Research and Development of Red Mud and Slag Alkali Activation Light Filling Materials Preparation by Ultra-High Water Content and Analysis of Microstructure Formation Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Red Mud
2.1.2. Ground Granulated Blast Furnace Slag and Calcium Hydroxide
2.1.3. Activator
2.1.4. Foaming Agent
2.2. Sample Preparation
2.2.1. Mix Proportioning
2.2.2. Preparation and Curing of Samples
2.3. Methods
2.3.1. Compressive Strength Test
2.3.2. Dry/Wet Density Test
2.3.3. Microscopic Analysis
Mercury Intrusion Porosimetry
Scanning Electron Microscopy
3. Results and Discussion
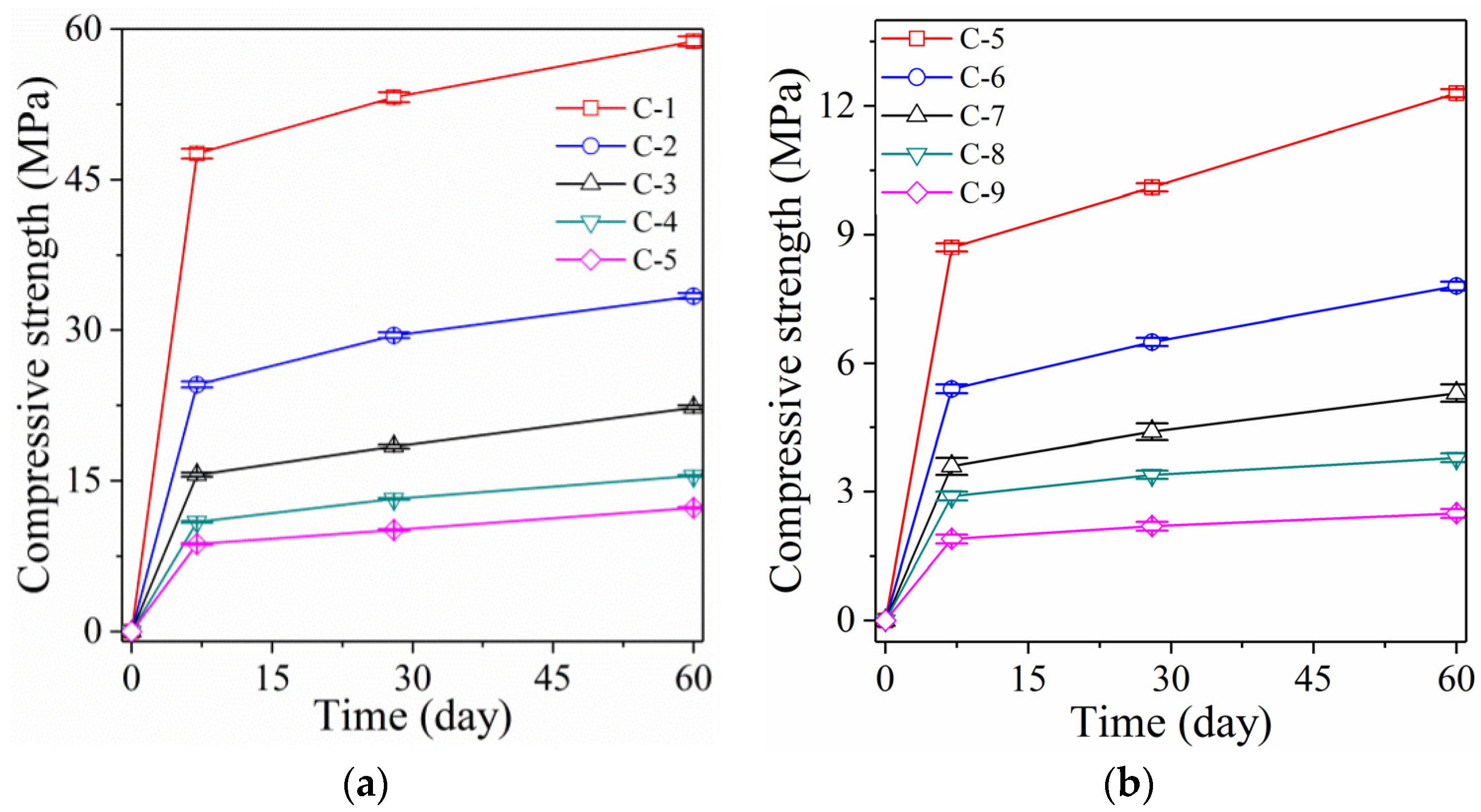
3.1. Analysis of Compressive Strength
3.1.1. Samples with High L/S
3.1.2. Samples with the Foaming Agent
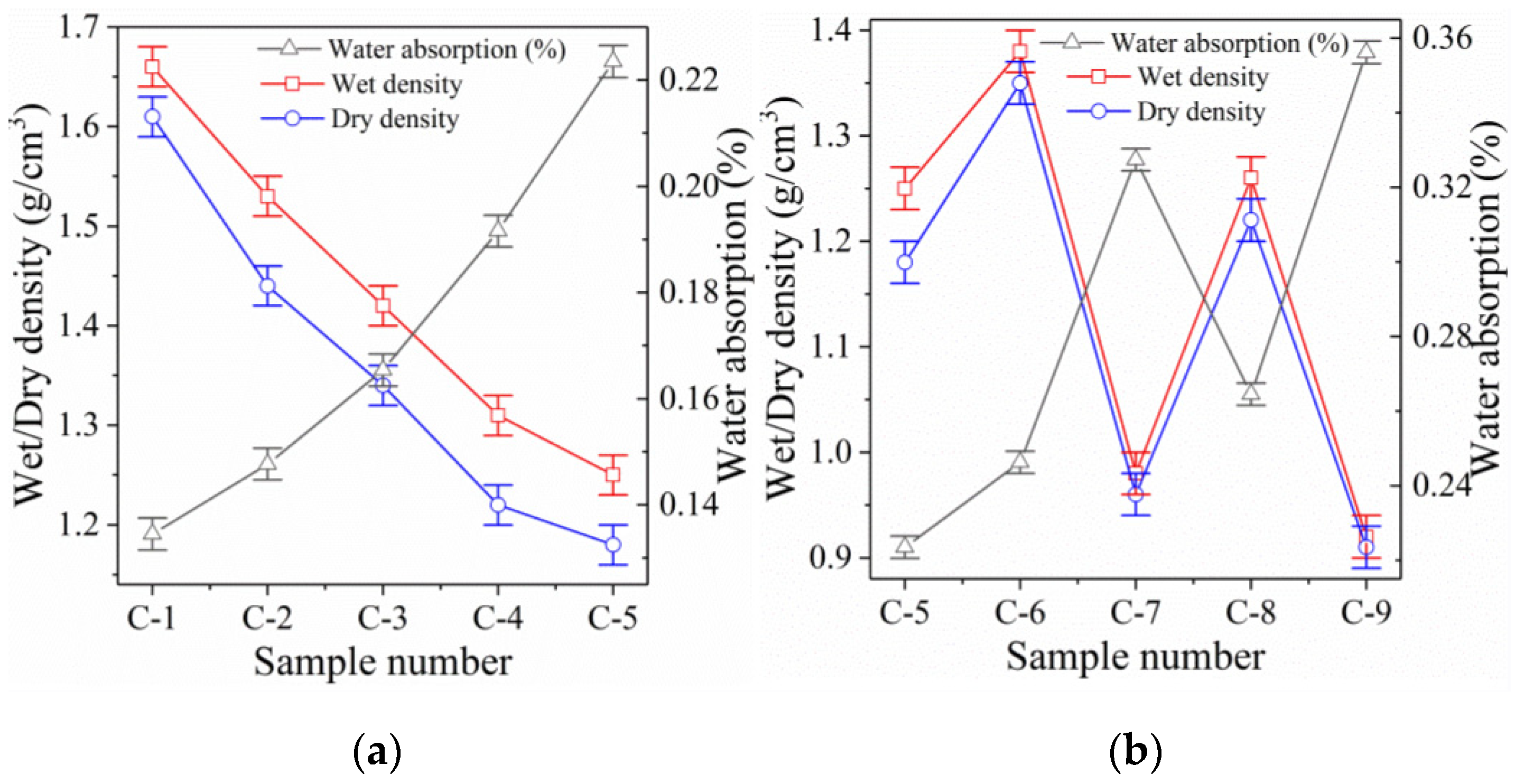
3.2. Dry/Wet Density and Water Absorption
3.2.1. Dry/Wet Density
Ultra-High L/S Samples
Foaming Samples
3.2.2. Water Absorption Analysis
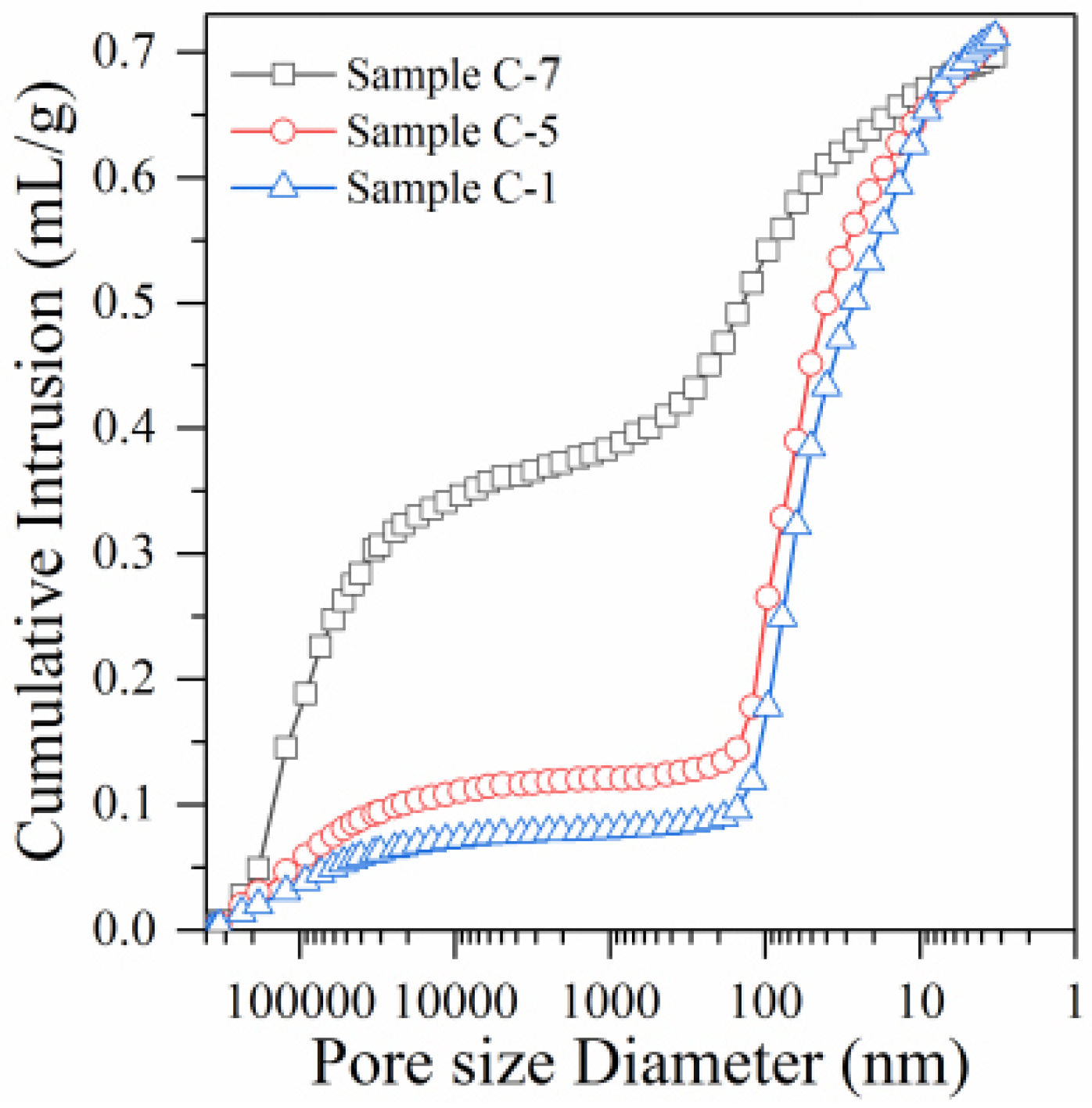
3.3. Results of the Microscopic Analysis
3.3.1. Mercury Injection Test Analysis
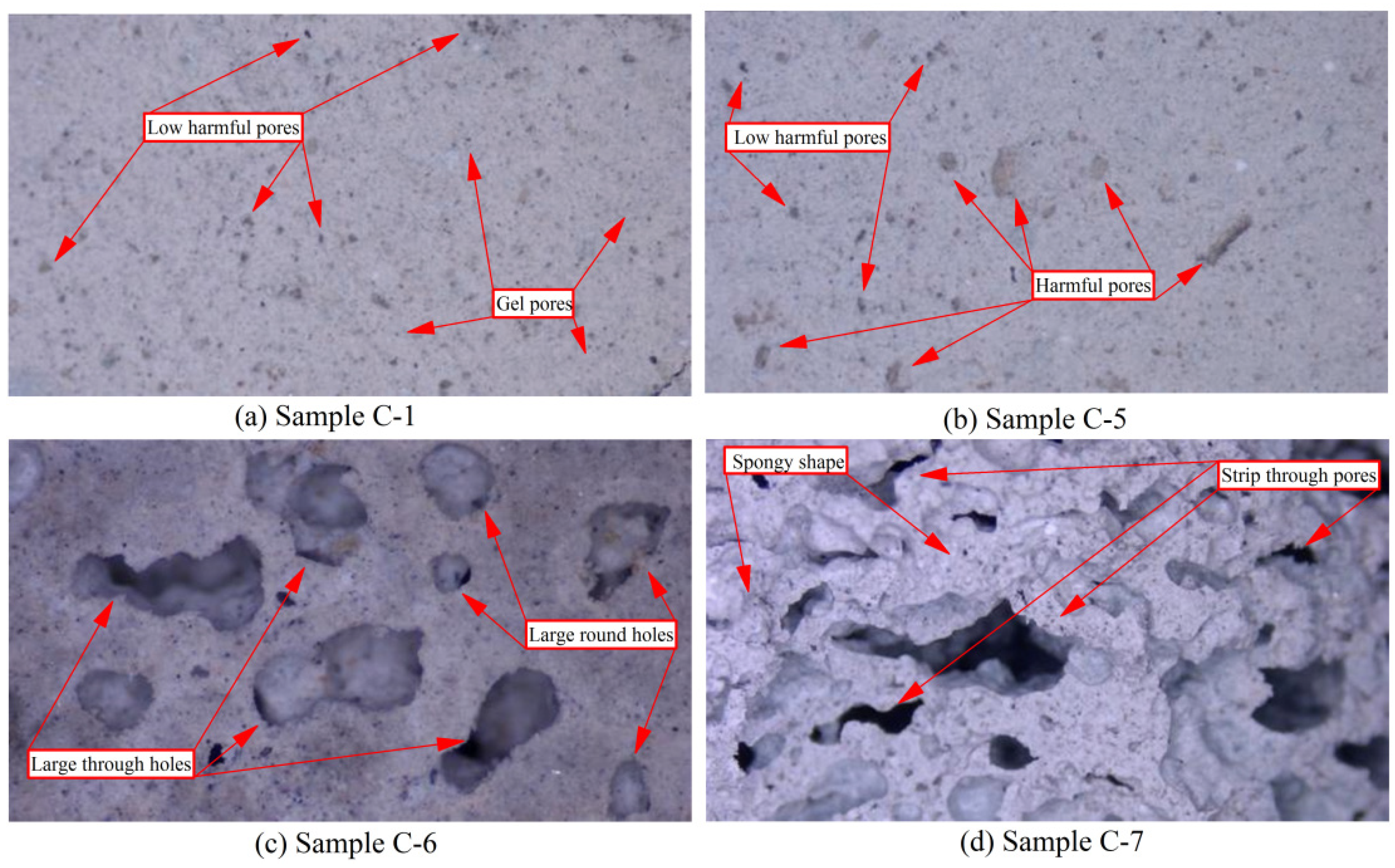
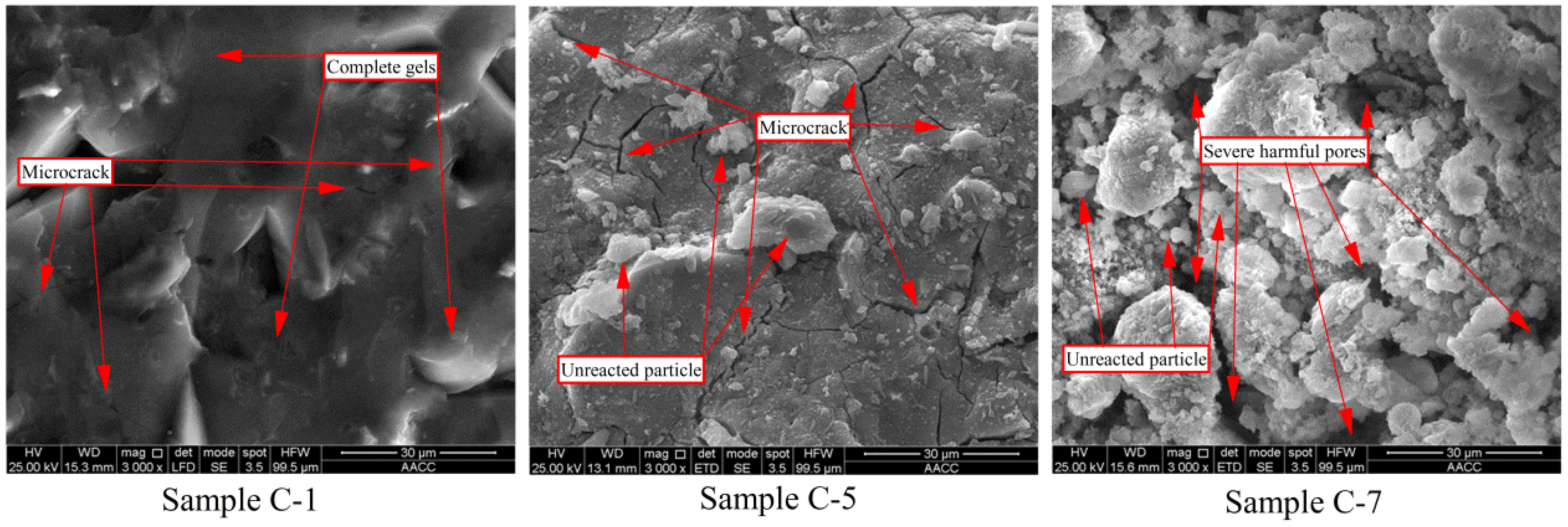
3.3.2. Scanning Electron Microscopy Analysis
Low Power Electron Microscopy Analysis (Magnification ×100)
High Power Electron Microscopy Analysis (Magnification ×3000)
4. Conclusions
- (1)
- The samples’ dry density decreases notably with the increase in L/S. At an L/S of 1.98, the dry density decreases to 1.02 g/cm3, which fully meets the requirement for lightweight materials and is even lighter than the sample prepared by adding 1 g foaming agent (1.35 g/cm3).
- (2)
- The alkali-activated RM lightweight samples prepared using the ultra-high L/S do not destroy the originally smooth and dense microstructure. This effectively alleviates mechanical properties’ deterioration and achieves a clearly better result than the lightweight samples prepared by adding a foaming agent.
- (3)
- Although increasing the L/S significantly improves the porosity of samples, it only increases the number of harmless and low-harmful pores, while the number of harmful and seriously harmful pores does not increase. This method effectively alleviates the increased water absorption and ensures that the fabricated lightweight samples have good durability compared to the lightweight samples prepared with a foaming agent.
Author Contributions
Funding
Conflicts of Interest
References
- Pundienė, I.; Pranckevičienė, J.; Kligys, M.; Kizinievič, O. The synergetic interaction of chemical admixtures on the properties of eco-friendly lightweight concrete from industrial technogenic waste. Constr. Build. Mater. 2020, 256, 119461. [Google Scholar] [CrossRef]
- Shang, X.; Li, J.; Zhan, B. Properties of sustainable cellular concrete prepared with environment-friendly capsule aggregates. J. Clean. Prod. 2020, 267, 122018. [Google Scholar] [CrossRef]
- Falliano, D.; de Domenico, D.; Ricciardi, G.; Gugliandolo, E. 3D-printable lightweight foamed concrete and comparison with classical foamed concrete in terms of fresh state properties and mechanical strength. Constr. Build. Mater. 2020, 254, 119271. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Lu, L.; Feng, D. Preparation and load-deformation characterization of carbon nanotubereinforced foam concrete. Constr. Build. Mater. 2020, 254, 119294. [Google Scholar] [CrossRef]
- Kim, J.; Chung, S.; Han, T.; Stephan, D.; Elrahman, M.A. Correlation between microstructural characteristics from micro-CT of foamed concrete and mechanical behaviors evaluated by experiments and simulations. Cem. Concr. Compos. 2020, 112, 103657. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, L.; Ren, F.; Qiu, J.; Ding, H. Rheological and mechanical properties of cemented foam backfill: Effect of mineral admixture type and dosage. Cem. Concr. Compos. 2020, 112, 103689. [Google Scholar] [CrossRef]
- Shi, X.; Huang, J.; Su, Q. Experimental and numerical analyses of lightweight foamed concrete as filler for widening embankment. Constr. Build. Mater. 2020, 250, 118897. [Google Scholar] [CrossRef]
- Bhosale, A.; Zade, N.P.; Sarkar, P.; Davis, R. Mechanical and physical properties of cellular lightweight concrete block masonry. Constr. Build. Mater. 2020, 248, 118621. [Google Scholar] [CrossRef]
- Raj, B.; Sathyan, D.; Madhavan, M.K.; Raj, A. Mechanical and durability properties of hybrid fiber reinforced foam concrete. Constr. Build. Mater. 2020, 245, 118373. [Google Scholar] [CrossRef]
- Phavongkham, V.; Wattanasiriwech, S.; Cheng, T.; Wattanasiriwech, D. Effects of surfactant on thermo-mechanical behavior of geopolymer foam paste made with sodium perborate foaming agent. Constr. Build. Mater. 2020, 243, 118282. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Q.; Deng, Y. The reinforcement attributes of multi-scale hybrid fiber throughout the uniaxial compression of ultra-low-weight foamed cement-based composites. Constr. Build. Mater. 2020, 242, 118184. [Google Scholar] [CrossRef]
- Chung, S.; Kim, J.; Lehmann, C.; Stephan, D.; Han, T.; Elrahman, M.A. Investigation of phase composition and microstructure of foamed cement paste with different supplementary cementing materials. Cem. Concr. Compos. 2020, 109, 103560. [Google Scholar] [CrossRef]
- Wang, R.; Qin, L.; Gao, X. Mechanical strength and water resistance of magnesium oxysulfate cement based lightweight materials. Cem. Concr. Compos. 2020, 109, 103554. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, X.; Lu, X.; Qi, C.; Luan, B.; Liu, F. The mechanical and microstructural properties of refuse mudstone-GGBS-red mud based geopolymer composites made with sand. Constr. Build. Mater. 2020, 253, 119193. [Google Scholar] [CrossRef]
- Wang, F.; Pan, H.; Xu, J. Evaluation of red mud based binder for the immobilization of copper, lead and zinc. Environ. Pollut. 2020, 263, 114416. [Google Scholar] [CrossRef]
- Salih, W.T.; Yu, W.; Dong, X.; Hao, W. Study on stress-strain-resistivity and microscopic mechanism of red mud waste modified by desulphurization gypsum-fly ash under drying-wetting cycles. Constr. Build. Mater. 2020, 249, 118772. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Shi, C.; Fernández-Jiménez, A.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Puertas, F.; González-Fonteboa, B.; González-Taboada, I.; Alonso, M.M.; Torres-Carrasco, M.; Rojo, G.; Martínez-Abella, F. Alkali-activated slag concrete: Fresh and hardened behavior. Cem. Concr. Compos. 2018, 85, 22–31. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- GB/T18046-2008; Ground Granulated Blast Furnace Slag Used for Cement and Concrete. AQSIQ: Beijing, China, 2008.
- GB/T17671-2021; Method of Testing Cements—Determination of Strength–ISO China. AQSIQ: Beijing, China, 2021.
- GB/T11969-2008; Test Methods for Autoclaved Aerated Concrete. AQSIQ: Beijing, China, 2008.
- Huang, G.; Ji, Y.; Li, J.; Zhang, L.; Liu, X.; Liu, B. Effect of activated silica on polymerization mechanism and strength development of MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2019, 201, 90–99. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, L.; Zheng, K.; Ling, C.; Zhu, H.; Liu, H.; Wang, P.; Li, K.; Liu, Z.; Wang, M. Electrical resistivity and capillary absorption in mortar with styrene-acrylic emulsion and air-entrained agent: Improvement and correlation with pore structure. Constr. Build. Mater. 2020, 255, 119287. [Google Scholar] [CrossRef]
- Gui, Q.; Qin, M.; Li, K. Gas permeability and electrical conductivity of structural concretes: Impact of pore structure and pore saturation. Cem. Concr. Res. 2016, 89, 109–119. [Google Scholar] [CrossRef]
- Tang, S.; Cai, X.; He, Z.; Zhou, W.; Shao, H.; Li, Z.; Wu, T.; Chen, E. The review of pore structure evaluation in cementitious materials by electrical methods. Constr. Build. Mater. 2016, 117, 273–284. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Zhang, L.; Li, J.; Hou, Z. The influence of curing methods on the strength of MSWI bottom ash-based alkali-activated mortars: The role of leaching of OH- and free alkali. Constr. Build. Mater. 2018, 186, 978–985. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Sun, Y.; Lu, Z.; Zhang, X.; Zuo, L.; Feng, Y.; Qian, R.; Qi, Y.; Ji, Y.; et al. Influence of NaOH content on the alkali conversion mechanism in MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 248, 118582. [Google Scholar] [CrossRef]
- He, R.; Ma, H.; Hafiz, R.B.; Fu, C.; Jin, X.; He, J. Determining porosity and pore network connectivity of cement-based materials by a modified non-contact electrical resistivity measurement: Experiment and theory. Mater. Des. 2018, 156, 82–92. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-Chong, T.; Dangla, P. Pore structure of cement pastes through NAD and MIP analysis. Adv. Cem. Res. 2015, 28, 23–32. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Chen, L.; Lu, Z.; Sun, Y.; Zhang, X.; Feng, Y.; Ji, Y.; Xu, Z. Use of pretreatment to prevent expansion and foaming in highperformance MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 245, 118471. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-Chong, T.; Dangla, P. Analysis of pore structure, contact angle and pore entrapment of blended cement pastes from mercury porosimetry data. Cem. Concr. Compos. 2012, 34, 1053–1060. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, K.; Fen-chong, T.; Dangla, P. Pore structure characterization of cement pastes blended with high-volume fly-ash. Cem. Concr. Res. 2012, 42, 194–204. [Google Scholar] [CrossRef]
- Huang, G.; Yuan, L.; Ji, Y.; Liu, B.; Xu, Z. Cooperative action and compatibility between Portland cement and MSWI bottom ash alkali-activated double gel system materials. Constr. Build. Mater. 2019, 209, 445–453. [Google Scholar] [CrossRef]
- Wen, F.; Fan, H.; Zhai, S.; Zhang, K.; Liu, F. Pore characteristics analysis and numerical seepage simulation of antifreeze permeable concrete. Constr. Build. Mater. 2020, 255, 119310. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Hatanaka, S.; Chareerat, T.; Mishima, N.; Yuasa, Y. Cement paste characteristics and porous concrete properties. Constr. Build. Mater. 2008, 22, 894–901. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Zhang, L.; Hou, Z.; Zhang, L.; Wu, S. Influence of calcium content on structure and strength of MSWI bottom ash-based geopolymer. Mag. Concr. Res. 2019, 71, 362–372. [Google Scholar] [CrossRef]
- Huang, G.; Li, Y.; Zhang, Y.; Zhu, J.; Li, D.; Wang, B. Effect of Sodium Hydroxide, Liquid Sodium Silicate, Calcium Hydroxide, and Slag on the Mechanical Properties and Mineral Crystal Structure Evolution of Polymer Materials. Crystals 2021, 11, 1586. [Google Scholar] [CrossRef]
- Huang, G.; Zhu, J.; Zhang, Y.; Li, D.; Wang, B.; Li, M.; Jin, L.; Gong, J. The Effect of Slag on the Mechanical Properties of Coralline-Activated Materials and the Formation and Transformation of Mineral Crystals. Crystals 2022, 12, 470. [Google Scholar] [CrossRef]
- Huang, G.; Li, D.; Cui, Y.; Feng, J.; Gao, Q.; Lu, T.; Zhang, Y.; Zhu, J. Compressive Strength Enhancement in Early Age Acid Activated Mortars: Mechanical Properties and Analysis. Crystals 2022, 12, 804. [Google Scholar] [CrossRef]
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | Loss | |
|---|---|---|---|---|---|---|---|---|---|
| RM | 16.98 | 13.35 | 7.43 | 30.29 | 1.5 | 2.82 | 0.38 | 2.29 | 24.96 |
| GBFS | 31.35 | 18.65 | 0.57 | 34.65 | 9.31 | 1.26 | 0.84 | - | 0.7 |
| Density (g/cm3) | Water Requirement for Normal Consistency (%) | Fineness (Residual 0.08 mm Square Pore Screen %) | Specific Surface Area (m2/kg) | |
|---|---|---|---|---|
| RM | 2.42 | 112 | 0.63 | 812 |
| GBFS | 2.70 | 100 | 0.82 | 416 |
| Raw Materials | Activators | Foaming Agent and Water | L/S | |||||
|---|---|---|---|---|---|---|---|---|
| RM 45% | GBFS 50% | Ca(OH)2 5% | Water | Sodium Silicate | NaOH | |||
| C-1 | 303.75 | 337.5 | 33.75 | 180 | 180 | 30 | / | 0.44 |
| C-2 | 303.75 | 337.5 | 33.75 | 360 | 360 | 60 | / | 0.88 |
| C-3 | 303.75 | 337.5 | 33.75 | 540 | 540 | 90 | / | 1.32 |
| C-4 | 303.75 | 337.5 | 33.75 | 720 | 720 | 120 | / | 1.76 |
| C-5 | 303.75 | 337.5 | 33.75 | 810 | 810 | 135 | / | 1.98 |
| C-6 | 303.75 | 337.5 | 33.75 | 180 | 180 | 30 | 1 + 30 | 0.44 |
| C-7 | 303.75 | 337.5 | 33.75 | 180 | 180 | 30 | 2 + 30 | 0.44 |
| C-8 | 303.75 | 337.5 | 33.75 | 330 | 360 | 60 | 1 + 30 | 0.88 |
| C-9 | 303.75 | 337.5 | 33.75 | 330 | 360 | 60 | 2 + 30 | 0.88 |
| L/S | Foaming Agent (g) | Wet Density (g/cm3) | Dry Density (g/cm3) | Density Difference (g/cm3) | Water Absorption (%) | |
|---|---|---|---|---|---|---|
| C-1 | 0.44 | 0 | 1.66 | 1.59 | 0.07 | 11.45 |
| C-2 | 0.88 | 0 | 1.53 | 1.42 | 0.11 | 12.27 |
| C-3 | 1.32 | 0 | 1.42 | 1.27 | 0.15 | 13.54 |
| C-4 | 1.76 | 0 | 1.31 | 1.13 | 0.18 | 15.16 |
| C-5 | 1.98 | 0 | 1.25 | 1.02 | 0.23 | 17.25 |
| C-6 | 0.44 | 1 | 1.38 | 1.35 | 0.03 | 24.63 |
| C-7 | 0.44 | 2 | 0.98 | 0.96 | 0.02 | 32.74 |
| C-8 | 0.88 | 1 | 1.26 | 1.22 | 0.04 | 26.45 |
| C-9 | 0.88 | 2 | 0.92 | 0.91 | 0.01 | 35.62 |
| L/S | Pore Size Range | Pore Type | Pore Ratio | Total Porosity (%) | |
|---|---|---|---|---|---|
| C-1 | 0.44 | <20 nm | 1 | 32.84 | 31.62 |
| 20–100 nm | 2 | 50.42 | |||
| 100–1000 nm | 3 | 6.21 | |||
| >1000 nm | 4 | 10.53 | |||
| C-5 | 1.98 | <20 nm | 1 | 29.32 | 46.82 |
| 20–100 nm | 2 | 45.11 | |||
| 100–1000 nm | 3 | 10.83 | |||
| >1000 nm | 4 | 14.74 | |||
| C-7 | 0.44 | <20 nm | 1 | 11.61 | 63.29 |
| 20–100 nm | 2 | 12.66 | |||
| 100–1000 nm | 3 | 22.59 | |||
| >1000 nm | 4 | 53.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Zhang, Y.; Mi, H.; Zhang, X.; Liu, M.; Fang, B.; Wang, C. Research and Development of Red Mud and Slag Alkali Activation Light Filling Materials Preparation by Ultra-High Water Content and Analysis of Microstructure Formation Mechanism. Polymers 2022, 14, 5176. https://doi.org/10.3390/polym14235176
Huang G, Zhang Y, Mi H, Zhang X, Liu M, Fang B, Wang C. Research and Development of Red Mud and Slag Alkali Activation Light Filling Materials Preparation by Ultra-High Water Content and Analysis of Microstructure Formation Mechanism. Polymers. 2022; 14(23):5176. https://doi.org/10.3390/polym14235176
Chicago/Turabian StyleHuang, Guodong, Yuting Zhang, Huafeng Mi, Xukang Zhang, Meng Liu, Bin Fang, and Chengcheng Wang. 2022. "Research and Development of Red Mud and Slag Alkali Activation Light Filling Materials Preparation by Ultra-High Water Content and Analysis of Microstructure Formation Mechanism" Polymers 14, no. 23: 5176. https://doi.org/10.3390/polym14235176
APA StyleHuang, G., Zhang, Y., Mi, H., Zhang, X., Liu, M., Fang, B., & Wang, C. (2022). Research and Development of Red Mud and Slag Alkali Activation Light Filling Materials Preparation by Ultra-High Water Content and Analysis of Microstructure Formation Mechanism. Polymers, 14(23), 5176. https://doi.org/10.3390/polym14235176






