Enhancement of Mechanical Properties and Bonding Properties of Flake-Zinc-Powder-Modified Epoxy Resin Composites
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
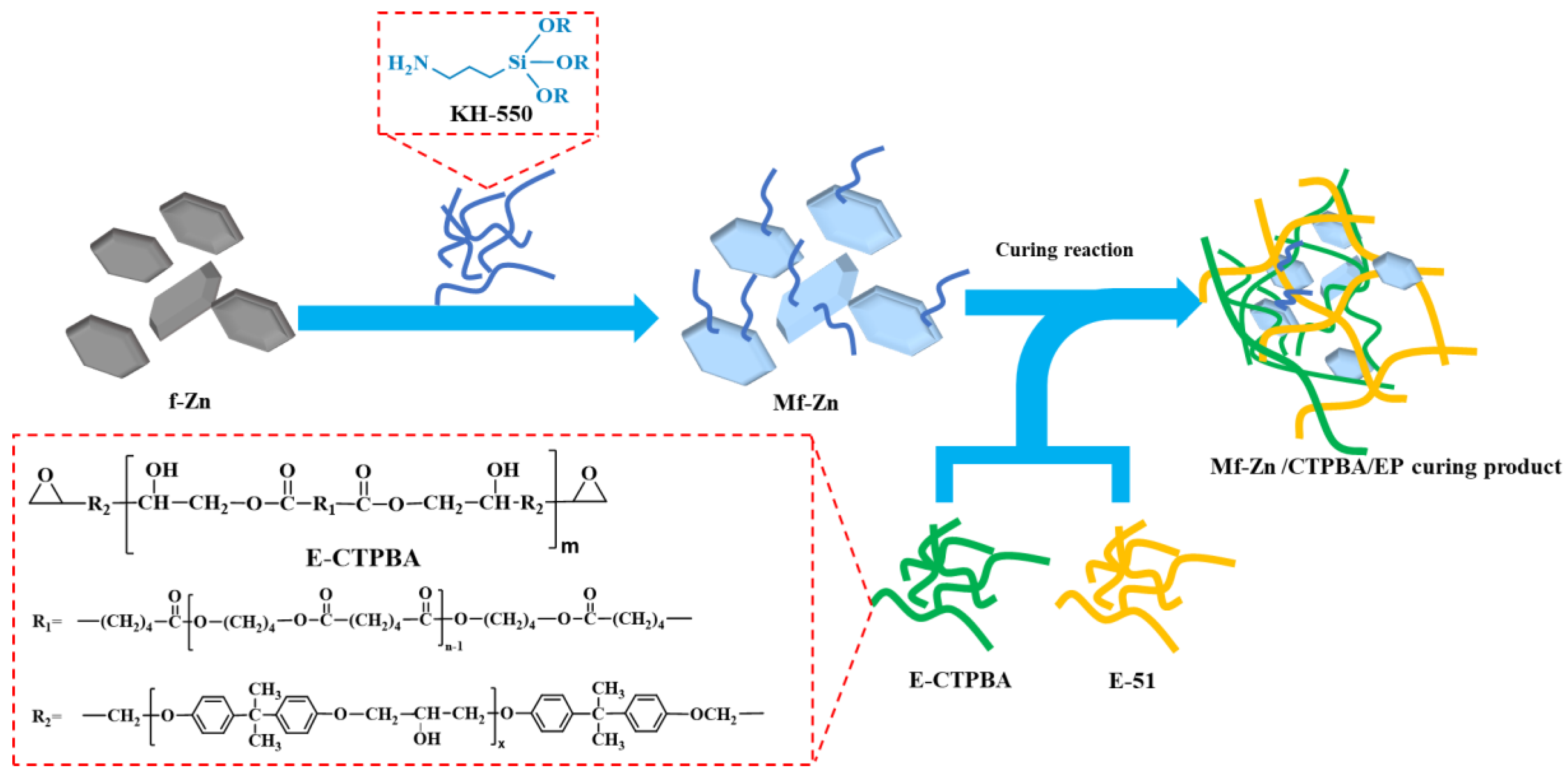
2.2. Modification of Zinc Powder
2.3. Preparation of Zn/CTPBA/EP Epoxy Resin
2.4. Characterization and Performance Testing
3. Results and Discussion
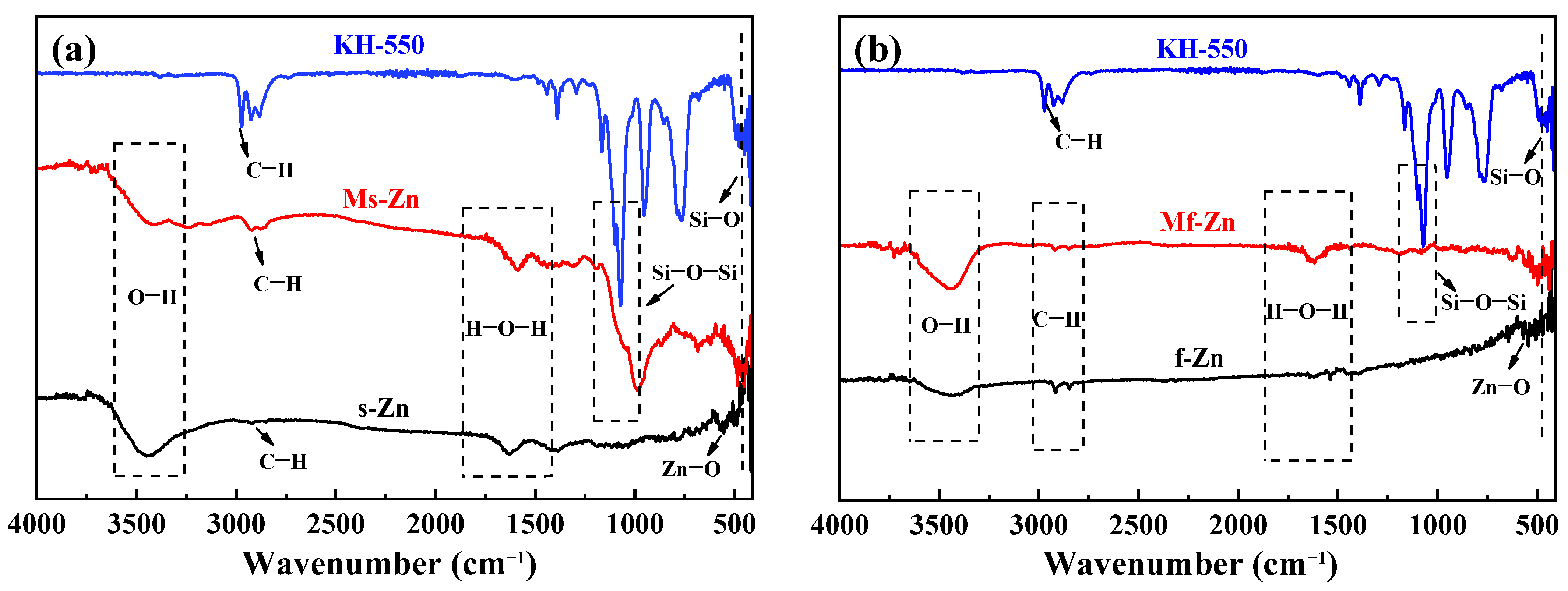
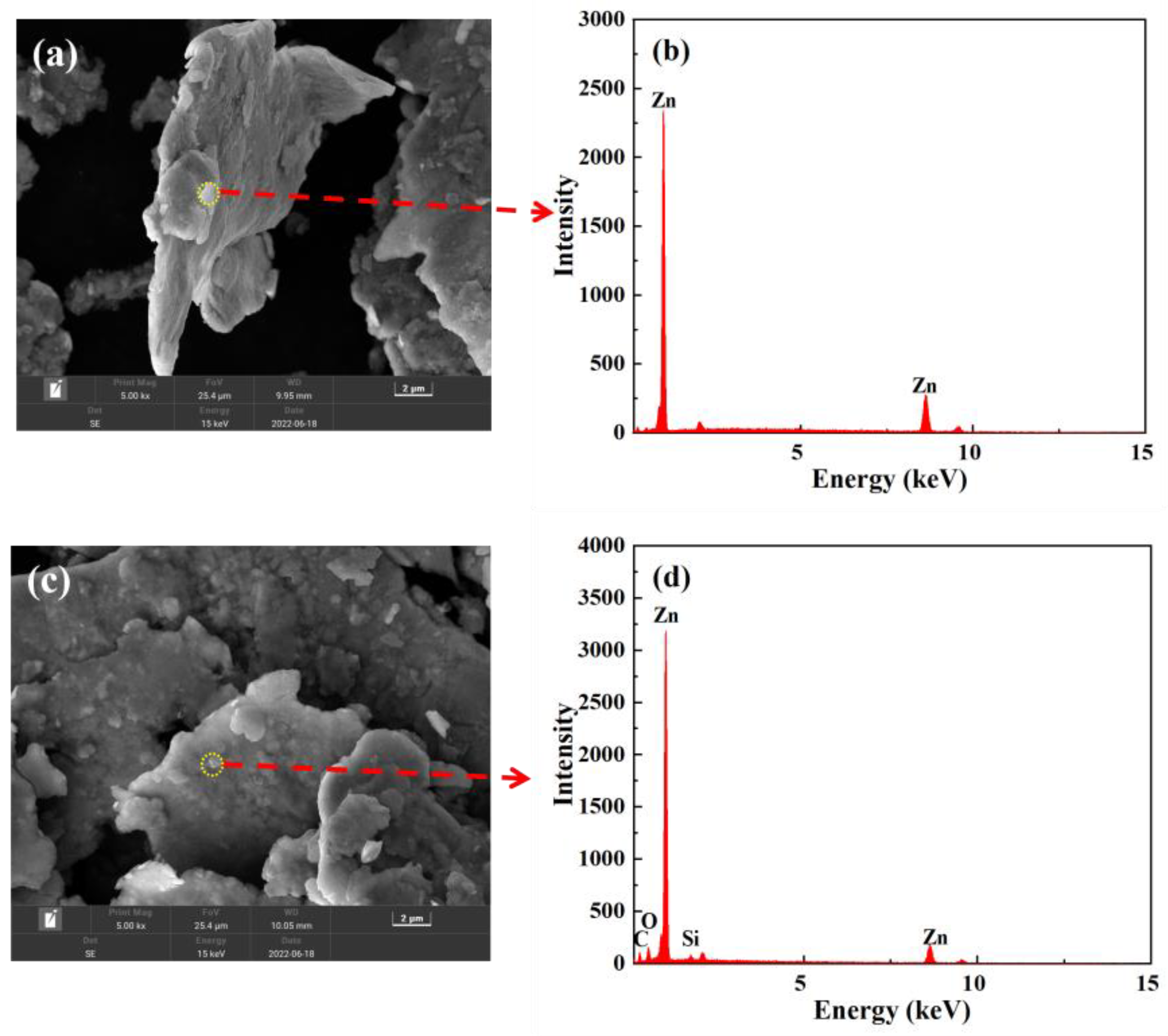
3.1. Characterization of Modified Zinc Powder
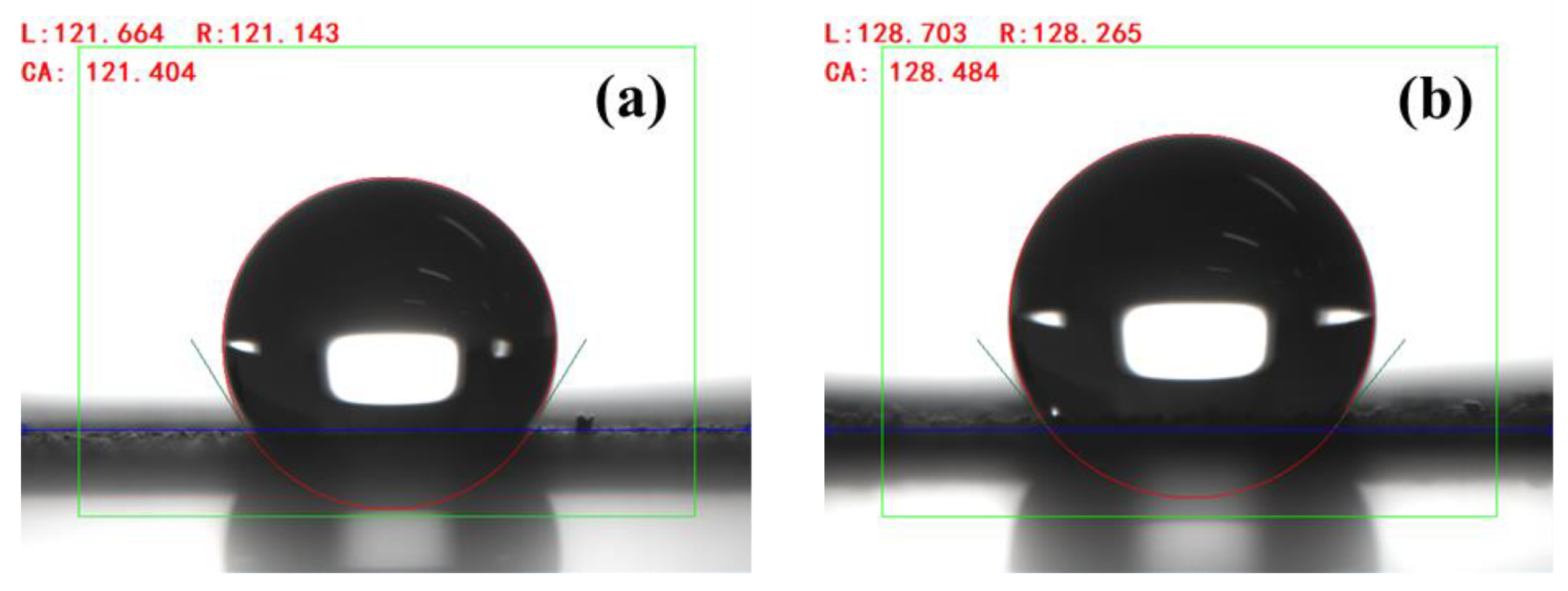
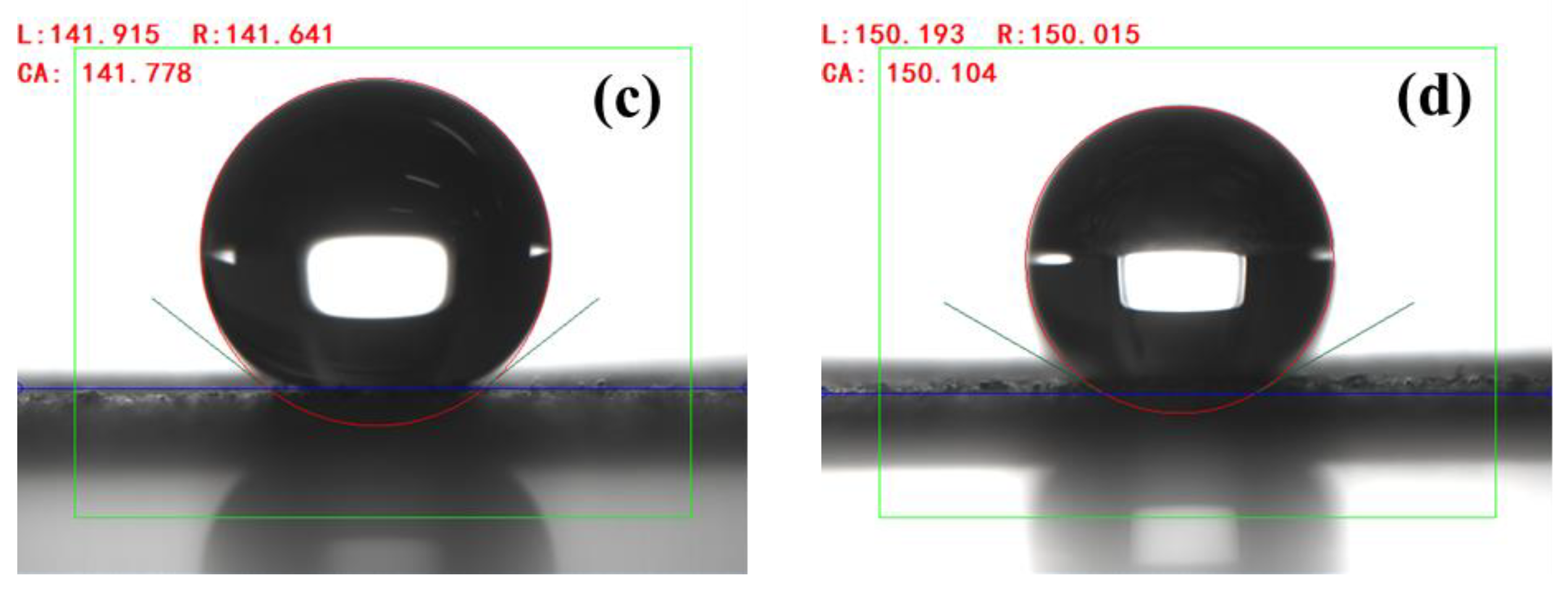
3.2. Effect of Coupling Agent on Material Properties
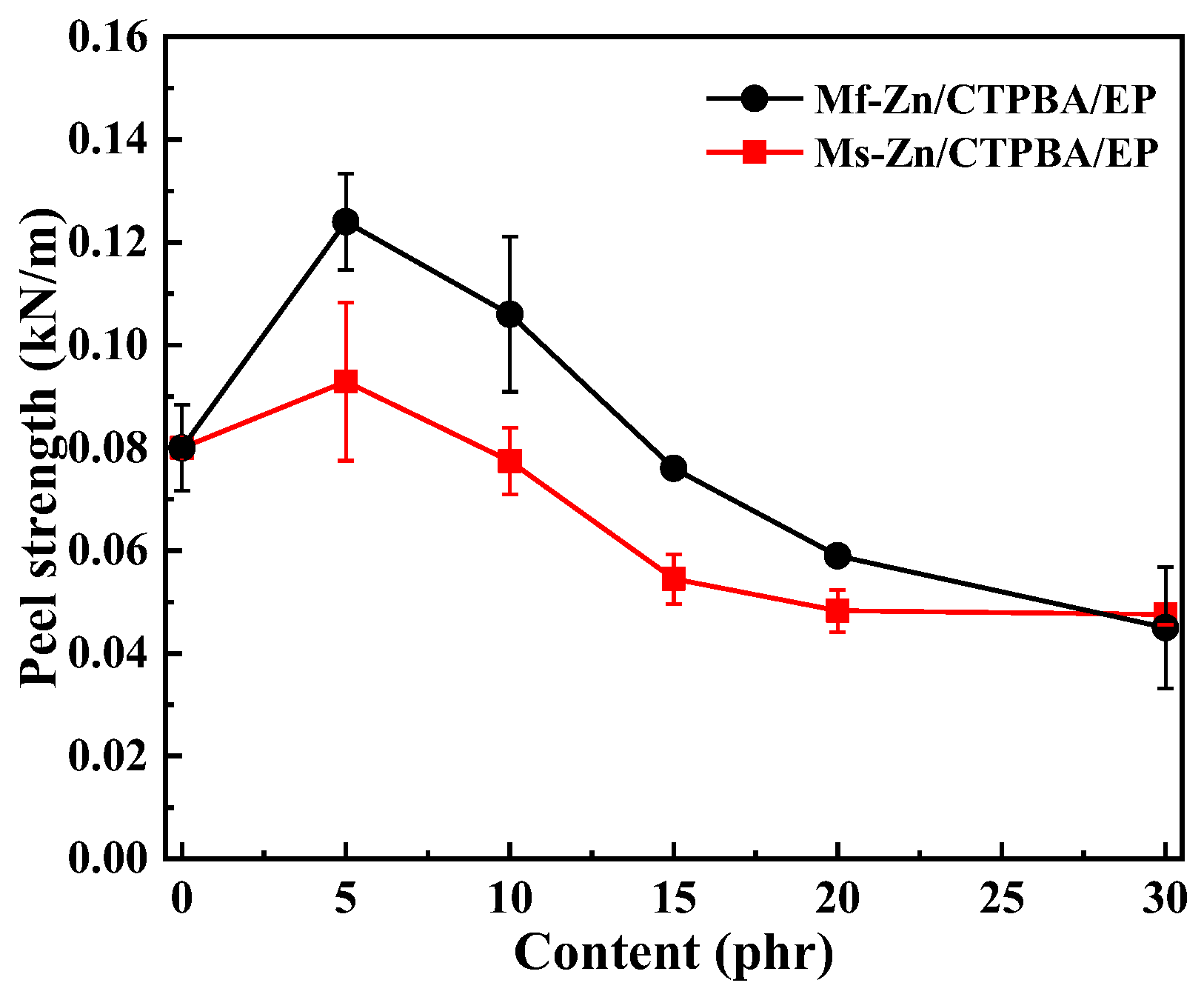
3.3. Effect of Zinc Powder on Bonding Properties of CTPBA/EP
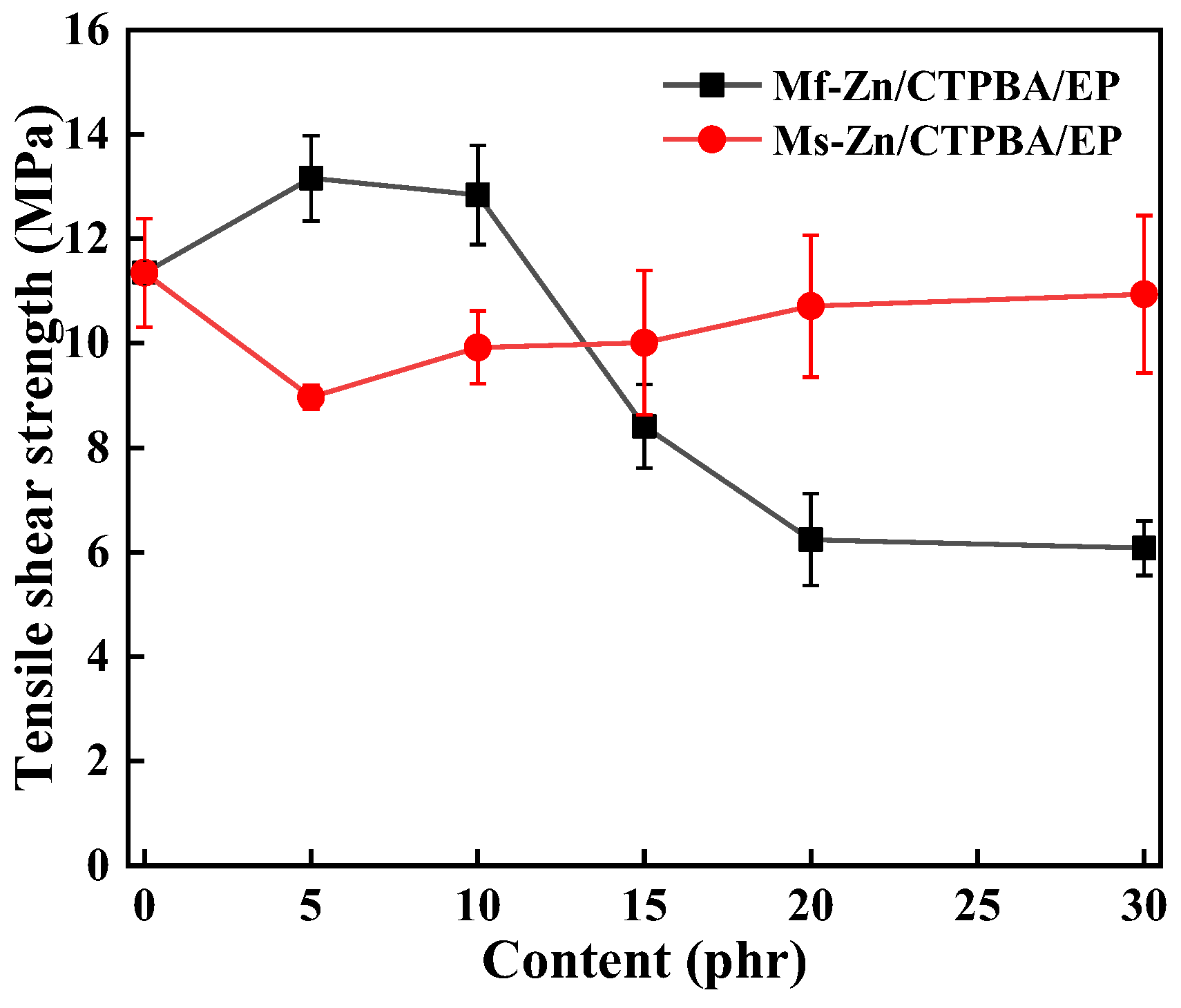
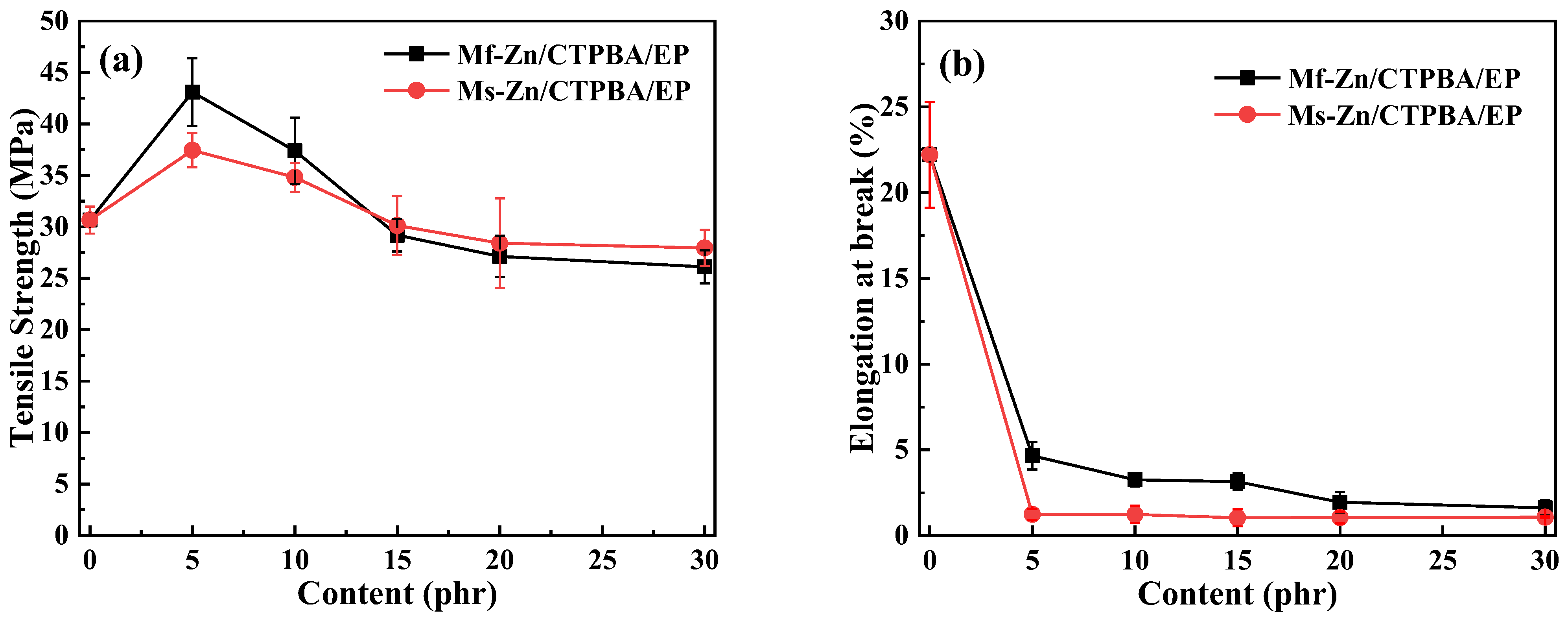
3.4. Effect of Zinc Powder on Mechanical Properties of CTPBA/EP
3.5. Thermal Stability
3.6. Dynamic Thermal Mechanical Properties
3.7. Medium Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Baig, M.M.A.; Samad, M.A. Epoxy\Epoxy Composite\Epoxy Hybrid Composite Coatings for Tribological Applications-A Review. Polymers 2021, 13, 179. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Liu, M.; Peng, C.; Wu, Z. Epoxy-functionalized polysiloxane reinforced epoxy resin for cryogenic application. J. Appl. Polym. Sci. 2018, 136, 46930. [Google Scholar] [CrossRef]
- Guo, Y.-K.; Li, H.; Zhao, P.-X.; Wang, X.-F.; Astruc, D.; Shuai, M.-B. Thermo-reversible MWCNTs/epoxy polymer for use in self-healing and recyclable epoxy adhesive. Chin. J. Polym. Sci. 2017, 35, 728–738. [Google Scholar] [CrossRef]
- Chen, D.; Li, J.; Yuan, Y.; Gao, C.; Cui, Y.; Li, S.; Wang, H.; Peng, C.; Liu, X.; Wu, Z.; et al. A new strategy to improve the toughness of epoxy thermosets by introducing the thermoplastic epoxy. Polymer 2022, 240, 124518. [Google Scholar] [CrossRef]
- Sprenger, S. Nanosilica-Toughened Epoxy Resins. Polymers 2020, 12, 1777. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ran, Q.; Sun, D.; Yin, H.; Wan, Y.; Liu, Y.; Mao, Y.; Liu, J. Self-synthesized epoxy terminated polydimethylsiloxane with various molecular weights toughening epoxy resin. J. Vinyl Addit. Technol. 2016, 24, 268–274. [Google Scholar] [CrossRef]
- Zhao, F.; Fei, X.; Wei, W.; Ye, W.; Luo, J.; Chen, Y.; Zhu, Y.; Liu, X. A random acrylate copolymer with epoxy-amphiphilic structure as an efficient toughener for an epoxy/anhydride system. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Du, X.; Xu, P.; Cong, P.; Zhou, Z. Compatibilization and toughness modification of linear aliphatic epoxy compound on paving epoxy asphalt. Mater. Struct. 2020, 53, 42. [Google Scholar] [CrossRef]
- Wu, J.; Li, C.; Hailatihan, B.; Mi, L.; Baheti, Y.; Yan, Y. Effect of the Addition of Thermoplastic Resin and Composite on Mechanical and Thermal Properties of Epoxy Resin. Polymers 2022, 14, 1087. [Google Scholar] [CrossRef]
- Bao, Q.; Wang, B.; Liu, Y.; Wang, Q.; Yang, Z. Epoxy resin flame retarded and toughed via flexible siloxane chain containing phosphaphenanthrene. Polym. Degrad. Stab. 2019, 172, 109055. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Chen, C.; Wu, Y.; Chen, J. MoS2 nanosheets modified SiO2 to enhance the anticorrosive and mechanical performance of epoxy coating. Prog. Org. Coat. 2019, 132, 316–327. [Google Scholar] [CrossRef]
- Akbari, R.; Beheshty, M.H.; Shervin, M. Toughening of dicyandiamide-cured DGEBA-based epoxy resins by CTBN liquid rubber. Iran. Polym. J. 2013, 22, 313–324. [Google Scholar] [CrossRef]
- XU, S.; SONG, X.; CAI, Y. Mechanical Properties and Morphologies of Carboxyl-Terminated Butadiene Acrylonitrile Liquid Rubber/Epoxy Blends Compatibilized by Pre-Crosslinking. Materials 2016, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Xie, Z.; Liu, Q.; Yuan, X.; Hou, X.; Zhao, J. Synergistic toughening of epoxy resin by CTBN and CM-β-CD. J. Appl. Polym. Sci. 2021, 138, 51248. [Google Scholar] [CrossRef]
- Bian, X.; Tuo, R.; Yang, W.; Zhang, Y.; Xie, Q.; Zha, J.; Lin, J.; He, S. Mechanical, Thermal, and Electrical Properties of BN–Epoxy Composites Modified with Carboxyl-Terminated Butadiene Nitrile Liquid Rubber. Polymers 2019, 11, 1548. [Google Scholar] [CrossRef]
- Zewde, B.; Pitliya, P.; Raghavan, D. The role of surface modified TiO2 nanoparticles on the mechanical and thermal properties of CTBN toughened epoxy nanocomposite. J. Mater. Sci. 2016, 51, 9314–9329. [Google Scholar] [CrossRef]
- Ma, S.; Liu, W.; Gao, N.; Yan, Z.; Zhao, Y. Synthesis and properties of LED-packaging epoxy resin toughened by a novel polysiloxane from hydrolysis and condensation. Macromol. Res. 2011, 19, 972–979. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.; Qiao, L.; Jiang, L.; Cao, T.; Zhang, Y. Developing an epoxy resin with high toughness for grouting material via co-polymerization method. e-Polymers 2019, 19, 489–498. [Google Scholar] [CrossRef]
- Dai, X.; Li, P.; Sui, Y.; Zhang, C. Synthesis and performance of flexible epoxy resin with long alkyl side chains via click reaction. J. Polym. Sci. 2021, 59, 627–637. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, L.; Bu, Y.; Sun, Q. Synergistical Performance Modification of Epoxy Resin by Nanofillers and Carboxyl-Terminated Liquid Nitrile–Butadiene Rubber. Materials 2021, 14, 4601. [Google Scholar] [CrossRef]
- Wang, J.; Xue, Z.; Li, Y.; Li, G.; Wang, Y.; Zhong, W.-H.; Yang, X. Synergistically effects of copolymer and core-shell particles for toughening epoxy. Polymer 2018, 140, 39–46. [Google Scholar] [CrossRef]
- Chen, C.-H.; Jian, J.-Y.; Yen, F.-S. Morphology, thermal, and mechanical properties of κ-aluminum oxide/CTBN/epoxy nanocomposites. Polym. Bull. 2020, 78, 3821–3834. [Google Scholar] [CrossRef]
- Cao, Y.; Weng, M.; Mahmoud, M.H.H.; Elnaggar, A.Y.; Zhang, L.; El Azab, I.H.; Chen, Y.; Huang, M.; Huang, J.; Sheng, X. Flame-retardant and leakage-proof phase change composites based on MXene/polyimide aerogels toward solar thermal energy harvesting. Adv. Compos. Hybrid Mater. 2022, 5, 1253–1267. [Google Scholar] [CrossRef]
- Wang, P.; Song, T.; Abo-Dief, H.M.; Song, J.; Alanazi, A.K.; Fan, B.; Huang, M.; Lin, Z.; Altalhi, A.A.; Gao, S.; et al. Effect of carbon nanotubes on the interface evolution and dielectric properties of polylactic acid/ethylene-vinyl acetate copolymer nanocomposites. Adv. Compos. Hybrid Mater. 2022, 5, 1100–1110. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, M.; Ibrahim, M.M.; Wu, H.; Wu, Y.; Li, Y.; Mersal, G.A.M.; El Azab, I.H.; El-Bahy, S.M.; Huang, M.; et al. Flexible polystyrene/graphene composites with epsilon-near-zero properties. Adv. Compos. Hybrid Mater. 2022, 5, 1054–1066. [Google Scholar] [CrossRef]
- Cai, J.; Murugadoss, V.; Jiang, J.; Gao, X.; Lin, Z.; Huang, M.; Guo, J.; Alsareii, S.A.; Algadi, H.; Kathiresan, M. Waterborne polyurethane and its nanocomposites: A mini-review for anti-corrosion coating, flame retardancy, and biomedical applications. Adv. Compos. Hybrid Mater. 2022, 5, 641–650. [Google Scholar] [CrossRef]
- Mohammadkhani, R.; Ramezanzadeh, M.; Saadatmandi, S.; Ramezanzadeh, B. Designing a dual-functional epoxy composite system with self-healing/barrier anti-corrosion performance using graphene oxide nano-scale platforms decorated with zinc doped-conductive polypyrrole nanoparticles with great environmental stability and non-toxicity. Chem. Eng. J. 2020, 382, 122819. [Google Scholar] [CrossRef]
- Taheri, N.N.; Ramezanzadeh, B.; Mandavian, M.; Bahlakeh, G. In-situ synthesis of Zn doped polyaniline on graphene oxide for inhibition of mild steel corrosion in 3.5 wt.% chloride solution. J. Ind. Eng. Chem. 2018, 63, 322–339. [Google Scholar] [CrossRef]
- Luo, X.; Li, Y.; Sun, Z.; Wang, G.; Xin, J.J.R.a. Preparation and research of epoxy modified by carboxyl-terminated polybutylene adipate at room temperature. RSC Adv. 2022, 12, 20471–20480. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, D.; Kumar, A.; Jain, A. An Analysis of Mechanical and Viscoelastic Behaviour of Nano-SiO2 Dispersed Epoxy Composites. Silicon 2020, 12, 2465–2477. [Google Scholar] [CrossRef]
- Kumar, K.; Goyat, M.S.; Solanki, A.; Kumar, A.; Kant, R.; Ghosh, P.K. Improved mechanical performance and unique toughening mechanisms of UDM processed epoxy-SiO2 nanocomposites. Polym. Compos. 2021, 42, 6000–6009. [Google Scholar] [CrossRef]
- ZHI, C.; Bando, Y.; TANG, C.; Kuwahara, H.; Golberg, D.J.A.M. Large-scale fabrication of boron nitride nanosheets and their utilization in polymeric composites with improved thermal and mechanical properties. Adv. Mater. 2009, 21, 2889–2893. [Google Scholar] [CrossRef]
- Ren, L.; Pashayi, K.; Fard, H.R.; Kotha, S.P.; Borca-Tasciuc, T.; Ozisik, R.J.C.P.B.E. Engineering the coefficient of thermal expansion and thermal conductivity of polymers filled with high aspect ratio silica nanofibers. Compos. Part B Eng. 2014, 58, 228–234. [Google Scholar] [CrossRef]



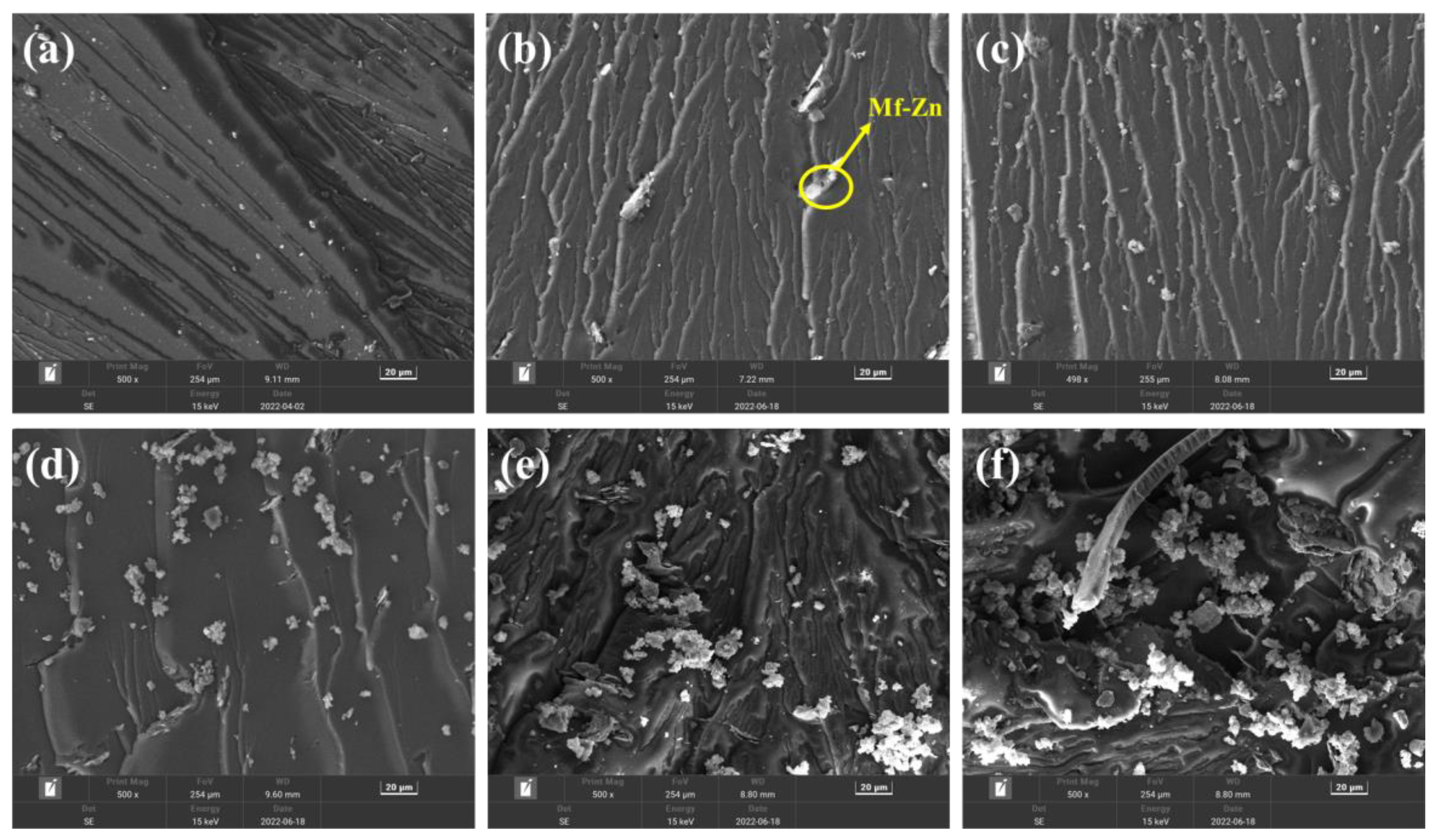
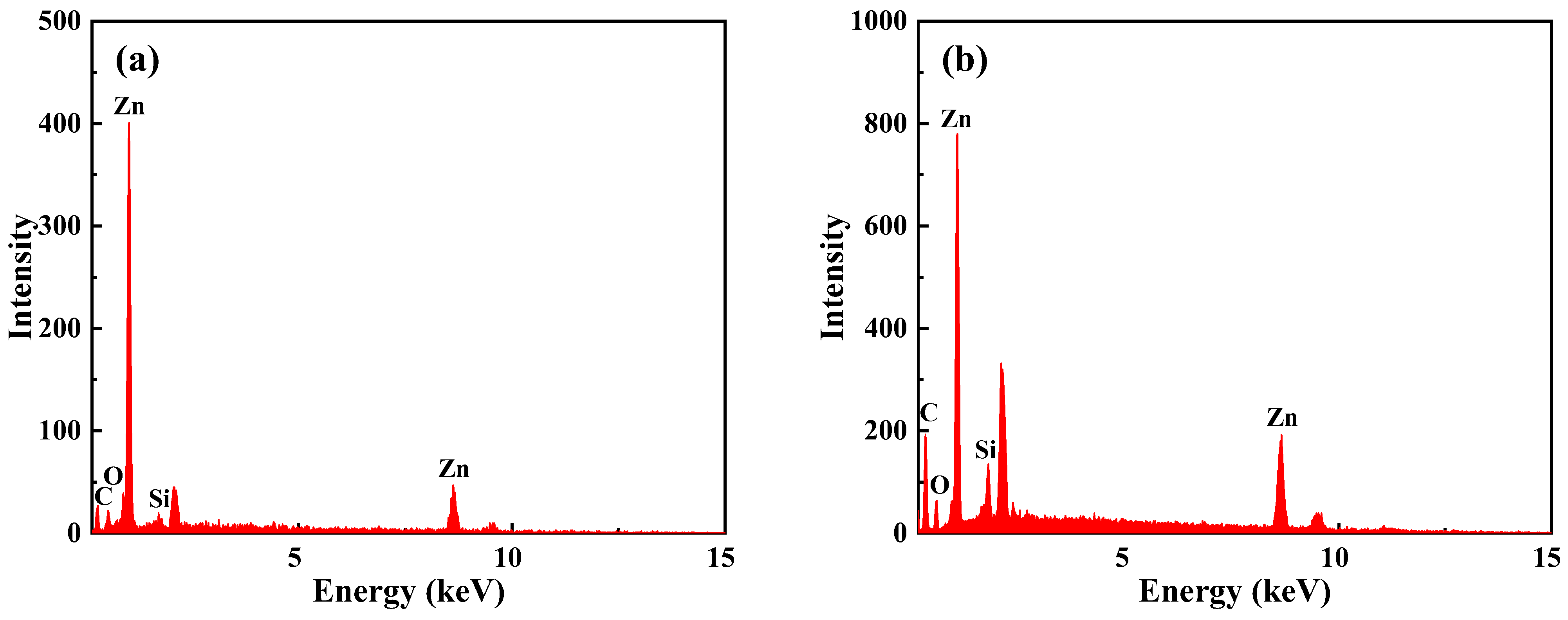


| Sample | E-CTPBA (phr) | EP (phr) | T-31 (phr) | Ms-Zn (phr) | Mf-Zn (phr) |
|---|---|---|---|---|---|
| CTPBA/EP | 20 | 100 | 25 | 0 | 0 |
| Ms-Zn/CTPBA/EP-5 | 20 | 100 | 25 | 5 | 0 |
| Ms-Zn/CTPBA/EP-10 | 20 | 100 | 25 | 10 | 0 |
| Ms-Zn/CTPBA/EP-15 | 20 | 100 | 25 | 15 | 0 |
| Ms-Zn/CTPBA/EP-20 | 20 | 100 | 25 | 20 | 0 |
| Ms-Zn/CTPBA/EP-30 | 20 | 100 | 25 | 30 | 0 |
| Mf-Zn/CTPBA/EP-5 | 20 | 100 | 25 | 0 | 5 |
| Mf-Zn/CTPBA/EP-10 | 20 | 100 | 25 | 0 | 10 |
| Mf-Zn/CTPBA/EP-15 | 20 | 100 | 25 | 0 | 15 |
| Mf-Zn/CTPBA/EP-20 | 20 | 100 | 25 | 0 | 20 |
| Mf-Zn/CTPBA/EP-30 | 20 | 100 | 25 | 0 | 30 |
| Sample | Tensile Shear Strength (MPa) | Peel Strength (kN/m) | Tensile Strength (MPa) | Impact Strength (kJ/m2) | Elongation at Break (%) |
|---|---|---|---|---|---|
| CTPBA/EP | 11.35 | 0.08 | 30.66 | 3.71 | 22.21 |
| s-Zn/CTPBA/EP | 7.84 | 0.03 | 33.02 | 2.80 | 0.97 |
| Ms-Zn/CTPBA/EP | 8.97 | 0.09 | 37.44 | 6.12 | 1.25 |
| f-Zn/CTPBA/EP | 6.00 | 0.05 | 35.01 | 6.46 | 1.39 |
| Mf-Zn/CTPBA/EP | 13.16 | 0.12 | 43.09 | 7.09 | 4.66 |
| Sample | IDT (°C) | T50 (°C) | Tmax (°C) | Residual Carbon Rate at 700 °C (%) |
|---|---|---|---|---|
| 0 phr | 324.39 | 401.50 | 378.80 | 9.19 |
| 5 phr | 328.41 | 393.71 | 380.31 | 18.19 |
| 10 phr | 331.47 | 394.19 | 381.32 | 19.62 |
| 15 phr | 334.51 | 396.24 | 380.67 | 22.65 |
| 20 phr | 336.25 | 398.32 | 380.05 | 26.42 |
| 30 phr | 339.88 | 401.74 | 380.13 | 30.55 |
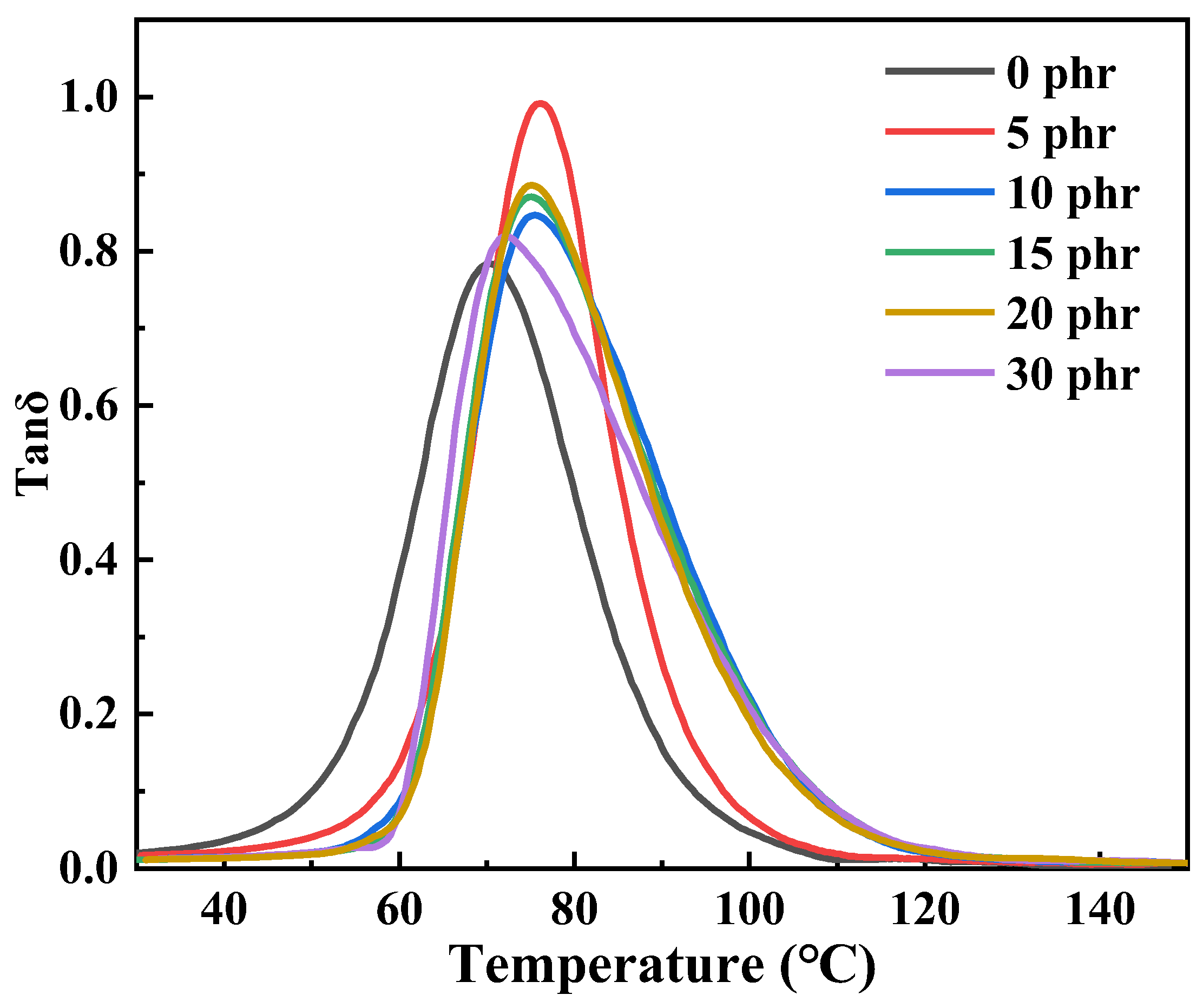
| Content | Tg/°C | DTR/°C (tanδ ≥ 0.3) | Tan δmax |
|---|---|---|---|
| 0 phr | 70.3 | 26 (58–84) | 0.78 |
| 5 phr | 75.6 | 24 (65–89) | 1.03 |
| 10 phr | 75.4 | 31 (65–96) | 0.85 |
| 15 phr | 75.2 | 31 (65–96) | 0.87 |
| 20 phr | 74.7 | 30 (65–95) | 0.89 |
| 30 phr | 72.6 | 31 (64–95) | 0.81 |
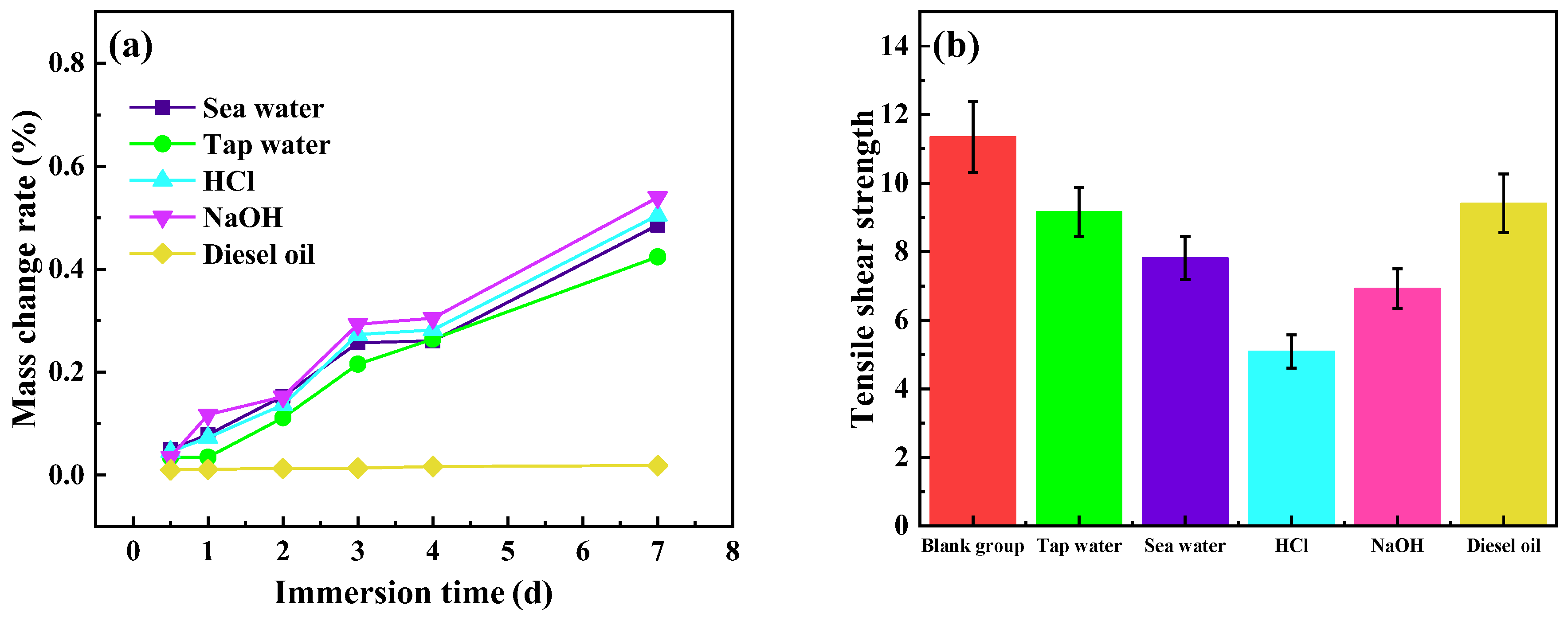
| Medium | CTPBA/EP | Mf-Zn/CTPBA/EP | ||
|---|---|---|---|---|
| Tensile Shear Strength (MPa) | Change Rate of Strength (%) | Tensile Shear Strength (MPa) | Change Rate of Strength (%) | |
| Blank group | 11.35 | 0 | 13.16 | 0 |
| Tap water | 9.16 | 19.30 | 10.30 | 21.73 |
| Sea water | 7.82 | 31.10 | 9.69 | 26.37 |
| HCl (10%) | 5.09 | 55.15 | 8.71 | 33.81 |
| NaOH (10%) | 6.92 | 39.03 | 8.53 | 35.18 |
| Diesel oil | 9.41 | 17.09 | 11.66 | 11.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Li, Y.; Li, S.; Liu, X. Enhancement of Mechanical Properties and Bonding Properties of Flake-Zinc-Powder-Modified Epoxy Resin Composites. Polymers 2022, 14, 5323. https://doi.org/10.3390/polym14235323
Luo X, Li Y, Li S, Liu X. Enhancement of Mechanical Properties and Bonding Properties of Flake-Zinc-Powder-Modified Epoxy Resin Composites. Polymers. 2022; 14(23):5323. https://doi.org/10.3390/polym14235323
Chicago/Turabian StyleLuo, Xu, Yu Li, Shuaijie Li, and Xin Liu. 2022. "Enhancement of Mechanical Properties and Bonding Properties of Flake-Zinc-Powder-Modified Epoxy Resin Composites" Polymers 14, no. 23: 5323. https://doi.org/10.3390/polym14235323
APA StyleLuo, X., Li, Y., Li, S., & Liu, X. (2022). Enhancement of Mechanical Properties and Bonding Properties of Flake-Zinc-Powder-Modified Epoxy Resin Composites. Polymers, 14(23), 5323. https://doi.org/10.3390/polym14235323






