Effective and Easy Techniques of Collagen Deposition onto Polylactide Films: DC-Discharge Plasma Treatment vs. Chemical Entrapment
Abstract
1. Introduction
2. Experimental
2.1. Material
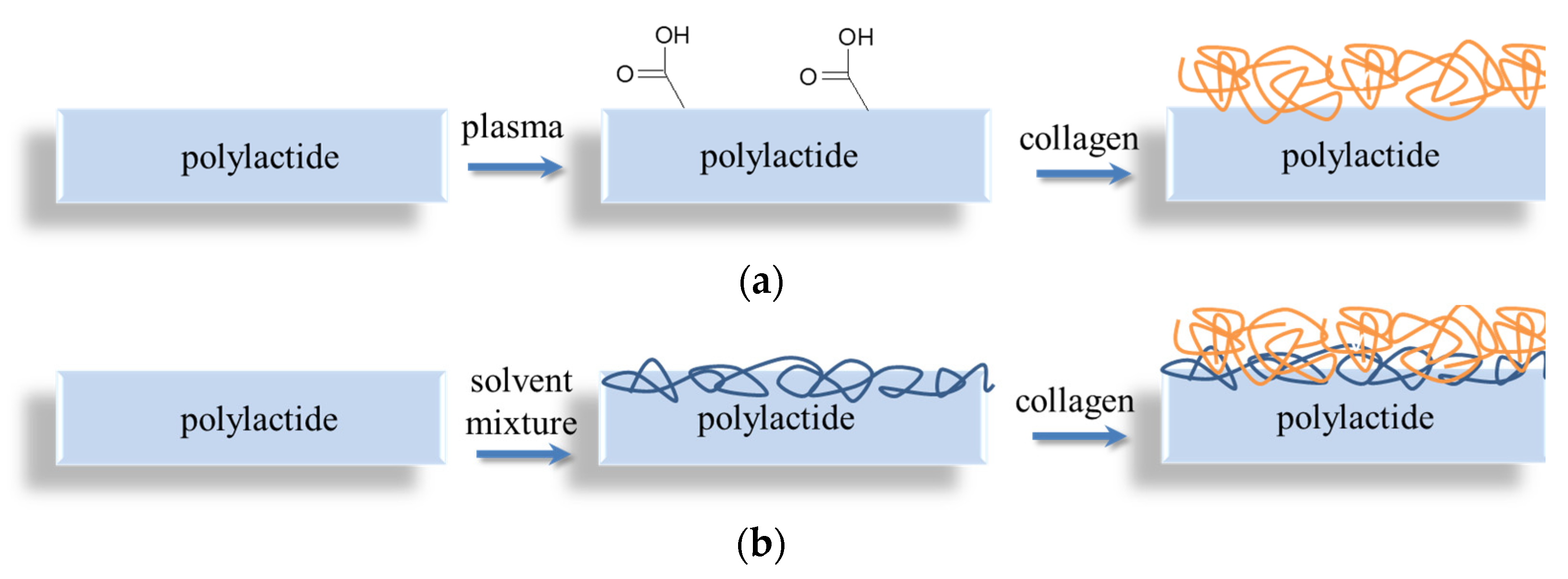
2.2. Preparation of Native and Collagen-Coated PLLA Films
2.3. Characterization of the Film Samples
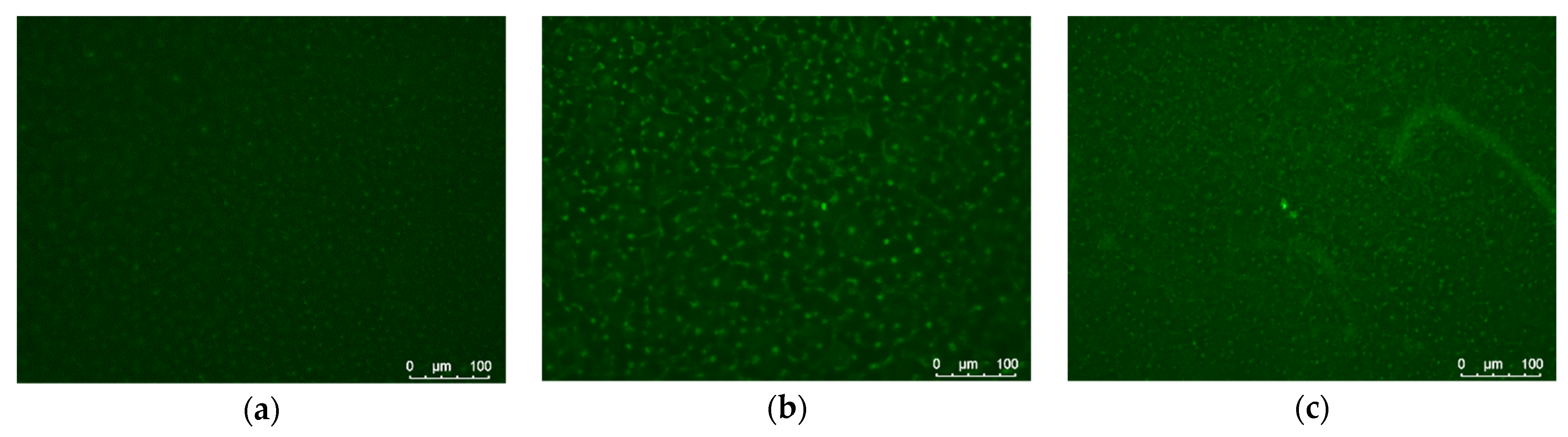
2.4. Cell Adhesion
2.5. Cytotoxicity
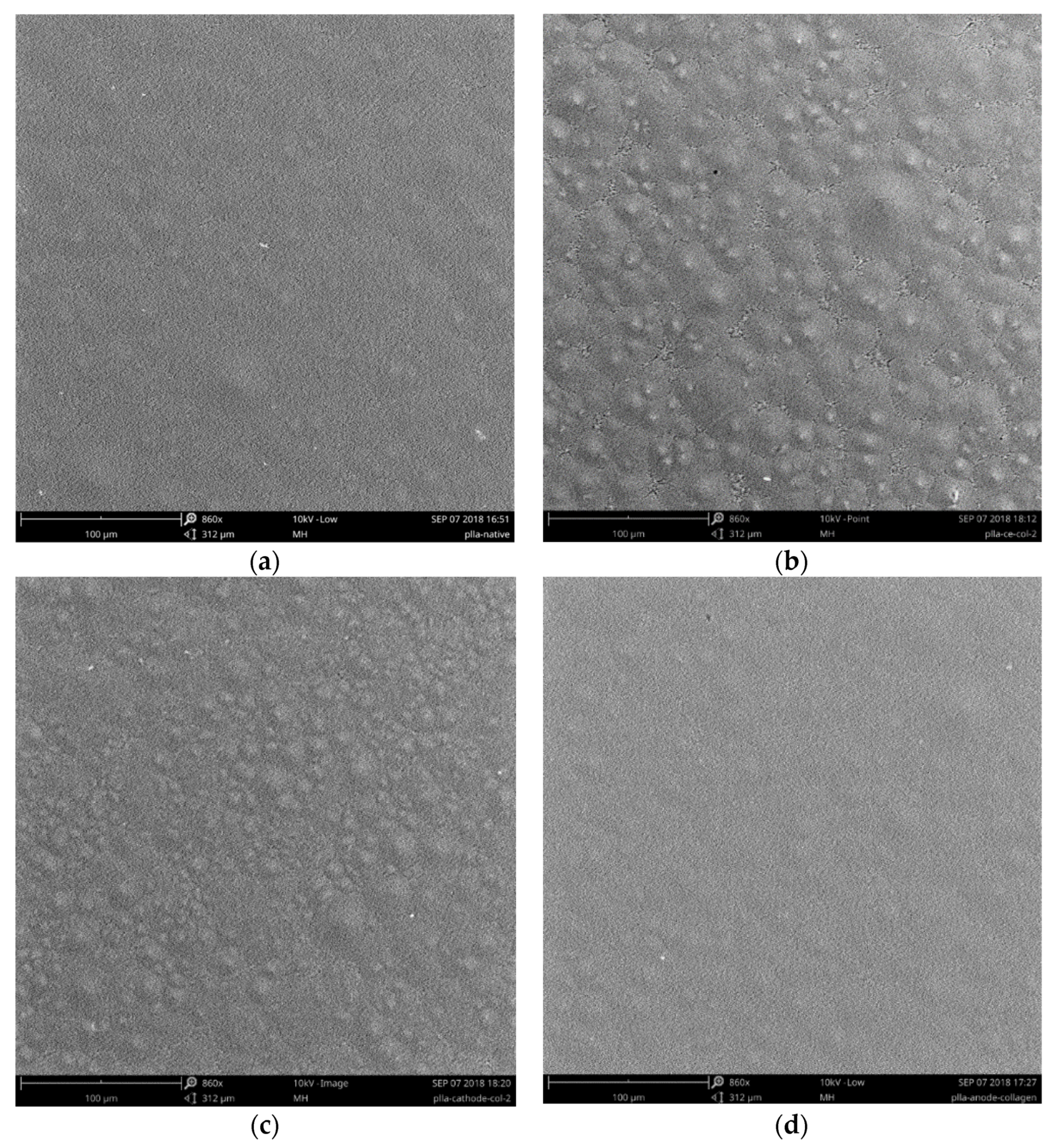
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Cell Adhesion and Cytotoxicity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.; Winkeljann, B.; Lieleg, O. Biopolymer-Based Coatings: Promising Strategies to Improve the Biocompatibility and Functionality of Materials Used in Biomedical Engineering. Adv. Mater. Interfaces 2020, 7, 2000850. [Google Scholar] [CrossRef]
- Bu, Y.; Ma, J.; Bei, J.; Wang, S. Surface Modification of Aliphatic Polyester to Enhance Biocompatibility. Front. Bioeng. Biotechnol. 2019, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Mao, Z.; Gao, C. Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloids Surf. B Biointerfaces 2007, 60, 137–157. [Google Scholar] [CrossRef]
- Demina, T.S.; Frolova, A.A.; Istomin, A.V.; Kotova, S.L.; Piskarev, M.S.; Bardakova, K.N.; Yablokov, M.Y.; Altynov, V.A.; Kravets, L.I.; Gilman, A.B.; et al. Coating of polylactide films by chitosan: Comparison of methods. J. Appl. Polym. Sci. 2020, 137, 48287. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Rogachev, A.V.; Yarmolenko, V.A.; Rogachev, A.A.; Pyzh, A.E.; Jiang, X.; Yarmolenko, M.A. Features of electron beam deposition of polymer coatings with the prolonged release of the drug component. Mater. Sci. Eng. C 2020, 110, 110730. [Google Scholar] [CrossRef]
- Tambe, N.; Di, J.; Zhang, Z.; Bernacki, S.; El-Shafei, A.; King, M.W. Novel genipin-collagen immobilization of polylactic acid (PLA) fibers for use as tissue engineering scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1188–1197. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, X.; He, T.; Shen, J. Immobilization of Biomacromolecules onto Aminolyzed Poly(L-lactic acid) toward Acceleration of Endothelium Regeneration. Tissue Eng. 2004, 10, 53–61. [Google Scholar] [CrossRef]
- Goreninskii, S.I.; Guliaev, R.O.; Stankevich, K.S.; Danilenko, N.V.; Bolbasov, E.N.; Golovkin, A.S.; Mishanin, A.I.; Filimonov, V.D.; Tverdokhlebov, S.I. “Solvent/non-solvent” treatment as a method for non-covalent immobilization of gelatin on the surface of poly(L-lactic acid) electrospun scaffolds. Colloids Surf. B Biointerfaces 2019, 177, 137–140. [Google Scholar] [CrossRef]
- Cui, Y.L.; Qi, A.D.; Liu, W.G.; Wang, X.H.; Wang, H.; Ma, D.M.; Yao, K.D. Biomimetic surface modification of poly(L-lactic acid) with chitosan and its effects on articular chondrocytes in vitro. Biomaterials 2003, 24, 3859–3868. [Google Scholar] [CrossRef]
- Jacobs, T.; Morent, R.; De Geyter, N.; Dubruel, P.; Leys, C. Plasma surface modification of biomedical polymers: Influence on cell-material interaction. Plasma Chem. Plasma Process. 2012, 32, 1039–1073. [Google Scholar] [CrossRef]
- Demina, T.S.; Piskarev, M.S.; Romanova, O.A.; Gatin, A.K.; Senatulin, B.R.; Skryleva, E.A.; Zharikova, T.M.; Gilman, A.B.; Kuznetsov, A.A.; Akopova, T.A.; et al. Plasma treatment of poly(ethylene terephthalate) films and chitosan deposition: DC- vs. AC-discharge. Materials 2020, 13, 508. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, V.; Stankevich, K.; Gudima, A.; Kibler, E.; Zhukov, Y.; Bolbasov, E.; Malashicheva, A.; Zhuravlev, M.; Riabov, V.; Liu, T.; et al. Atmospheric pressure plasma assisted immobilization of hyaluronic acid on tissue engineering PLA-based scaffolds and its effect on primary human macrophages. Mater. Des. 2017, 127, 261–271. [Google Scholar] [CrossRef]
- Romanova, O.A.; Tenchurin, T.H.; Demina, T.S.; Sytina, E.V.; Shepelev, A.D.; Rudyak, S.G.; Klein, O.I.; Krasheninnikov, S.V.; Safronova, E.I.; Kamyshinsky, R.A.; et al. Non-woven bilayered biodegradable chitosan-gelatin-polylactide scaffold for bioengineering of tracheal epithelium. Cell Prolif. 2019, 52, e12598. [Google Scholar] [CrossRef] [PubMed]
- Vesel, A.; Mozetic, M. New developments in surface functionalization of polymers using controlled plasma treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Mangindaan, D.; Kuo, W.-H.; Kurniawan, H.; Wang, M.-J. Creation of biofunctionalized plasma polymerized allylamine gradients. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1361–1367. [Google Scholar] [CrossRef]
- Bardakova, K.N.; Grebenik, E.A.; Minaev, N.V.; Churbanov, S.N.; Moldagazyeva, Z.; Krupinov, G.E.; Kostjuk, S.V.; Timashev, P.S. Tailoring the collagen film structural properties via direct laser crosslinking of star-shaped polylactide for robust scaffold formation. Mater. Sci. Eng. C 2020, 107, 110300. [Google Scholar] [CrossRef]
- Demina, T.; Zaytseva-Zotova, D.; Yablokov, M.; Gilman, A.; Akopova, T.; Markvicheva, E.; Zelenetskii, A. DC discharge plasma modification of chitosan/gelatin/PLLA films: Surface properties, chemical structure and cell affinity. Surf. Coat. Technol. 2012, 207, 508–516. [Google Scholar] [CrossRef]
- Wu, S. Polymer Interface and Adhesion; CRC Press: Boca Raton, FL, USA, 2017; Volume 79, ISBN 9781351423533. [Google Scholar]
- Gorkun, A.A.; Shpichka, A.I.; Zurina, I.M.; Koroleva, A.V.; Kosheleva, N.V.; Nikishin, D.A.; Butnaru, D.V.; Timashev, P.S.; Repin, V.S.; Saburina, I.N. Angiogenic potential of spheroids from umbilical cord and adipose-derived multipotent mesenchymal stromal cells within fibrin gel. Biomed. Mater. 2018, 13, 044108. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bikmulina, P.; Kosheleva, N.; Efremov, Y.; Antoshin, A.; Heydari, Z.; Kapustina, V.; Royuk, V.; Mikhaylov, V.; Fomin, V.; Vosough, M.; et al. 3D or not 3D: A guide to assess cell viability in 3D cell systems. Soft Matter 2022. [Google Scholar] [CrossRef] [PubMed]
- Nakamatsu, J.; Delgado-Aparicio, L.F.; Da Silva, R.; Soberon, F. Ageing of plasma-treated poly(tetrafluoroethylene) surfaces. J. Adhes. Sci. Technol. 1999, 13, 753–761. [Google Scholar] [CrossRef]
- Sharif, S.; Ai, J.; Azami, M.; Verdi, J.; Atlasi, M.A.; Shirian, S.; Samadikuchaksaraei, A. Collagen-coated nano-electrospun PCL seeded with human endometrial stem cells for skin tissue engineering applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
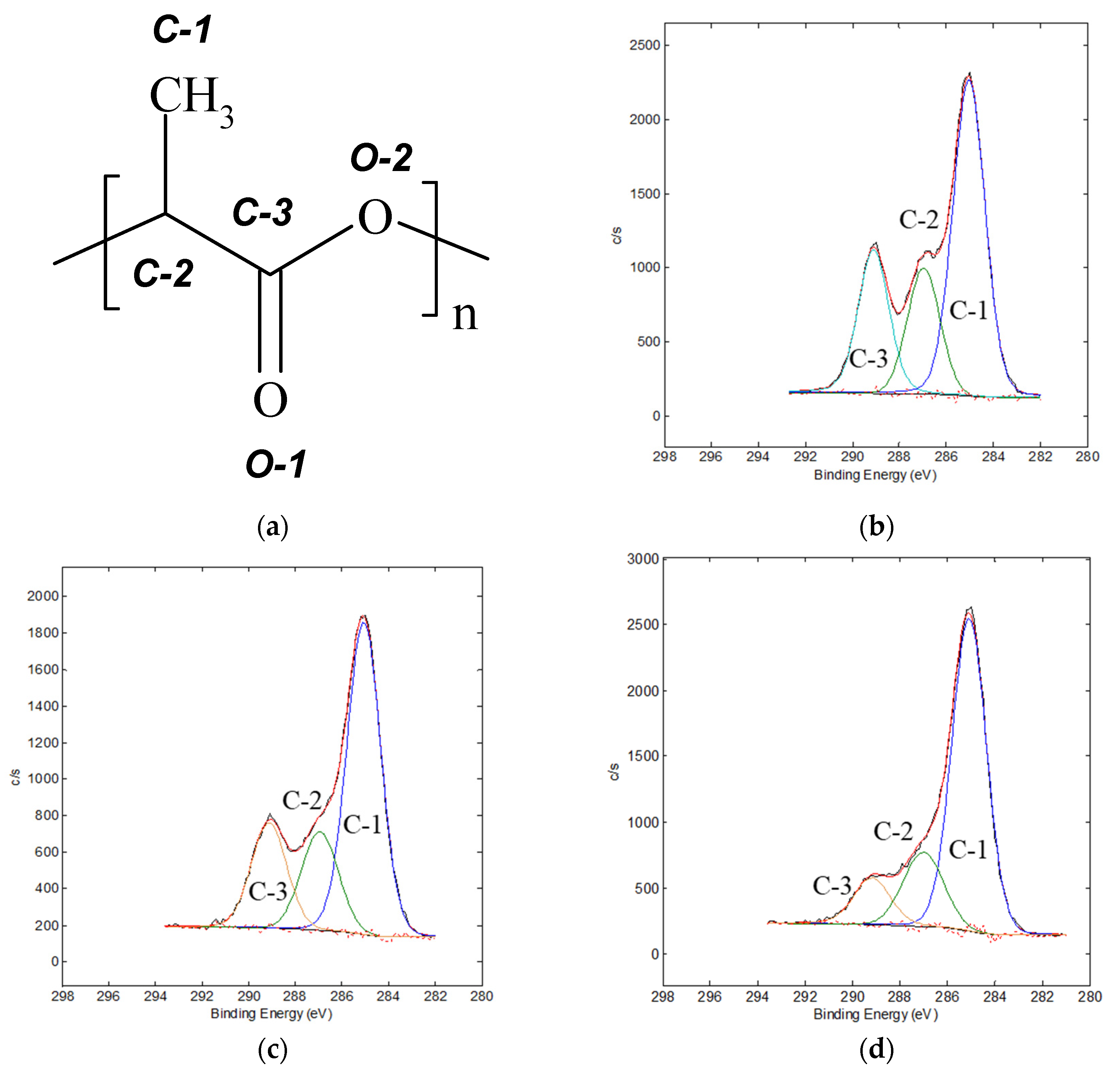


| Sample | Atomic Concentration, % | ||||
|---|---|---|---|---|---|
| C | O | N | Al | O/C | |
| PLA | 67.0 | 33.0 | - | - | 0.49 |
| PLA-C | 61.5 | 34.2 | 2.0 | 2.3 | 0.56 |
| PLA-A | 72.5 | 23.8 | 2.5 | 1.2 | 0.33 |
| Sample | Parameter | C1s | O1s | N1s | |||
|---|---|---|---|---|---|---|---|
| C-1 | C-2 | C-3 | O-1 | O-2 | |||
| PLA | Binding energy, eV | 285.0 | 286.9 | 289.1 | 532.2 | 533.6 | − |
| Peak half-width, eV | 1.61 | 1.63 | 1.54 | 1.63 | 1.86 | − | |
| area, % | 55 | 21 | 24 | 46 | 54 | − | |
| PLA-C | Binding energy, eV | 285.0 | 286.9 | 289.1 | 532.3 | 400.3 | |
| Peak half-width, eV | 1.71 | 1.87 | 1.79 | − | − | − | |
| area, % | 60 | 20 | 20 | − | − | − | |
| PLA-A | Binding energy, eV | 285.0 | 286.9 | 289.1 | 532.7 | 400.4 | |
| Peak half-width, eV | 1.71 | 2.0 | 1.8 | − | − | − | |
| area, % | 70 | 19 | 11 | − | − | − | |
| Sample | θ, deg. | Wa, mJ/m2 | γ, mJ/m2 | ||||
|---|---|---|---|---|---|---|---|
| Water | Glycerol | Water | Glycerol | γ | γp | γd | |
| PLA | 75 | 71 | 91.6 | 84.0 | 29.0 | 18.0 | 11.0 |
| PLA-C | 11 | 56 | 144.3 | 98.9 | 115.3 | 114.5 | 0.8 |
| PLA-A | 12 | 37 | 144.0 | 114.0 | 81.7 | 78.1 | 3.6 |
| Sample | θ, deg. | Wa, mJ/m2 | γ, mJ/m2 | ||||
|---|---|---|---|---|---|---|---|
| Water | Glycerol | Water | Glycerol | γ | γp | γd | |
| PLA | 75 | 71 | 91.6 | 84.0 | 29.0 | 18.0 | 11.0 |
| PLA-C-col | 60 | 43 | 109.2 | 109.8 | 48.0 | 15.2 | 32.8 |
| PLA-A-col | 60 | 33 | 109.2 | 97.7 | 40.9 | 28.6 | 12.3 |
| PLA-S-col | 73 | 63 | 94.1 | 92.8 | 33.5 | 13.3 | 20.2 |
| Collagen | 60 | 56 | 109.5 | 113.5 | 52.8 | 12.3 | 40.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demina, T.S.; Piskarev, M.S.; Birdibekova, A.V.; Veryasova, N.N.; Shpichka, A.I.; Kosheleva, N.V.; Gatin, A.K.; Skryleva, E.A.; Istranova, E.V.; Gilman, A.B.; et al. Effective and Easy Techniques of Collagen Deposition onto Polylactide Films: DC-Discharge Plasma Treatment vs. Chemical Entrapment. Polymers 2022, 14, 4886. https://doi.org/10.3390/polym14224886
Demina TS, Piskarev MS, Birdibekova AV, Veryasova NN, Shpichka AI, Kosheleva NV, Gatin AK, Skryleva EA, Istranova EV, Gilman AB, et al. Effective and Easy Techniques of Collagen Deposition onto Polylactide Films: DC-Discharge Plasma Treatment vs. Chemical Entrapment. Polymers. 2022; 14(22):4886. https://doi.org/10.3390/polym14224886
Chicago/Turabian StyleDemina, Tatiana S., Mikhail S. Piskarev, Aisylu V. Birdibekova, Nadezhda N. Veryasova, Anastasia I. Shpichka, Nastasia V. Kosheleva, Andrey K. Gatin, Elena A. Skryleva, Elena V. Istranova, Alla B. Gilman, and et al. 2022. "Effective and Easy Techniques of Collagen Deposition onto Polylactide Films: DC-Discharge Plasma Treatment vs. Chemical Entrapment" Polymers 14, no. 22: 4886. https://doi.org/10.3390/polym14224886
APA StyleDemina, T. S., Piskarev, M. S., Birdibekova, A. V., Veryasova, N. N., Shpichka, A. I., Kosheleva, N. V., Gatin, A. K., Skryleva, E. A., Istranova, E. V., Gilman, A. B., Akopova, T. A., & Timashev, P. S. (2022). Effective and Easy Techniques of Collagen Deposition onto Polylactide Films: DC-Discharge Plasma Treatment vs. Chemical Entrapment. Polymers, 14(22), 4886. https://doi.org/10.3390/polym14224886








