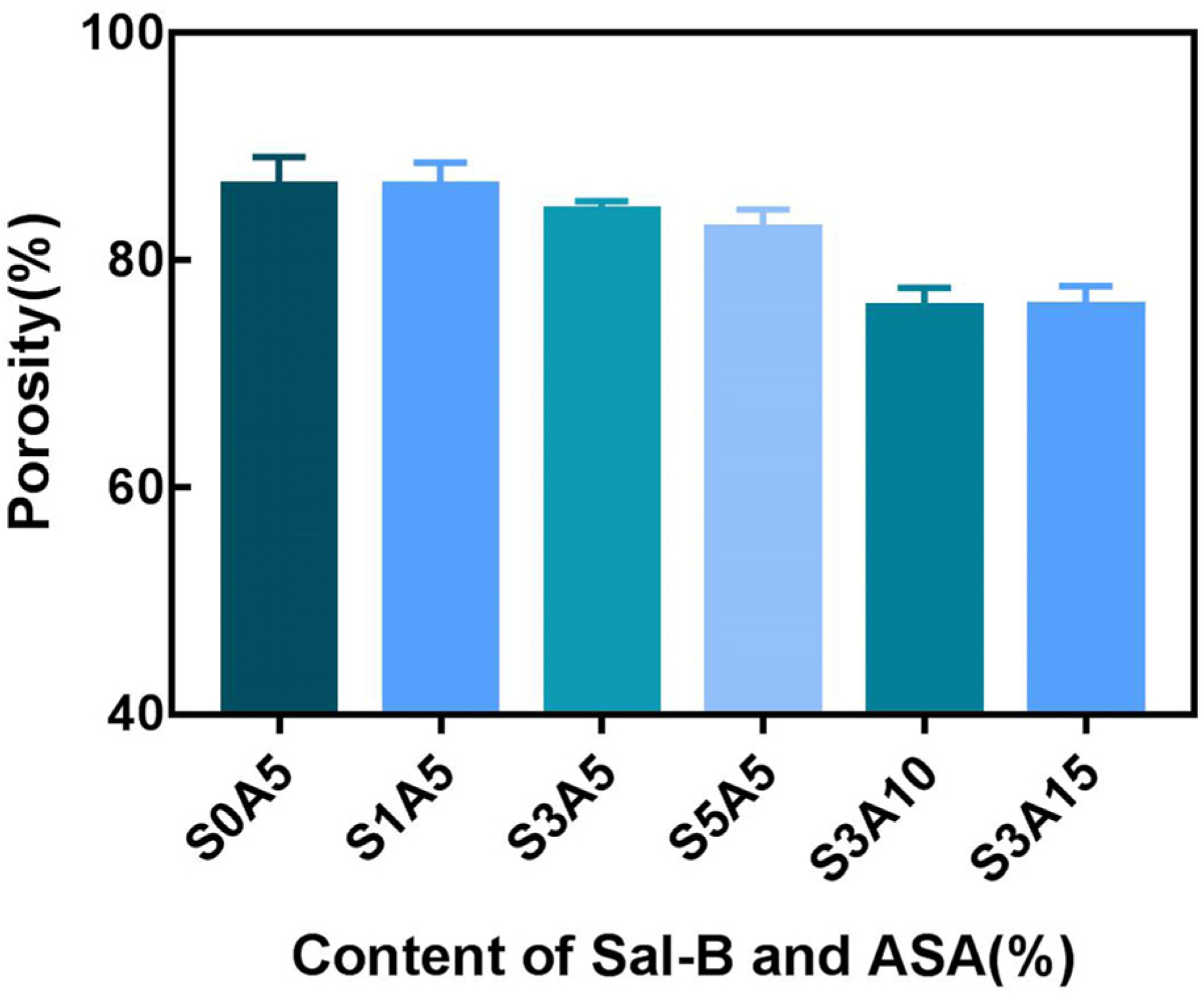
3.4. Porosity of PLA/GO/ASA/Sal-B Dual Drug-Loaded Biomimetic Composite Scaffolds
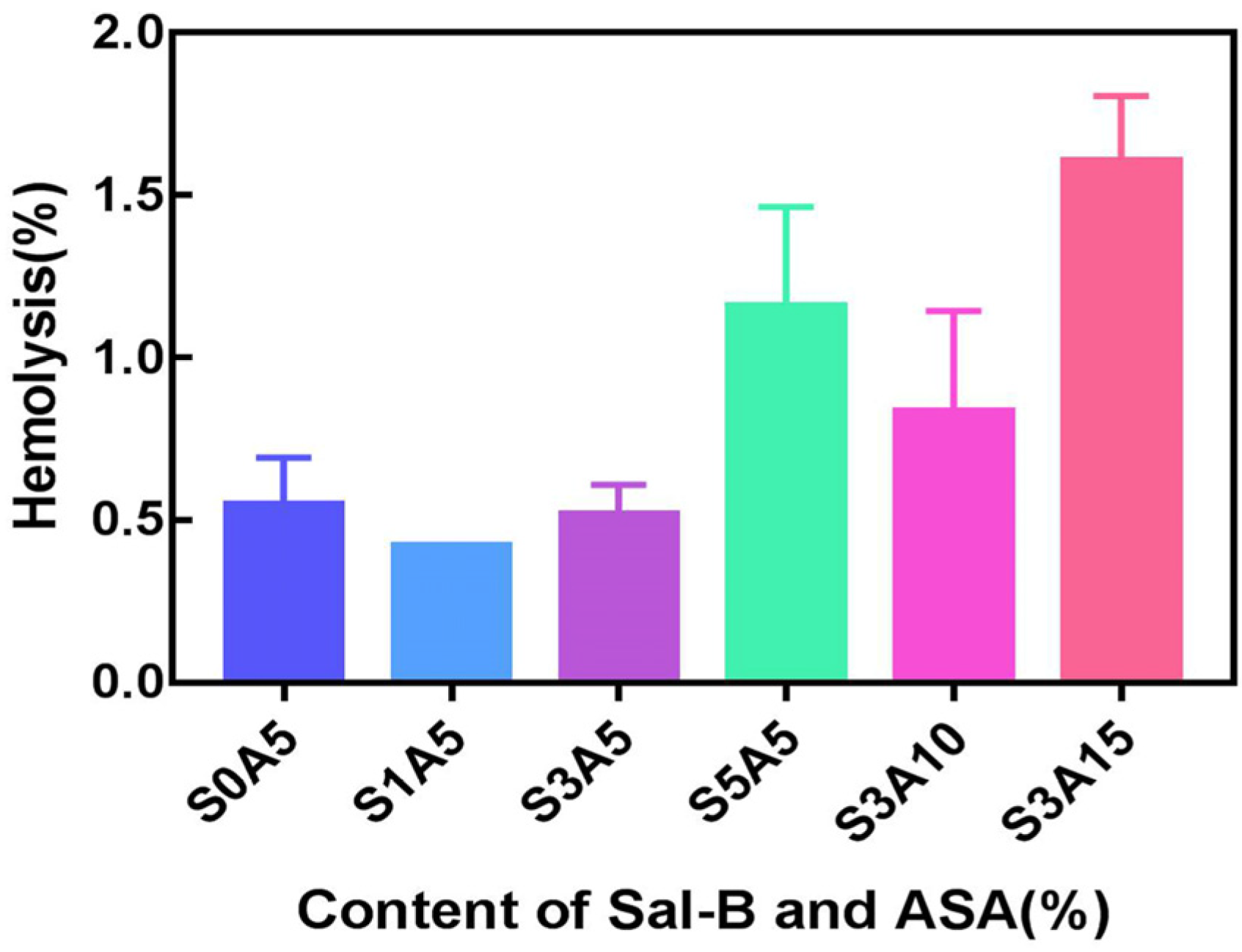
3.5. Haemocompatibility of PLA/GO/ASA/Sal-B Dual Drug-Loaded Biomimetic Composite Scaffolds
3.6. Cell Proliferation
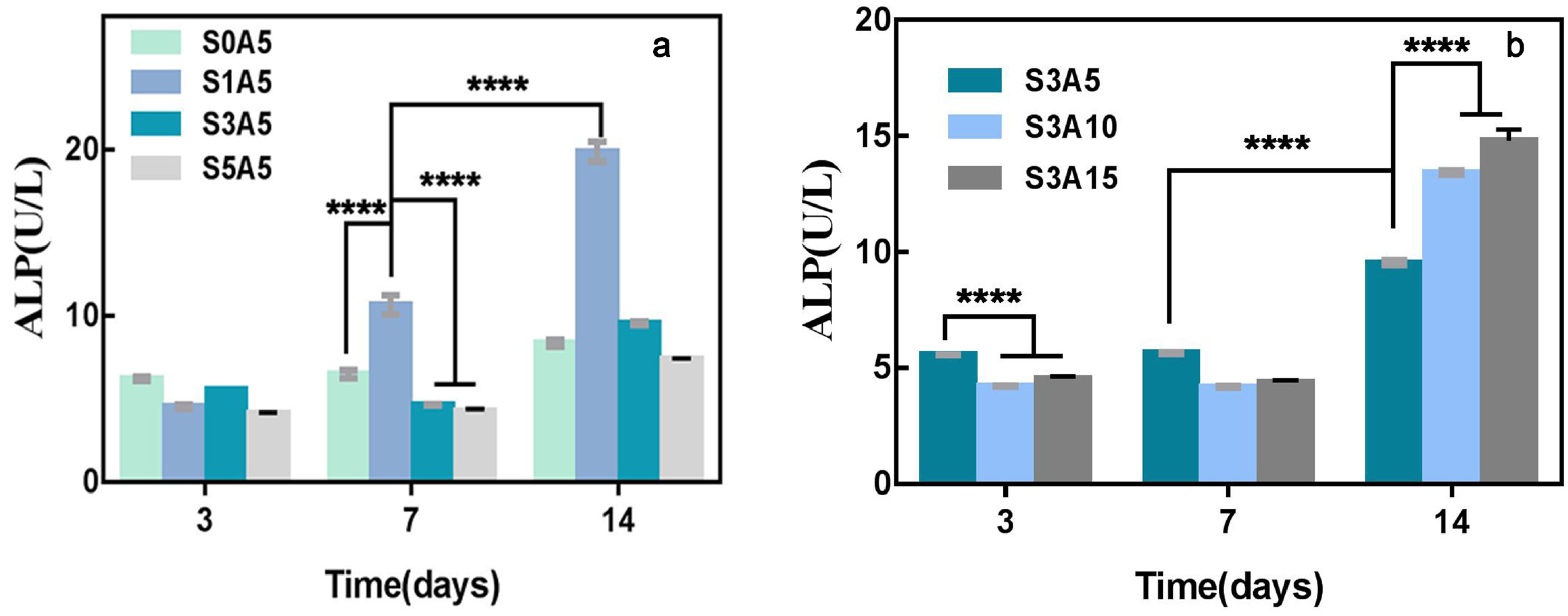
3.7. Alkaline Phosphatase Activity
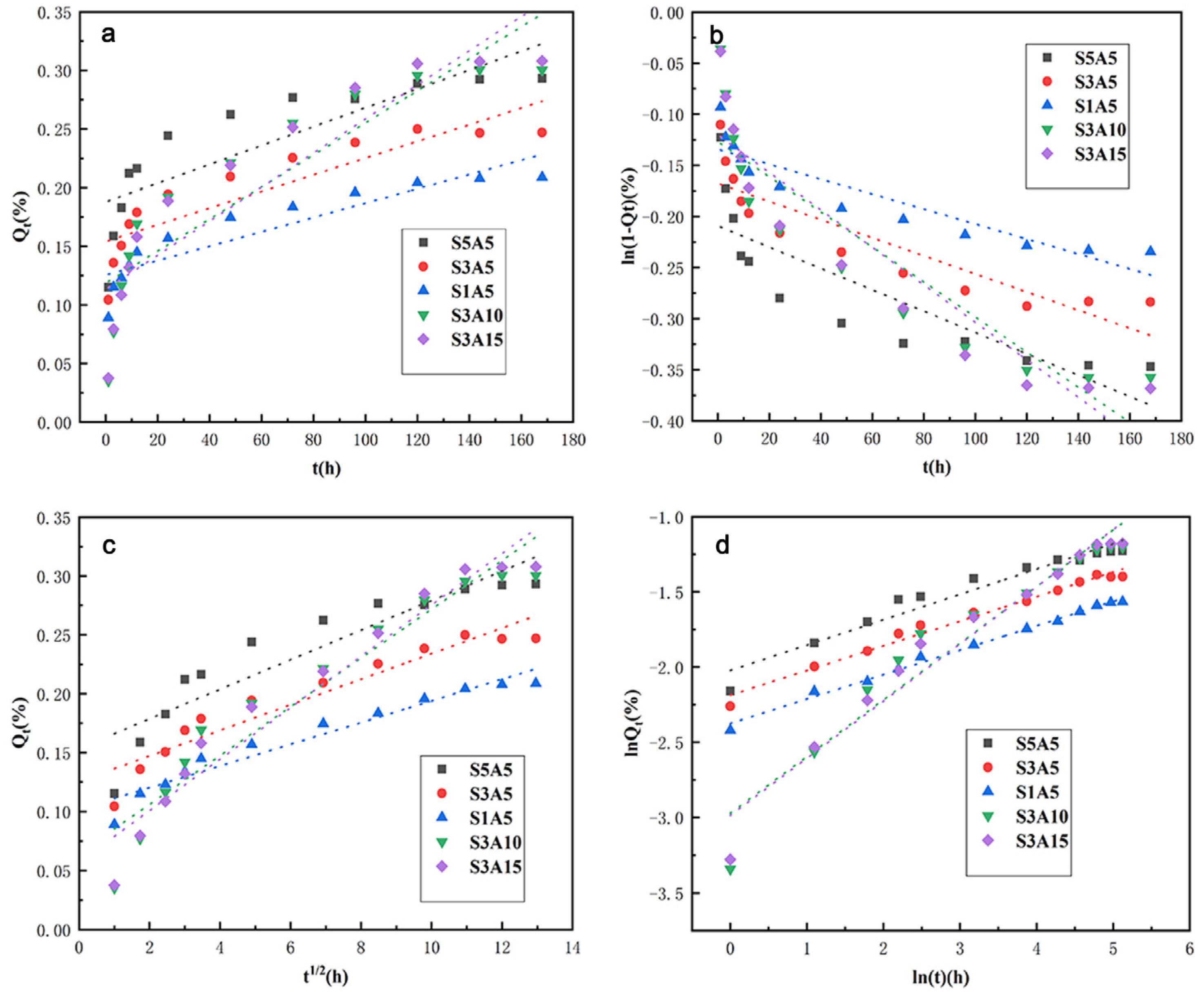
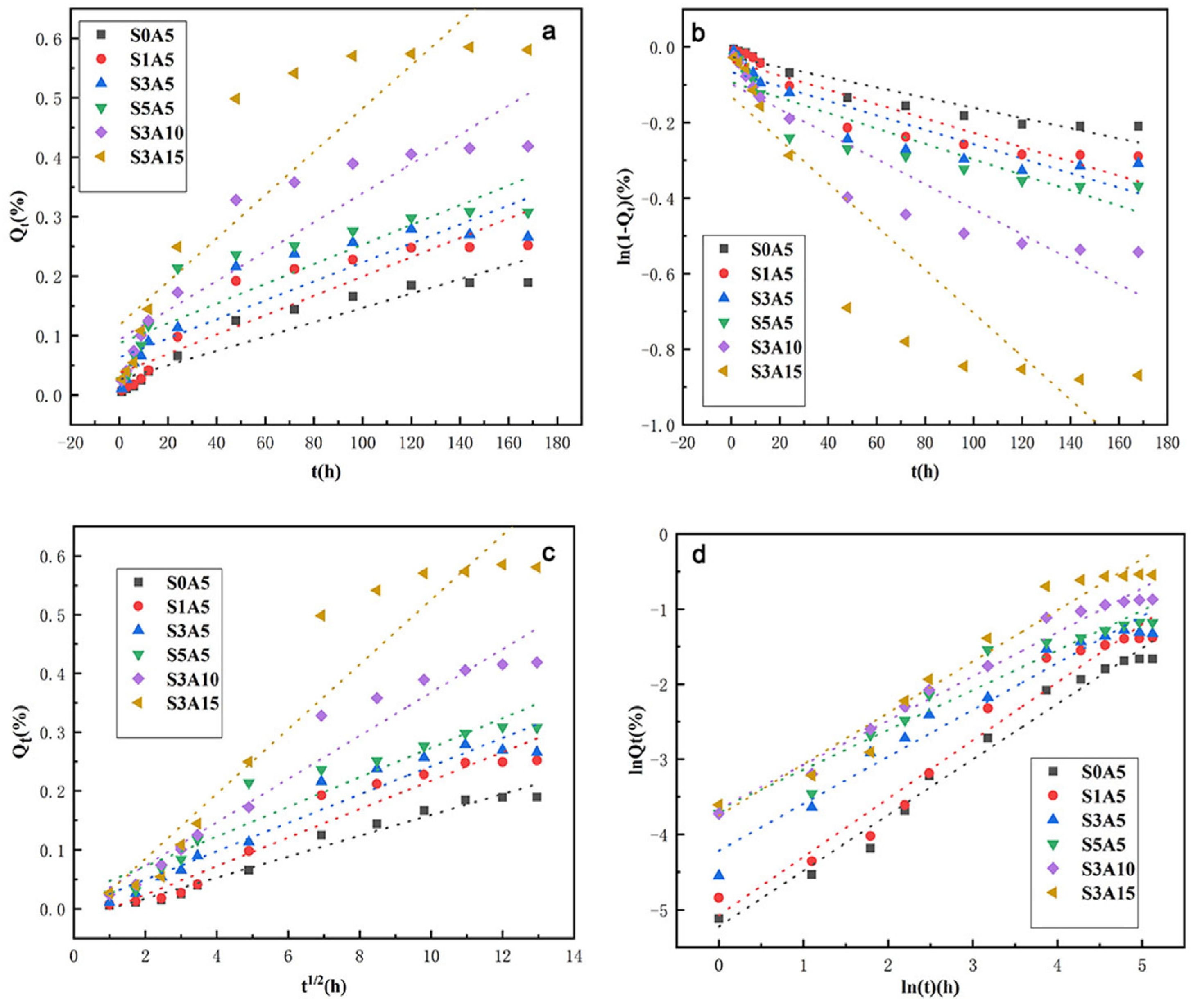
3.8. The Sustained Release of the Two Drugs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, G.; Zhang, W.; Ma, D.; Lu, B.; Wei, G.; Guang, Y.; Ru, C.; Wang, Y. Influence of VEGF/BMP-2 on the proliferation and osteogenetic differentiation of rat bone mesenchymal stem cells on PLGA/gelatin composite scaffold. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2316–2328. [Google Scholar] [PubMed]
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Shu-Yong, W.; Yi, C.; Xiao-Yu, X. Progress on the pharmacological research of puerarin: A review. Chin. J. Nat. Med. 2014, 12, 407–414. [Google Scholar]
- Chen, G.; Lv, Y. Immobilization and application of electrospun nanofiber scaffold-based growth factor in bone tissue engineering. Curr. Pharm. Des. 2015, 21, 1967–1978. [Google Scholar] [CrossRef]
- Strobel, C.; Bormann, N.; Kadow-Romacker, A.; Schmidmaier, G.; Wildemann, B. Sequential release kinetics of two (gentamicin and BMP-2) or three (gentamicin, IGF-I and BMP-2) substances from a one-component polymeric coating on implants. J. Control Release 2011, 156, 37–45. [Google Scholar] [CrossRef]
- Çakır-Özkan, N.; Eğri, S.; Bekar, E.; Altunkaynak, B.Z.; Kabak, Y.B.; Kıvrak, E.G. The use of sequential VEGF-and BMP2-releasing biodegradable scaffolds in rabbit mandibular defects. J. Oral Maxillofac. Surg. 2017, 75, 221.e1–221.e14. [Google Scholar] [CrossRef]
- Wang, L. Experimental Study on Bone Tissue-Engineering Seeded with Bone Marrow Stromal Cell. Master’s Thesis, Shandong University of Traditional Chinese Medicine, Jinan, China, 2007. [Google Scholar]
- Lv, Y.; Gao, C.-W.; Liu, B.; Wang, H.-Y.; Wang, H.-P. BMP-2 combined with salvianolic acid B promotes cardiomyocyte differentiation of rat bone marrow mesenchymal stem cells. Kaohsiung J. Med. Sci. 2017, 33, 477–485. [Google Scholar] [CrossRef]
- Shoba, E.; Lakra, R.; Kiran, M.S.; Korrapati, P.S. Fabrication of core–shell nanofibers for controlled delivery of bromelain and salvianolic acid B for skin regeneration in wound therapeutics. Biomed. Mater. 2017, 12, 035005. [Google Scholar] [CrossRef]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef]
- Yao, B. Effect of Chinese Patent Medicine Containing Salvia Miltiorrhiza Combined with Aspirin on Platelet Function. Master’s Thesis, Henan University of Traditional Chinese Medicine, Zhengzhou, China, 2013. [Google Scholar]
- Ren, L.; Pan, S.; Li, H.; Li, Y.; He, L.; Zhang, S.; Che, J.; Niu, Y. Effects of aspirin-loaded graphene oxide coating of a titanium surface on proliferation and osteogenic differentiation of MC3T3-E1 cells. Sci. Rep. 2018, 8, 15143. [Google Scholar] [CrossRef]
- Chin, K.-Y. A Review on the Relationship between Aspirin and Bone Health. J. Osteoporos. 2017, 2017, 3710959. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Kikuiri, T.; Akiyama, K.; Chen, C.; Xu, X.; Yang, R.; Chen, W.; Wang, S.; Shi, S. Mesenchymal stem cell–based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat. Med. 2011, 17, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Abd Rahman, F.; Mohd Ali, J.; Abdullah, M.; Abu Kasim, N.; Musa, S. Aspirin enhances osteogenic potential of periodontal ligament stem cells (PDLSCs) and modulates the expression profile of growth factor–associated genes in PDLSCs. Periodontol 2016, 87, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xiong, J.; Mei, S.; Wang, F.; Zhao, Z.; Wang, S.; Liu, Y. Aspirin promotes bone marrow mesenchymal stemcell-based calvarial bone regeneration in mini swine. Stem Cell Res. Ther. 2015, 6, 210. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, S.N.; Wu, H.T.; Qin, H.J.; Ren, M.L.; Lin, J.C.; Yu, B. Aspirin alleviates orthopedic implant-associated infection. Int. J. Mol. Med. 2019, 44, 1281–1288. [Google Scholar]
- Zhan, Y.; He, Z.; Liu, X.; Miao, N.; Lin, F.; Mu, S.; Mu, H.; Yuan, M.; Cao, X.; Jin, H. Aspirin-Induced attenuation of adipogenic differentiation of bone marrow mesenchymal stem cells is accompanied by the disturbed epigenetic modification. Int. J. Biochem. Cell Biol. 2018, 98, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xiong, J.; Wu, T.; Tang, Z.; Ding, G.; Zhang, C.; Wang, S.; Liu, Y. Aspirin treatment improved mesenchymal stem cell immunomodulatory properties via the 15d-PGJ2/PPARγ/TGF-β1 pathway. Stem Cells Dev. 2014, 23, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-S.; Lee, C.-C.; Wang, Y.-P.; Chen, H.-J.; Lai, C.-H.; Hsieh, W.-L.; Chen, Y.-W. Controlled-Release of tetracycline and lovastatin by poly (D,L-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs. Int. J. Nanomed. 2016, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, S.; Liu, Z.; Geng, H.; Lu, X.; Zhang, X.; Li, H.; Gao, C.; Zhang, X.; Gao, P. Guided bone regeneration with asymmetric collagen-chitosan membranes containing aspirin-loaded chitosan nanoparticles. Int. J. Nanomed. 2017, 12, 8855. [Google Scholar] [CrossRef]
- Li, G.; Chen, L.; Chen, K. Curcumin promotes femoral fracture healing in a rat model by activation of autophagy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 4064. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, X.; Feng, B. Cardiovascular effects of salvianolic acid B. Corp. Evid.-Based Complement. Altern. Med. 2013, 2013, 247948. [Google Scholar] [CrossRef]
- Yuan, Y.; Zheng, P. Advances in pharmacology of Dipsacus asreroides. Res. Tradit. Chin. Med. 2002, 5, 53–54. [Google Scholar]
- Cui, L.; Zhou, L.Y.; Liu, Y.Y.; Ai, C.M.; Wu, T.; Wu, Y. Preventing cancellous bone loss in steroid-treated rats and stimulating bone formation by water estract of salvia miltiorrhixa and danshensu. Chin. Pharmacol. Bull. 2004, 20, 286–290. [Google Scholar]
- He, X.; Shen, Q. Salvianolic acid B promotes bone formation by increasing activity of alkaline phosphatase in a rat tibia fracture model: A pilot study. BMC Complement. Altern. Med. 2014, 14, 493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huan, H.Q.; Cui, L. Effects of salvianolic acid B on osteoblast in vitro. Chin. Pharmacol. Bull. 2008, 24, 978–979. [Google Scholar]
- Che, C.-T.; Wong, M.S.; Lam, C.W.K. Natural products from Chinese medicines with potential benefits to bone health. Molecules 2016, 21, 239. [Google Scholar] [CrossRef]
- Xu, D.; Xu, L.; Zhou, C.; Lee, W.Y.; Wu, T.; Cui, L.; Li, G. Salvianolic acid B promotes osteogenesis of human mesenchymal stem cells through activating ERK signaling pathway. Int. J. Biochem. Cell Biol. 2014, 51, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lishchynskyi, O.; Stetsyshyn, Y.; Raczkowska, J.; Awsiuk, K.; Budkowski, A. Fabrication and Impact of Fouling-Reducing Temperature-Responsive POEGMA Coatings with Embedded CaCO3 Nanoparticles on Different Cell Lines. Materials 2021, 14, 1417. [Google Scholar] [CrossRef]
- Mao, Z.; Li, J.; Huang, W.; Jiang, H.; Zimba, B.L.; Chen, L.; Wan, J.; Wu, Q. Preparation of poly (lactic acid)/graphene oxide nanofiber membranes with different structures by electrospinning for drug delivery. RSC Adv. 2018, 8, 16619–16625. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, Y.; Huang, Y.; Huang, F.; Li, S.; Shen, Y.; Zhu, M.; Xie, A. A GO@PLA@HA composite microcapsule: Its preparation and multistage and controlled drug release. Eur. J. Inorg. Chem. 2017, 2017, 3312–3321. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Re, G.L.; Parisi, A.; Busacca, A. Advanced piezoresistive sensor achieved by amphiphilic nanointerfaces of graphene oxide and biodegradable polymer blends. Compos. Sci. Technol. 2018, 156, 166–176. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Y.; Wu, Z.; Hu, J.; Liu, R. Preparation and characterization of aspirin-loaded polylactic acid/graphene oxide biomimetic nanofibrous scaffolds. Polymer 2020, 211, 123093. [Google Scholar] [CrossRef]
- Liu, S.Q.; Liu, R.L.; Rao, R.Y. Effect of Aspirin on Structure of Polycaprolactone Carried Materials and Controlled-release Performance. Cailiao Yanjiu Xuebao/Chin. J. Mater. Res. 2018, 32, 913–920. [Google Scholar]
- Liu, S.; Wu, X.; Hu, J.; Wu, Z.; Zheng, Y. Preparation and characterisation of a novel polylactic acid/hydroxyapatite/graphene oxide/aspirin drug-loaded biomimetic composite scaffold. New J. Chem. 2021, 45, 10788–10797. [Google Scholar] [CrossRef]
- Yu, G.-B.; Zhang, Y.-Z.; Wang, S.-D.; Shi, D.-B.; Dong, Z.-H.; Fu, W.-G. Study of the electrospun PLA/silk fibroin-gelatin composite nanofibrous scaffold for tissue engineering. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 93, 158–163. [Google Scholar] [CrossRef]
- Haghjooy Javanmard, S.; Anari, J.; Zargar Kharazi, A.; Vatankhah, E. In vitro hemocompatibility and cytocompatibility of a three-layered vascular scaffold fabricated by sequential electrospinning of PCL, collagen, and PLLA nanofibers. J. Biomater. Appl. 2016, 31, 438–449. [Google Scholar] [CrossRef]
- Alippilakkotte, S.; Kumar, S.; Sreejith, L. Fabrication of PLA/Ag nanofibers by green synthesis method using Momordica charantia fruit extract for wound dressing applications. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 771–782. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Y.; Hu, J.; Wu, Z.; Chen, H. Fabrication and characterization of polylactic acid/polycaprolactone composite macroporous micro-nanofiber scaffolds by phase separation. New J. Chem. 2020, 44, 17382–17390. [Google Scholar] [CrossRef]
- He, L.; Liao, S.; Quan, D.; Ma, K.; Chan, C.; Ramakrishna, S.; Lu, J. Synergistic effects of electrospun PLLA fiber dimension and pattern on neonatal mouse cerebellum C17.2 stem cells. Acta Biomater. 2010, 6, 2960–2969. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Zhang, R. Synthetic nano-scale fibrous extracellular matrix. J. Biomed. Mater. Res. 1999, 46, 60–72. [Google Scholar] [CrossRef]
- Liu, S.Q.; Xiao, X.F.; Liu, R.F.; Lin, Y.H.; Zhong, Z.Y.; Jiao, J.J. Fabrication of Poly (L-lacticacid) Nanofibrous Scaffolds by Thermally Induced Phase Separatio. Chem. J. Chin. Univ. 2011, 32, 372–378. [Google Scholar]
- Ghavimi, M.A.; Shahabadi, A.B.; Jarolmasjed, S.; Memar, M.Y.; Dizaj, S.M.; Sharifi, S. Nanofibrous asymmetric collagen/curcumin membrane containing aspirin-loaded PLGA nanoparticles for guided bone regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Zhi, W.; Wang, J.; Feng, B.; Qu, S.; Mu, Y.; Weng, J. Immobilization of salvianolic acid B-loaded chitosan microspheres distributed three-dimensionally and homogeneously on the porous surface of hydroxyapatite scaffolds. Biomed. Mater. 2016, 11, 055014. [Google Scholar] [CrossRef]
- Jiang, Y. Preparation and Property Study of Electrospun Drug-Loaded PLA/SF Composite Membranes. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2012. [Google Scholar]
- Wang, X.; Song, G.; Lou, T. Fabrication and characterization of nano composite scaffold of poly (L-lactic acid)/hydroxyapatite. J. Mater. Sci. Mater. Med. 2010, 21, 183–188. [Google Scholar] [CrossRef]
- Hajji, S.; Khedir, S.B.; Hamza-Mnif, I.; Hamdi, M.; Jedidi, I.; Kallel, R.; Boufi, S.; Nasri, M. Biomedical potential of chitosan-silver nanoparticles with special reference to antioxidant, antibacterial, hemolytic and in vivo cutaneous wound healing effects. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 241–254. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Zhang, Y.; Zhang, S.; Ye, J.; Wen, Z.; Ding, J.; Kunapuli, S.P.; Luo, X.; Ding, Z. Salvianolic acid B inhibits platelets as a P2Y12 antagonist and PDE inhibitor: Evidence from clinic to laboratory. Thromb. Res. 2014, 134, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lin, Y.-H.; Chang, S.-H.; Tai, C.-D.; Liu, S.-J.; Chu, Y.; Wang, C.-J.; Hsu, M.-Y.; Chang, H.; Chang, G.-J. Local sustained delivery of acetylsalicylic acid via hybrid stent with biodegradable nanofibers reduces adhesion of blood cells and promotes reendothelialization of the denuded artery. Int. J. Nanomed. 2014, 9, 311. [Google Scholar]
- Aslani, S.; Kabiri, M.; Kehtari, M.; Hanaee-Ahvaz, H. Vascular tissue engineering: Fabrication and characterization of acetylsalicylic acid-loaded electrospun scaffolds coated with amniotic membrane lysate. J. Cell. Physiol. 2019, 234, 16080–16096. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, X.; Wang, S.; Zhai, C.; He, Y.; Zhang, Y.; Qiao, Y. Mechanism of action of salvianolic acid B by module-based network analysis. Bio-Med. Mater. Eng. 2014, 24, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Bi, L.; Li, J.; Fan, J. Salvianolic acid B-loaded chitosan/hydroxyapatite scaffolds promotes the repair of segmental bone defect by angiogenesis and osteogenesis. Int. J. Nanomed. 2019, 14, 8271. [Google Scholar] [CrossRef] [PubMed]
- Saveleva, M.S.; Ivanov, A.N.; Chibrikova, J.A.; Abalymov, A.A.; Surmeneva, M.A.; Surmenev, R.A. Osteogenic Capability of Vaterite-coated Nonwoven Polycaprolactone Scaffolds for in vivo Bone Tissue Regeneration. Macromol. Biosci. 2021, 21, 2100266. [Google Scholar] [CrossRef]
- Soomherun, N.; Kreua-ongarjnukool, N.; Niyomthai, S.T.; Chumnanvej, S. Kinetics of Drug Release via Nicardipine Hydrochloride-loaded Carboxymethyl Cellulose/Poly (D,L-lactic-co-glycolic acid) Nanocarriers Using a Contemporary Emulsion Process. ChemNanoMat 2020, 6, 1754–1769. [Google Scholar] [CrossRef]
- Gouda, R.; Baishya, H.; Qing, Z. Application of mathematical models in drug release kinetics of carbidopa and levodopa ER tablets. J. Dev. Drugs 2017, 6, 1000171. [Google Scholar]
- Lotfy, V.F.; Basta, A.H. Optimizing the chitosan-cellulose based drug delivery system for controlling the ciprofloxacin release versus organic/inorganic crosslinker, characterization and kinetic study. Int. J. Biol. Macromol. 2020, 165, 1496–1506. [Google Scholar] [CrossRef]
- Hixson, A.; Crowell, J. Dependence of reaction velocity upon surface and agitation. Ind. Eng. Chem. 1931, 23, 923–931. [Google Scholar] [CrossRef]



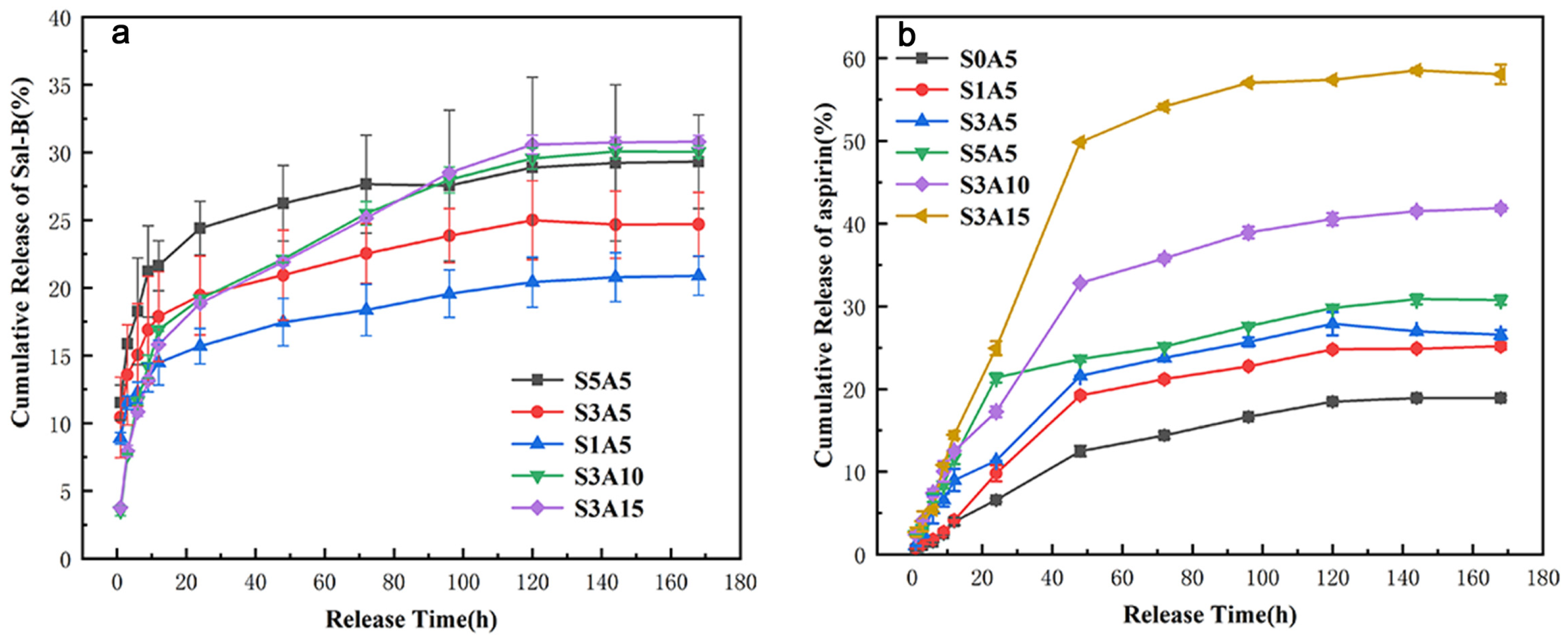
| Name | S0A5 | S1A5 | S3A5 | S5A5 | S3A10 | S3A15 | |
|---|---|---|---|---|---|---|---|
| Composition(%) | |||||||
| PLA | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | |
| GO | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | |
| Sal-B | 0.0 | 1.0 | 3.0 | 5.0 | 3.0 | 3.0 | |
| ASA | 5.0 | 5.0 | 5.0 | 5.0 | 10.0 | 15.0 | |
| Model | Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | R2 | k0 | R2 | k1 | R2 | kH | R2 | n | kkp | |
| Sample | ||||||||||
| S1A5 | 0.6513 | 8.03 × 10−4 | 0.6791 | 1.04 × 10−3 | 0.6700 | 3.19 × 10−4 | 0.9396 | 0.1686 | 0.1324 | |
| S3A5 | 0.7500 | 7.11 × 10−4 | 0.7695 | 8.85 × 10−4 | 0.7631 | 2.74 × 10−4 | 0.9795 | 0.1637 | 0.1123 | |
| S5A5 | 0.8075 | 6.12 × 10−4 | 0.8224 | 7.32 × 10−4 | 0.8175 | 2.30 × 10−4 | 0.9923 | 0.1627 | 0.0929 | |
| S3A10 | 0.7789 | 1.37 × 10−3 | 0.8134 | 1.72 × 10−3 | 0.8023 | 5.30 × 10−4 | 0.9263 | 0.3770 | 0.0512 | |
| S3A15 | 0.8194 | 1.45 × 10−3 | 0.8501 | 1.83 × 10−3 | 0.8402 | 5.65 × 10−4 | 0.9557 | 0.3802 | 0.0504 | |
| Model | Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | R2 | k0 | R2 | k1 | R2 | kH | R2 | n | kKP | |
| Sample | ||||||||||
| S0A5 | 0.8887 | 1.21 × 10−3 | 0.9002 | 1.35 × 10−3 | 0.8964 | 4.33 × 10−4 | 0.9790 | 0.7402 | 0.0054 | |
| S1A5 | 0.8345 | 1.63 × 10−3 | 0.8500 | 1.89 × 10−3 | 0.8449 | 6.00 × 10−4 | 0.9623 | 0.7744 | 0.0063 | |
| S3A5 | 0.8058 | 1.60 × 10−3 | 0.8219 | 1.91 × 10−3 | 0.8167 | 6.00 × 10−4 | 0.9598 | 0.6249 | 0.0147 | |
| S5A5 | 0.7742 | 1.66 × 10−3 | 0.8044 | 2.04 × 10−3 | 0.7944 | 6.35 × 10−4 | 0.9412 | 0.5299 | 0.0255 | |
| S3A10 | 0.8283 | 2.46 × 10−3 | 0.8582 | 3.31 × 10−3 | 0.8485 | 9.97 × 10−4 | 0.9778 | 0.5882 | 0.0257 | |
| S3A15 | 0.7785 | 3.64 × 10−3 | 0.8156 | 5.73 × 10−3 | 0.8035 | 1.63 × 10−3 | 0.9557 | 0.6837 | 0.0235 | |
| Drug Release Mechanism | Release Index (n) | ||
|---|---|---|---|
| Ball | Cylinder | Film | |
| Fickian diffusion | 0.43 | 0.45 | 0.50 |
| Non-fick diffusion (Random diffusion) | 0.43 < n < 0.85 | 0.45 < n < 0.89 | 0.50 < n < 1.00 |
| Swelling/dissolution of polymer | 0.85 | 0.89 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Li, W.; Xu, Z.; Hu, J.; Wu, F.; Zheng, Y. Preparation and Synergistic Effect of Biomimetic Poly(lactic acid)/Graphene Oxide Composite Scaffolds Loaded with Dual Drugs. Polymers 2022, 14, 5348. https://doi.org/10.3390/polym14245348
Liu S, Li W, Xu Z, Hu J, Wu F, Zheng Y. Preparation and Synergistic Effect of Biomimetic Poly(lactic acid)/Graphene Oxide Composite Scaffolds Loaded with Dual Drugs. Polymers. 2022; 14(24):5348. https://doi.org/10.3390/polym14245348
Chicago/Turabian StyleLiu, Shuqiong, Wanzhu Li, Zhenyi Xu, Jiapeng Hu, Fangfang Wu, and Yuying Zheng. 2022. "Preparation and Synergistic Effect of Biomimetic Poly(lactic acid)/Graphene Oxide Composite Scaffolds Loaded with Dual Drugs" Polymers 14, no. 24: 5348. https://doi.org/10.3390/polym14245348
APA StyleLiu, S., Li, W., Xu, Z., Hu, J., Wu, F., & Zheng, Y. (2022). Preparation and Synergistic Effect of Biomimetic Poly(lactic acid)/Graphene Oxide Composite Scaffolds Loaded with Dual Drugs. Polymers, 14(24), 5348. https://doi.org/10.3390/polym14245348








