Properties in Langmuir Monolayers and Langmuir-Blodgett Films of a Block Copolymer Based on N-Isopropylacrylamide and 2,2,3,3-Tetrafluropropyl Methacrylate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
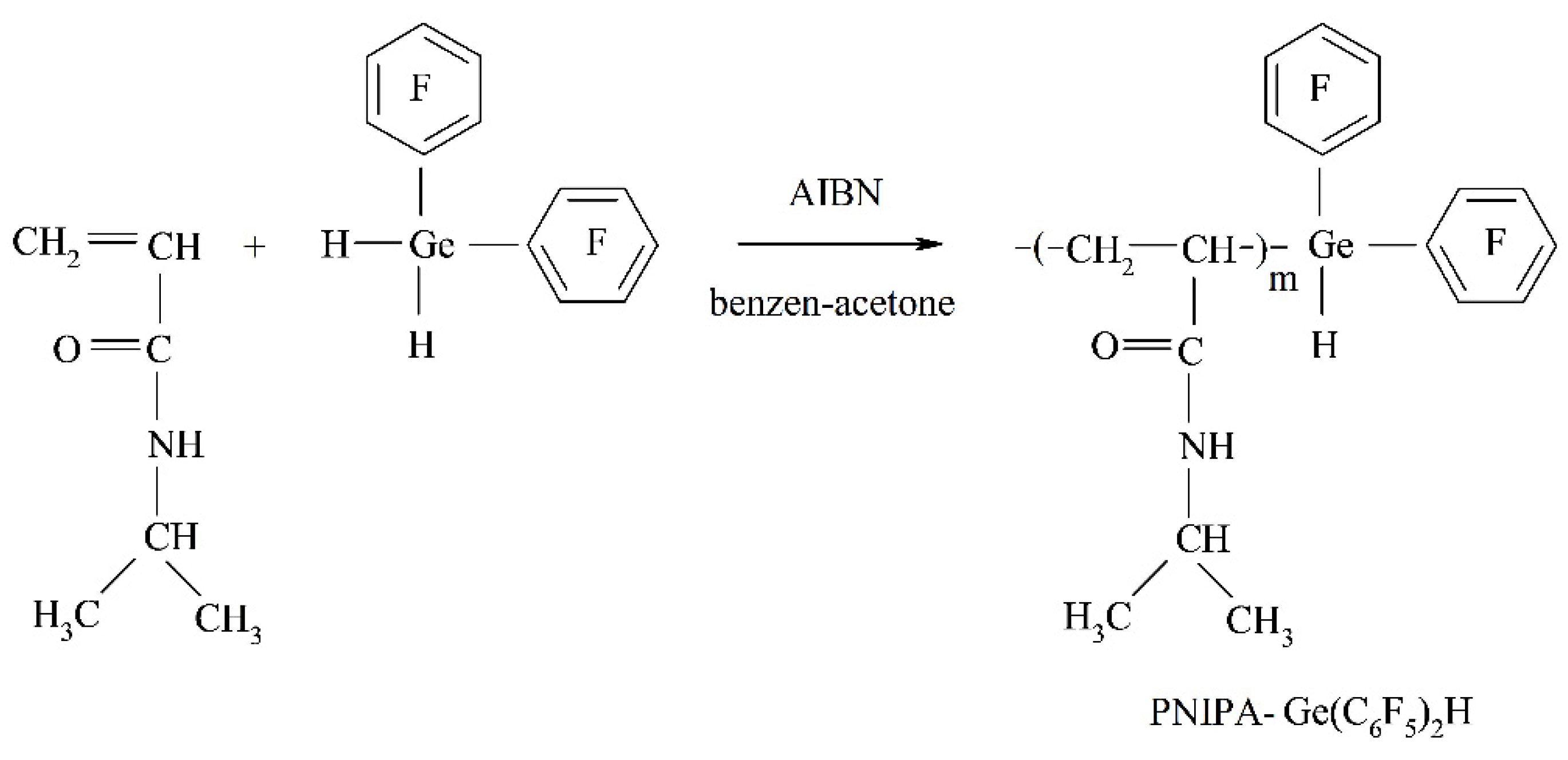
2.2. Synthesis of Functional Polymers PNIPAAm–Ge(C6F5)2H
2.3. Synthesis of Block Copolymers PNIPAAm–Ge(C6F5)2–PFMA
2.4. Polymer Characterization
2.5. Properties of Solution Polymers
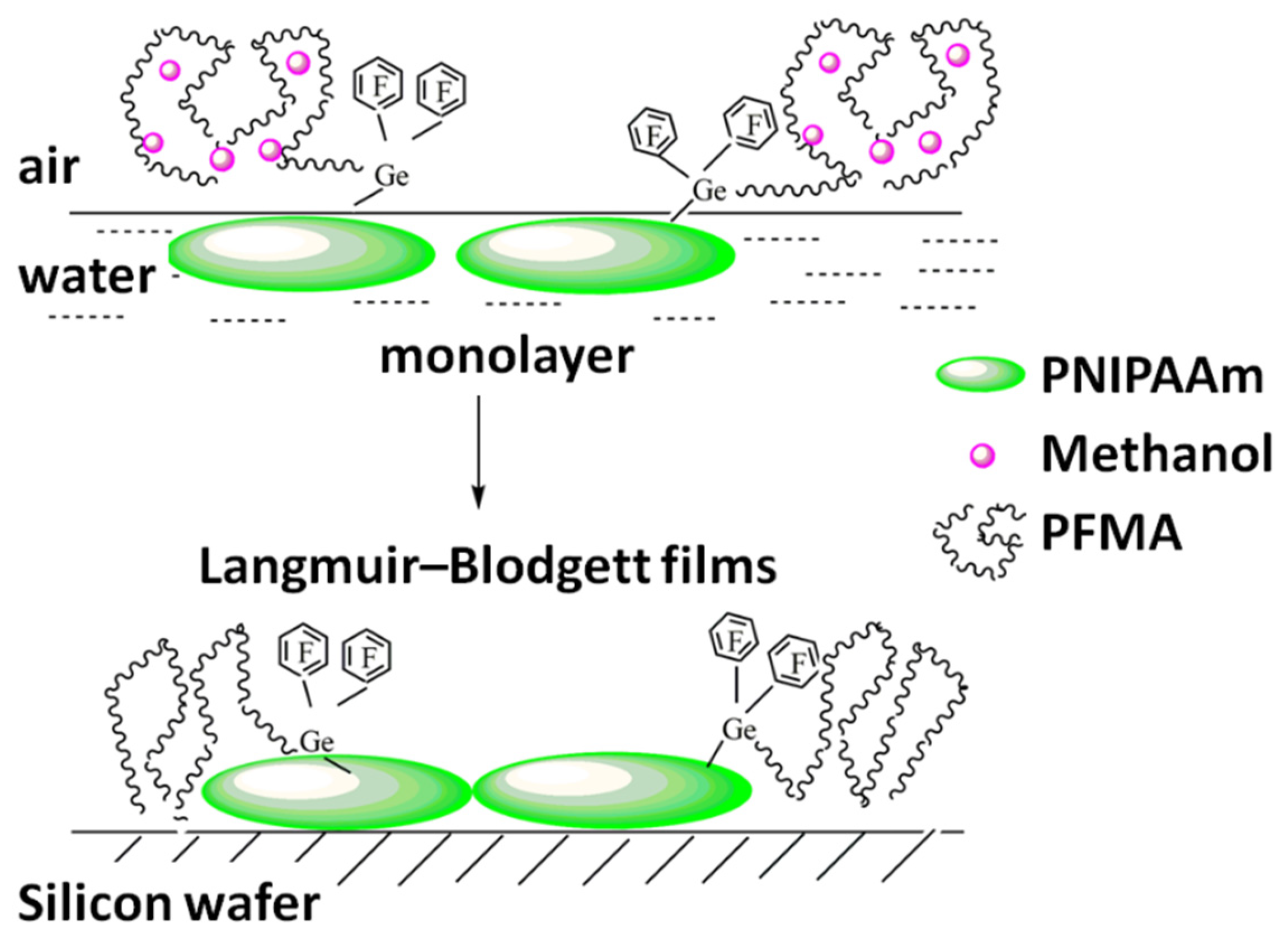
2.6. Properties of Monolayers Polymers
2.7. Properties of Langmuir-Blodgett Films Polymers
3. Results and Discussion
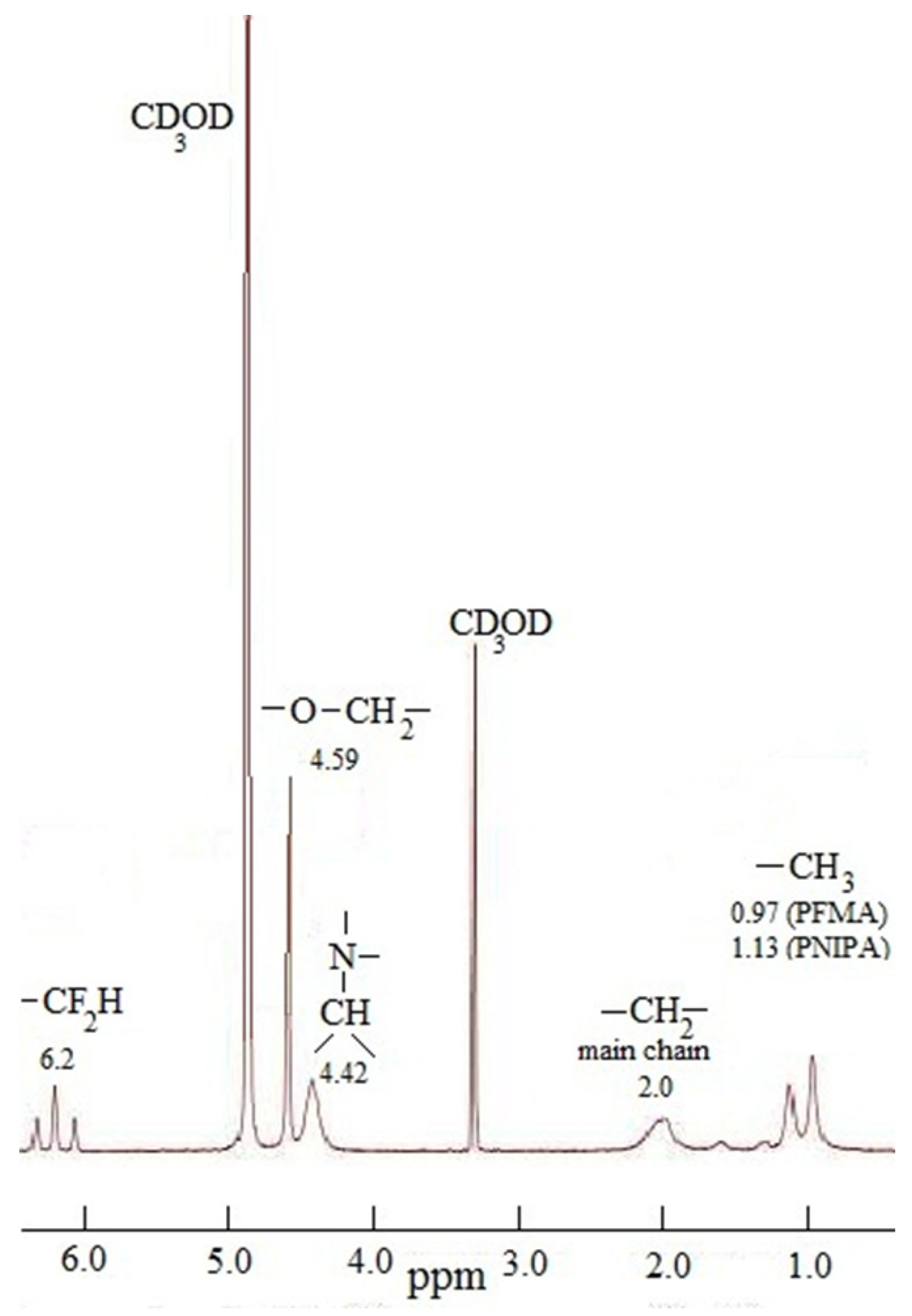
3.1. Synthesis and Characterization of Polymers
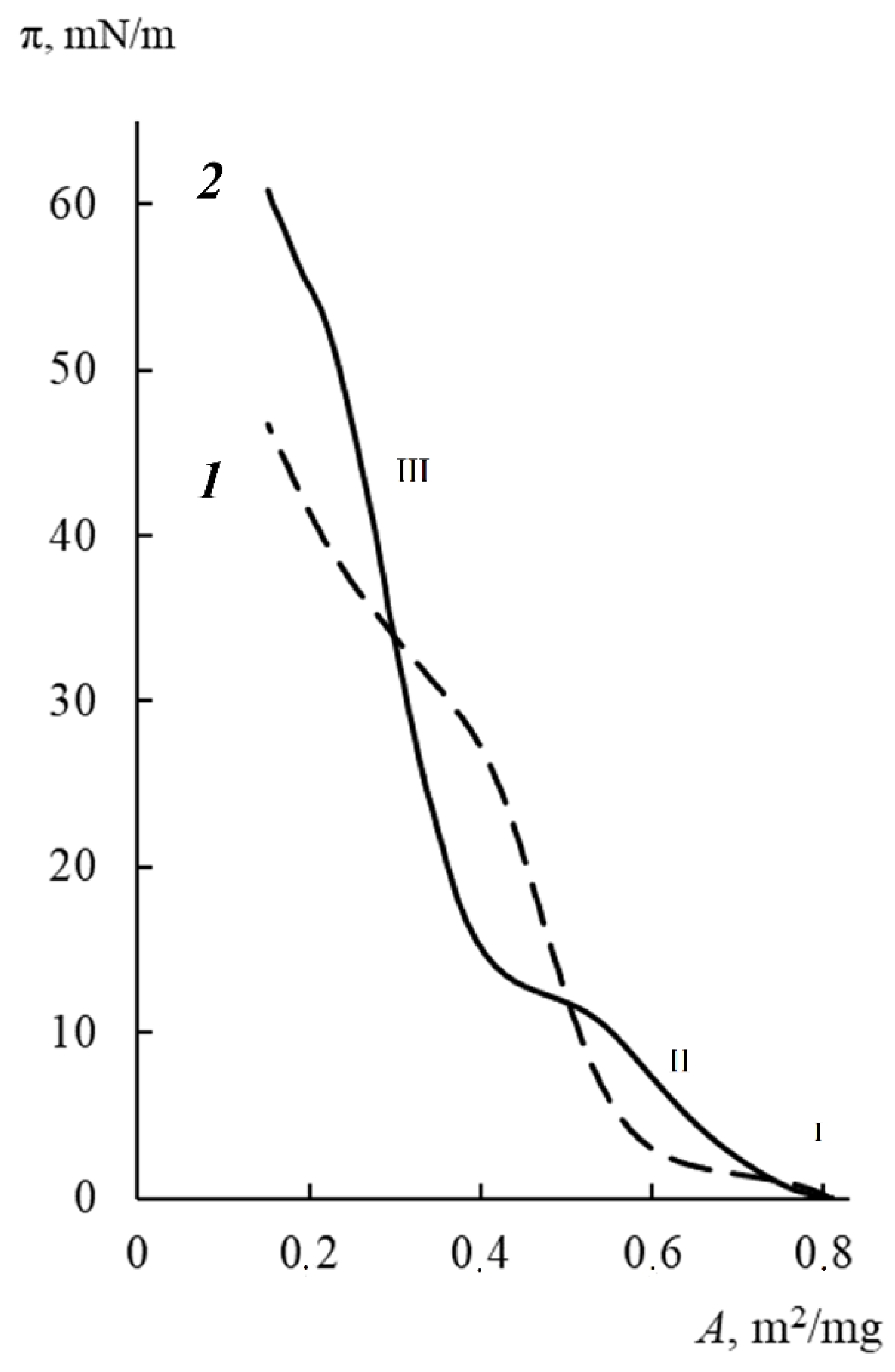
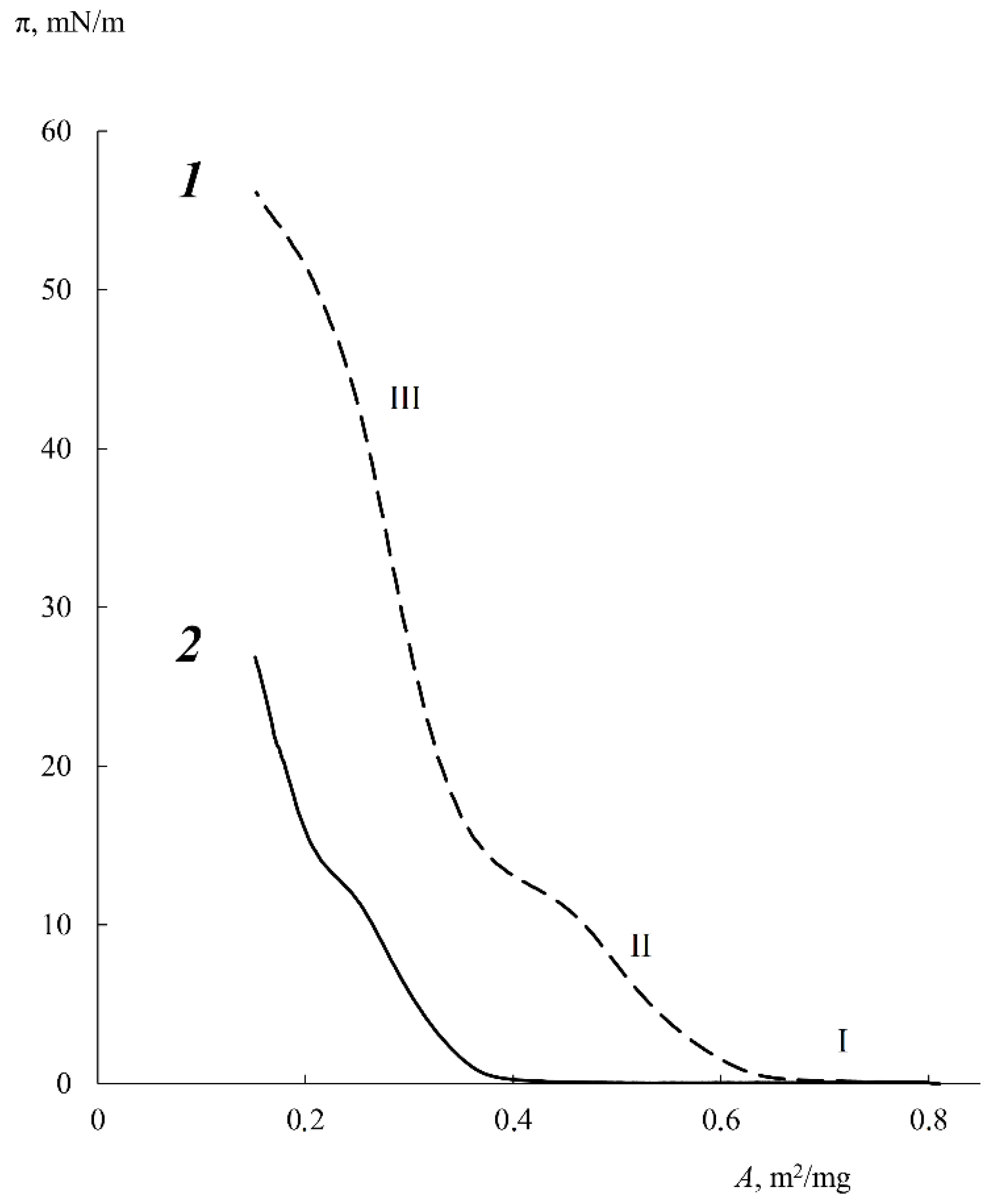
3.2. Langmuir Monolayers of Block Copolymers
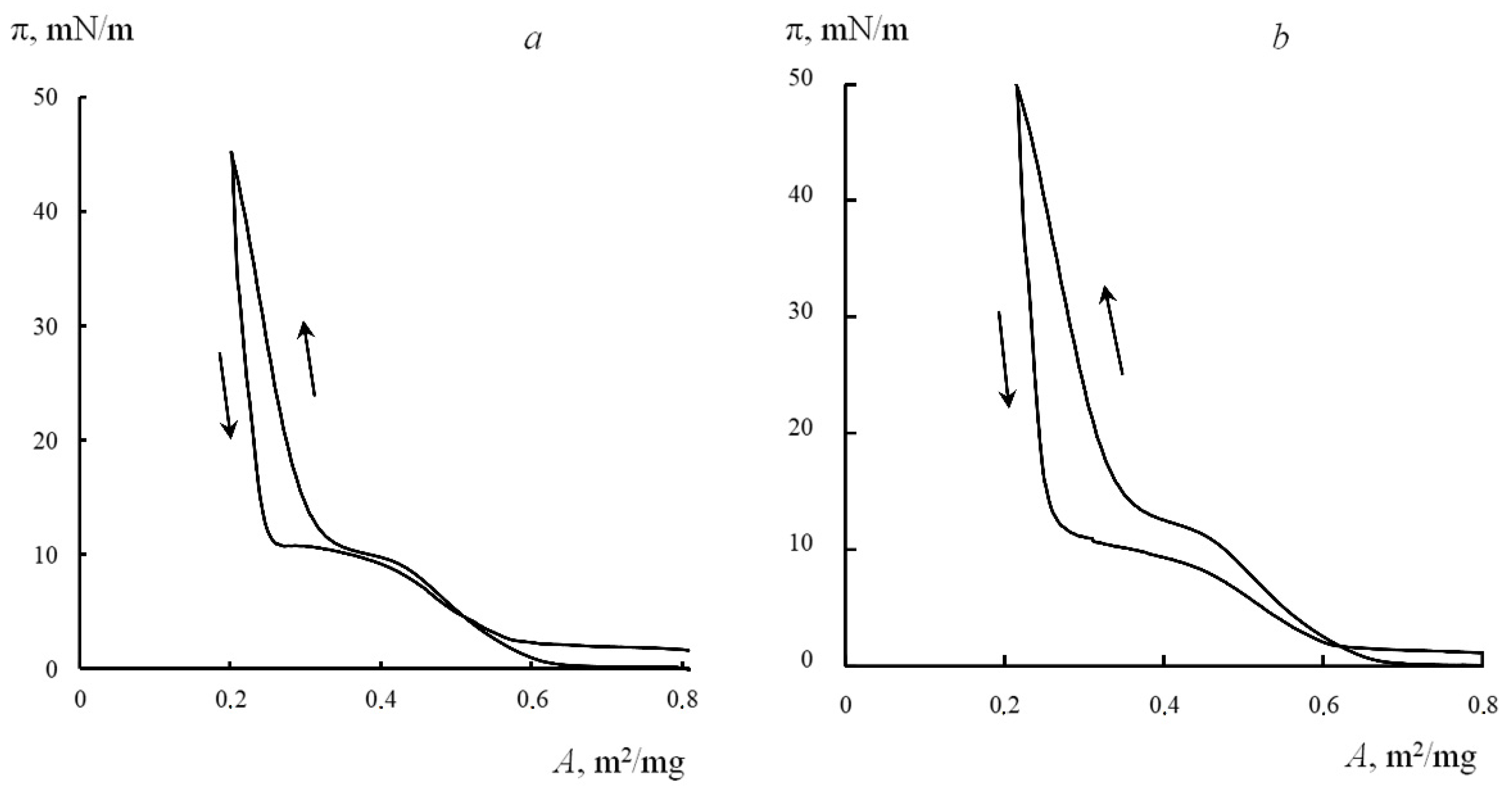
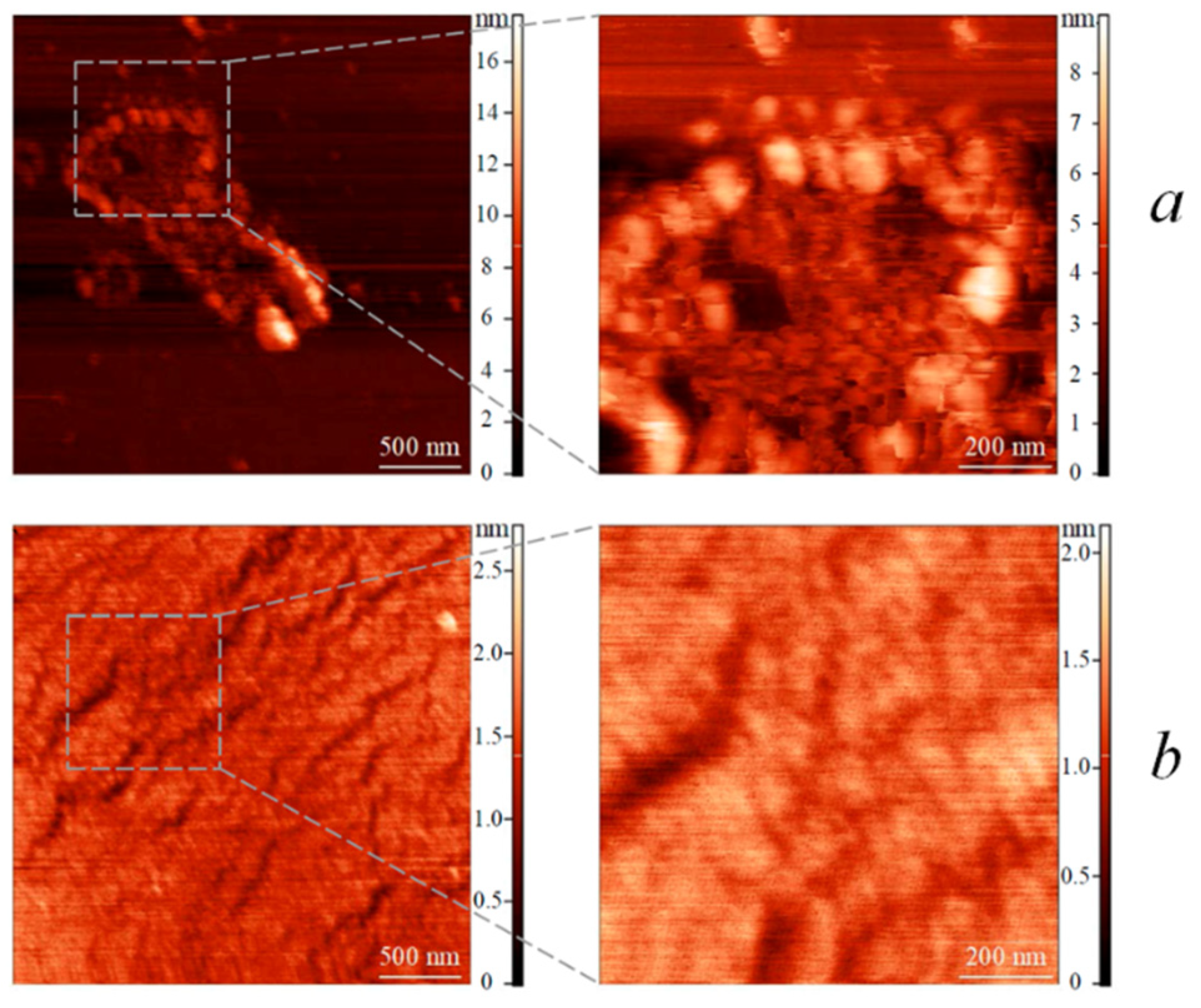
3.3. Surface Properties of LB Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, B.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef]
- Dong, Q.; Luo, C.; Li, N.; Chi, J.; Zhang, Q. Temperature and recognition dual responsive poly(N-isopropylacrylamide) and poly(N,N-dimethylacrylamide) with adamantly side group. Materials 2018, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and copolymers: A review on recent progresses in biomedical applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.L.; Wang, C.C.; Chen, C.Y. Preparation of shell crosslinked nanoencapsulate for drug carriers by using poly(N-isopropyl acrylamide)-co-poly(L-lysine) grafted copolymer. J. Polym. Res. 2018, 25, 134. [Google Scholar] [CrossRef]
- Pawar, K.; Kutcherlapati, S.N.R.; Yeole, N.; Jana, T.; Hundiwale, D. Synthesis of poly(N-isopropylacrylamide-b-N-vinylcarbazole) copolymers via RAFT polymerization and its stimuli responsive morphology in aqueous media. J. Polym. Res. 2018, 25, 91. [Google Scholar] [CrossRef]
- Zareie, M.H.; Dincer, S.; Piskin, E. Observation of phase transition of thermoresponsive poly(NIPA)-PEI block copolymers. J. Coll. Inter. Sci. 2002, 251, 424–428. [Google Scholar] [CrossRef]
- Dincer, S.; Tunce, A.; Piskin, E. A potential gene delivery vector: N-isopropylacrylamide-ethyleneimine block copolymers. Macromol. Chem. Phys. 2002, 203, 1460–1465. [Google Scholar] [CrossRef]
- Piskin, E. Molecularly designed water soluble, intelligent, nanosize polymeric carriers. Int. J. Pharm. 2004, 227, 105–118. [Google Scholar] [CrossRef]
- Piskin, E.; Dincer, S.; Turk, M. Gene delivery: Intelligent but just at the beginning. J. Biomater, Sci. Polym. Ed. 2004, 15, 1181–1202. [Google Scholar] [CrossRef] [PubMed]
- Arotcarena, D.M.; Heise, B.; Ishaya, S.; Laschewsky, A. Switching the inside and the outside of aggregates of water-soluble block copolymers with double thermoresponsivity. J. Am. Chem. Soc. 2002, 124, 3787–3793. [Google Scholar] [CrossRef] [PubMed]
- Nuopponen, M.; Ojala, J.; Tenhu, H. Aggregation behavior of well defined amphiphilic diblock copolymers with poly(N-isopropylacrylamide) and hydrophobic blocks. Polymer 2004, 45, 3643–3650. [Google Scholar] [CrossRef]
- Tarabukina, E.B.; Simonova, M.A.; Bucatariu, S.; Fundueanu, G.; Filippov, A.P. Behavior of thermo- and pH-responsive copolymer of N-isopropylacrylamide and maleic acid in aqueous solutions. Int. J. Polym. Anal. Charact. 2016, 21, 11–17. [Google Scholar] [CrossRef]
- Simonova, M.A.; Tarabukina, E.B.; Filippov, A.P.; Constantin, M.; Popescu, I. Effect of Concentration on the properties of heat- and PH-sensitive copolymers of poly(N-isopropylacrylamide) with maleic acid in aqueous solutions. Fiber. Chem. 2015, 47, 152–155. [Google Scholar] [CrossRef]
- Filippov, A.P.; Zamyshlyayeva, O.G.; Tarabukina, E.B.; Simonova, M.A.; Kozlov, A.V.; Semchikov, Y.D. Structural and conformational properties of hyperbranched copolymers based on perfluorinated germanium hydrides. Polym. Sci. Ser. A. 2012, 54, 319–329. [Google Scholar] [CrossRef]
- Pitois, C.; Vestberg, R.; Rodlert, M. Fluorinated dendritic polymers and dendrimers for waveguide applications. Opt. Mater. 2002, 21, 499–506. [Google Scholar] [CrossRef]
- Gasilova, E.R.; Koblyakova, M.A.; Filippov, A.P.; Zakharova, O.G.; Zaitsev, S.D.; Yu, D. Semchikov. Hydrodynamics and light scattering in solutions of a hyperbranched perfluorinated polyphenylenegermane–poly(methyl methacrylate) block copolymer. Polym. Sci. Ser. A. 2006, 48, 989. [Google Scholar] [CrossRef]
- Zakharova, O.G.; Tarasova, E.V.; Simonova, M.A.; Semchikov, Y.D.; Filippov, A.P. Synthesis and structural and conformational properties of hybrid polymers of styrene with perfluorinated compounds of germanium. Polym. Sci. Ser. A. 2009, 51, 512–517. [Google Scholar] [CrossRef]
- Tarabukina, E.; Kozlov, A.; Simonova, M.; Zamyshlyayeva, O.; Semchikov, Y. Hydrodynamic and molecular properties of hyperbranched copolymers formed by pentafluorophenylgermane hydrides. Int. J. Polym. Anal. Charact. 2011, 16, 369–376. [Google Scholar] [CrossRef]
- Simonova, M.A.; Zamyshlyayeva, O.G.; Simonova, A.A.; Tarasova, E.V.; Filippov, A.P. Model and hybrid polystyrenes containing trispentafluorophenylgermanium and groups. Int. J. Polym. Anal. Charact. 2009, 14, 454–467. [Google Scholar]
- Simonova, M.A.; Zamyshlyayeva, O.G.; Simonova, A.A.; Filippov, A.P. Conformation of the linear-dendritic block copolymers of hyperbranched polyphenylenegermane and linear poly(methylmethacrylate). Int. J. Polym. Anal. Charact. 2015, 20, 223–230. [Google Scholar] [CrossRef]
- Xian-Kai, L.; Xia, F.; Li, C.; Yi-Ping, Z. Characterization of temperature-sensitive membranes prepared from poly(vinylidenefluoride)-graft-poly(N-isopropylacrylamide) copolymers obtained by atom transfer radical polymerization. Front Mater Sci. China 2010, 4, 345–352. [Google Scholar]
- Zamyshlyayeva, O.G.; Smirnov, E.A.; Zakharycheva, N.S. Linear-dendritic block copolymers based on N-isopropylacrylamide and perfluorinated polyphenylengermane: Synthesis and properties at various interfacial boundaries. Polym. Sci. B 2017, 59, 708–717. [Google Scholar]
- Zamyshlyayeva, O.G.; Deniskina, I.V.; Filippov, A.P.; Semchikov, Y.D. Synthesis and self-organization of amphiphilic poly(N-vinylpyrrolidone-2,2,3,3-tetrafluoropropyl methacrylate) diblock copolymers in solution and bulk. Polym. Sci. A 2011, 53, 691–697. [Google Scholar] [CrossRef]
- Zamyshlyayeva, O.G.; Lapteva, O.S.; Baten’kin, M.A.; Semchikov, Y.D.; Mel’nikova, N.B. Self-organization and aggregation of amphiphilic block copolymers of N-vinylpyrrolidone-block-2,2,3,3-tetrafluoropropyl methacrylate at interfaces. Rus. Chem. Bull. 2014, 63, 1823–1836. [Google Scholar] [CrossRef]
- Kovylin, R.S.; Baten′kin, M.A.; Kulikova, T.I.; Ermolaeva, C.G.; Zamyshlyayeva, O.G.; Chesnokov, S.A. Amphiphilic fluorinated block-copolymer coating for the preparation of hydrophobic porous materials. J. Polym. Res. 2018, 25, 208. [Google Scholar] [CrossRef]
- Tritschler, U.; Pearce, S.; Gwyther, J.; Whittell, G.R.; Manners, I. Functional nanoparticles from the solution self-assembly of block copolymers. Macromolecules 2018, 50, 3439–3463. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. Self-Assembly of block and graft copolymers in organic solvents: An overview of recent advances. Polymers 2018, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Uda, K. Nanostructure of poly(N-isopropylacrylamide) brush at the air/water interface and its responsivity to temperature and salt. Langmuir 2016, 32, 8383–8391. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, M.; Kumashiro, Y.; Nakayama, M.; Tanaka, N.; Haraguchi, Y.; Umemura, K.; Shimizu, T.; Yamato, M.; Okano, T. Preparation of thermoresponsive nanostructured surfaces for tissue engineering. J. Visual. Exper. 2016, 109, 1–8. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Fujishige, S. Intrinsic viscosity-molecular weight relationships for poly(N-isopropylacrylamide) solutions. Polym. J. 1987, 19, 297–300. [Google Scholar] [CrossRef]
- Tsvetkov, V.N. Rigid-Chain Polymers, 1st ed.; Plenum Press: New York, NY, USA, 1989. [Google Scholar]
- Jaycock, M.J.; Parfitt, G.D. Chemistry of Interfaces; John Wiley and Sons: New York, NY, USA, 1981. [Google Scholar]
- Kaelble, D.H. Physical Chemistry of Adhesion; Wiley Interscience: New York, NY, USA, 1971. [Google Scholar]
- Rukenstein, E.; Lee, S.H. Estimation of the equilibrium surface free energy components of restructuring solid surfaces. J. Coll. Inter. Sci. 1987, 120, 153–161. [Google Scholar] [CrossRef]
- Bushuk, W.; Benoit, H. Light scattering studies of copolymers: I. effect of heterogeneity of chain composition on the molecular weight. Canad. J. Chem. 1958, 36, 1616–1626. [Google Scholar] [CrossRef]
- Kratochvil, P. Classical Light Scattering from Polymer Solution, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 1–346. [Google Scholar]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions, 1st ed.; Springer: Berlin, Germany, 2007; pp. 1–187. [Google Scholar]
- Scholz, C. Polymer for Biomedicine. Synthesis, Characterization, and Applications; John Wiley & Sons Ltd: New York, NY, USA, 2017. [Google Scholar]
- Pan, W.; Chen, H.; Wen, G.; Giaouzi, D.; Pispas, S.; Zuo, J. Surface micelle structures and monolayer compression moduli of double hydrophilic block copolymer. J. Phys. Chem. C. 2020, 124, 17150–17157. [Google Scholar] [CrossRef]
- Perepichka, I.I.; Lu, Q.; Badia, A.; Geraldine, C.B. Understanding and controlling morphology formation in Langmuir-Blodgett block copolymer films using PS-P4VP and PS-P4VP/PDP. Langmuir 2013, 29, 4502–4519. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Graf, K.; Kresseler, J. Langmuir monolayer and Langmuir-Blodgett films of amphiphilic triblock copolymers with water-soluble middle block. Colloid Polym. Sci. 2006, 285, 27–37. [Google Scholar]
- Robert, B.; Moffitt, C.M.G. Novel two-dimensional “ring and chain” morphologies in Langmuir-Blodgett monolayers of PS-b-PEO block copolymers: Effect of spreading solution concentration on self-assembly at the air-water interface. Langmuir 2005, 21, 5453–5460. [Google Scholar] [CrossRef]
- Rodriguez-Parada, J.M.; Kaku, M.; Sogah, D.Y. Monolayers and Langmuir-Blodgett films of poly(N-acylethylenimines) with hydrocarbon and fluorocarbon side chains. Macromolecules 1994, 27, 1571–1577. [Google Scholar] [CrossRef]
- Choi, M.; Chung, B.; Chun, B.; Chang, T. Surface micelle formation of polystyrene-b-poly(2-vynylpyridine) diblock copolymer at air-water interface. Macromol. Res. 2004, 12, 127–133. [Google Scholar]
- Claro, P.C.S.; Coustet, M.E.; Diaz, C.; Maza, E.; Cortizo, M.S.; Requejo, F.G.; Pietrasanta, L.I.; Ceolín, M.; Azzaroni, O. Self-assembly of PBzMA-b-PDMAEMA diblock copolymer films at the air-water interface and deposition on solid substrates via Langmuir-Blodgett transfer. Soft Matter. 2013, 9, 10891–10898. [Google Scholar] [CrossRef]
- Hood, J.; Van Gordon, K.; Thomson, P.; Coleman, B.R.; Burns, F.; Moffitt, M.G. Structural hierarchy in blends of amphiphilic block copolymers self-assembled at the air-water interface. J. Colloid Interface Sci. 2019, 556, 392–440. [Google Scholar] [CrossRef]


| Solvent | MM × 10−3, g/mol | Rh, nm | dn/dc, cm3/g |
|---|---|---|---|
| PNIPAAm–Ge(C6F5)2H | |||
| Chloroform | 52 a/44 b | 4.2 | 0.060 |
| Chloroform/Methanol (9/1) | 43 | 6.2 | 0.075 |
| PFMA | |||
| Methanol | 200 a | 14 | 0.080 |
| PNIPAAm–Ge(C6F5)2–PFMA | |||
| Chloroform/Methanol (9/1) | - | 40 | 0.040 |
| THF | 490 c | 10/100 | 0.010 |
| Polymer | Chemical Shifts of Protons (ppm) | ||||
| –N–CH< | –CH2– Main Chain | –CH3 | –O–CH2–CF2– | –CF2H | |
| PNIPAAm–Ge(C6F5)2H | 3.96 | 1.15–1.7 | 0.87, 1.15 | – | – |
| PNIPAAm–Ge(C6F5)2–PFMA | 4.42 | 1.15–1.7 | 0.97, 1.13 | 4.59 | 6.20 |
| Chemical shifts of carbon atom (ppm) | |||||
| –C(O)– | –NH–CH< | –CH< | –CH3 | ||
| PNIPAAm–Ge(C6F5)2H | 174.75 | 47.60 | 41.11 | 21.29 | |
| PNIPAAm–Ge(C6F5)2–PFMA | 175.72 | 47.60 | 44.82 | 18.11 | |
| Vs.sola (μL) | A0 b (m2/mg) | πmax c (mN/m) | |
|---|---|---|---|
| pH = 7.0 | |||
| 20 | 0.4 | 13 | 5.7 |
| 30 | 0.6 | 47 | 3.0 |
| 50 | 0.3 | 52 | 5.6 |
| 60 | 0.3 | 47 | 2.3 |
| pH = 1.3 | |||
| 20 | 0.4 | 33 | 7.8 |
| 30 | 0.4 | 61 | 5.1 |
| 50 | 0.4 | 48 | 4.4 |
| 60 | 0.3 | 53 | 5.6 |
| Polymer | θ (±1), ° | ∆θ, ° | Gibbs Surface Energy (mJ/m2) | ||||
|---|---|---|---|---|---|---|---|
| H2O | CH2I2 | H2O | CH2I2 | γs c | |||
| PNIPAAm–Ge(C6F5)2H | 34 | 48 | 6 | 1 | 34.3 | 28.2 | 62.5 |
| PNIPAAm–Ge(C6F5)2–PFMA | 64 | 61 | 20 | 1 | 17.1 | 23.9 | 41.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamyshlyayeva, O.; Shaliagina, Z.; Simonova, M.; Filippov, A.; Baten’kin, M. Properties in Langmuir Monolayers and Langmuir-Blodgett Films of a Block Copolymer Based on N-Isopropylacrylamide and 2,2,3,3-Tetrafluropropyl Methacrylate. Polymers 2022, 14, 5193. https://doi.org/10.3390/polym14235193
Zamyshlyayeva O, Shaliagina Z, Simonova M, Filippov A, Baten’kin M. Properties in Langmuir Monolayers and Langmuir-Blodgett Films of a Block Copolymer Based on N-Isopropylacrylamide and 2,2,3,3-Tetrafluropropyl Methacrylate. Polymers. 2022; 14(23):5193. https://doi.org/10.3390/polym14235193
Chicago/Turabian StyleZamyshlyayeva, Olga, Zarina Shaliagina, Maria Simonova, Alexander Filippov, and Maxim Baten’kin. 2022. "Properties in Langmuir Monolayers and Langmuir-Blodgett Films of a Block Copolymer Based on N-Isopropylacrylamide and 2,2,3,3-Tetrafluropropyl Methacrylate" Polymers 14, no. 23: 5193. https://doi.org/10.3390/polym14235193
APA StyleZamyshlyayeva, O., Shaliagina, Z., Simonova, M., Filippov, A., & Baten’kin, M. (2022). Properties in Langmuir Monolayers and Langmuir-Blodgett Films of a Block Copolymer Based on N-Isopropylacrylamide and 2,2,3,3-Tetrafluropropyl Methacrylate. Polymers, 14(23), 5193. https://doi.org/10.3390/polym14235193






