Chitosan-Graft-Poly(acrylic acid) Superabsorbent’s Water Holding in Sandy Soils and Its Application in Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
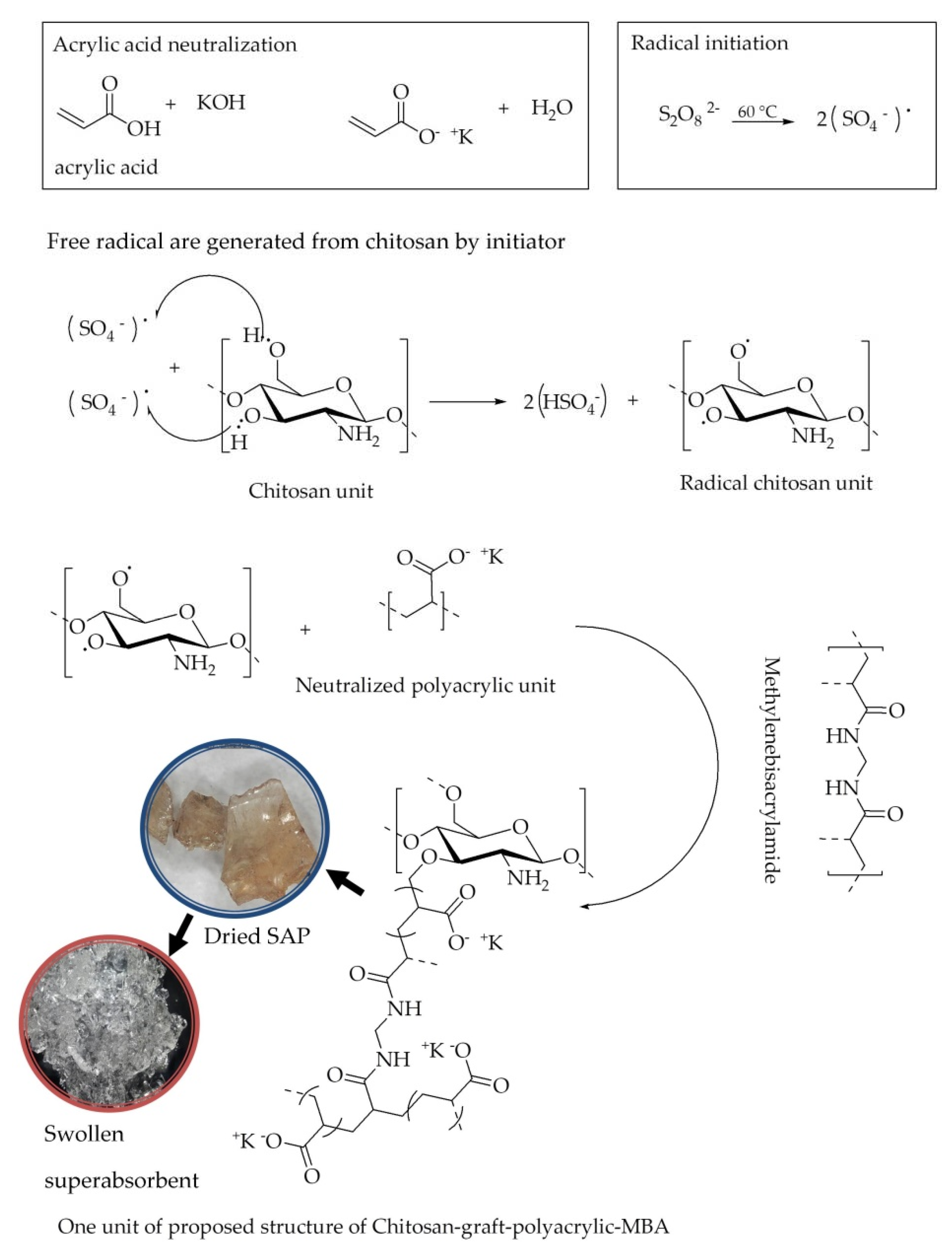
2.2. Preparation of Chitosan-Graft-Poly(acrylic acid) Superabsorbent
2.3. Determination of Gel Fraction
2.4. Measurement of Swelling and Kinetics
2.5. Reusability
2.6. Characteristics of Chitosan-Graft-Poly(acrylic acid) Superabsorbent
2.7. Water-Holding Capacity and Water Retention of Sandy Soils
3. Results and Discussion
3.1. Synthesis of Chitosan-Graft-Poly(acrylic acid) Superabsorbent
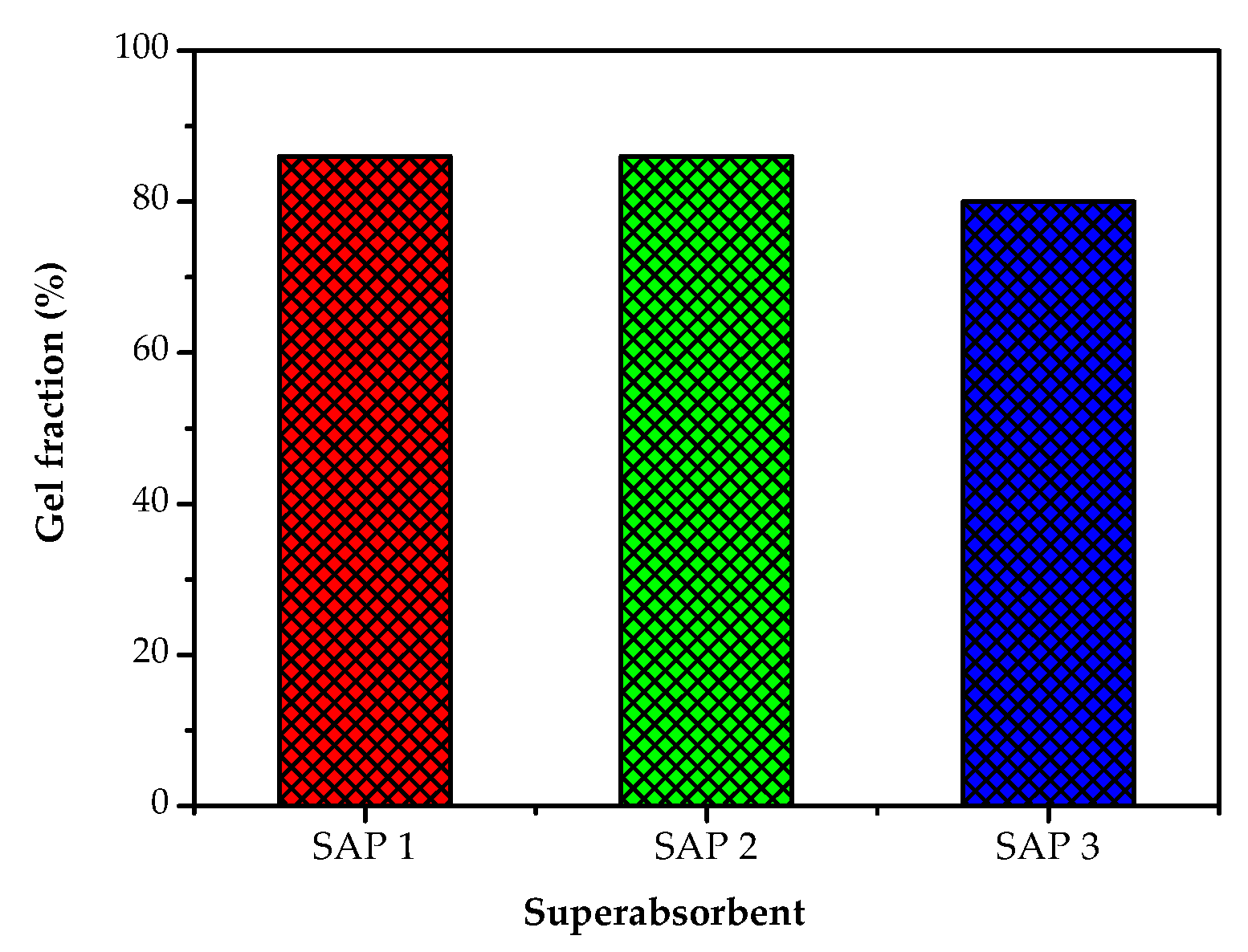
3.2. Gel Fraction
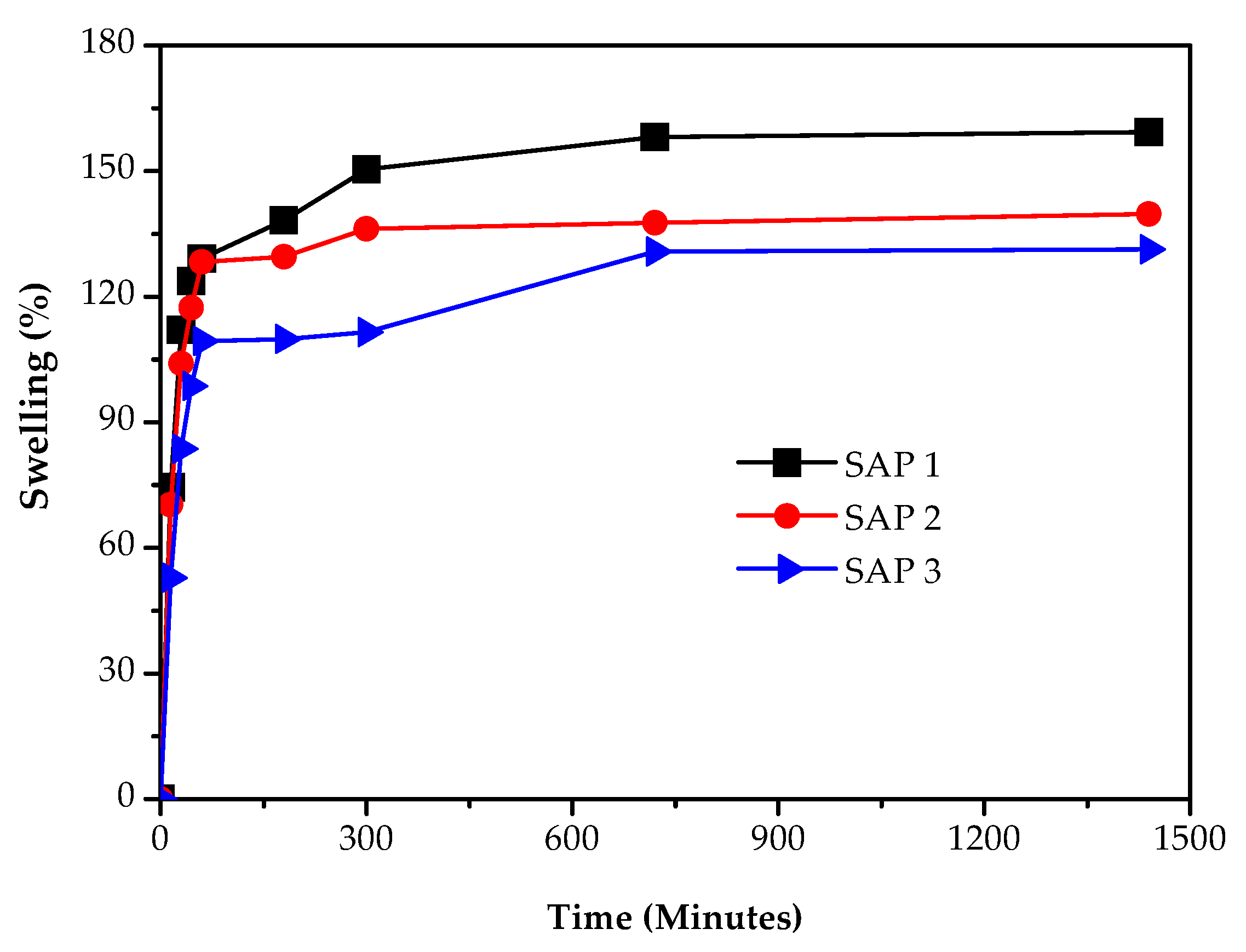
3.3. Measurements of Swelling Capacity and Kinetics
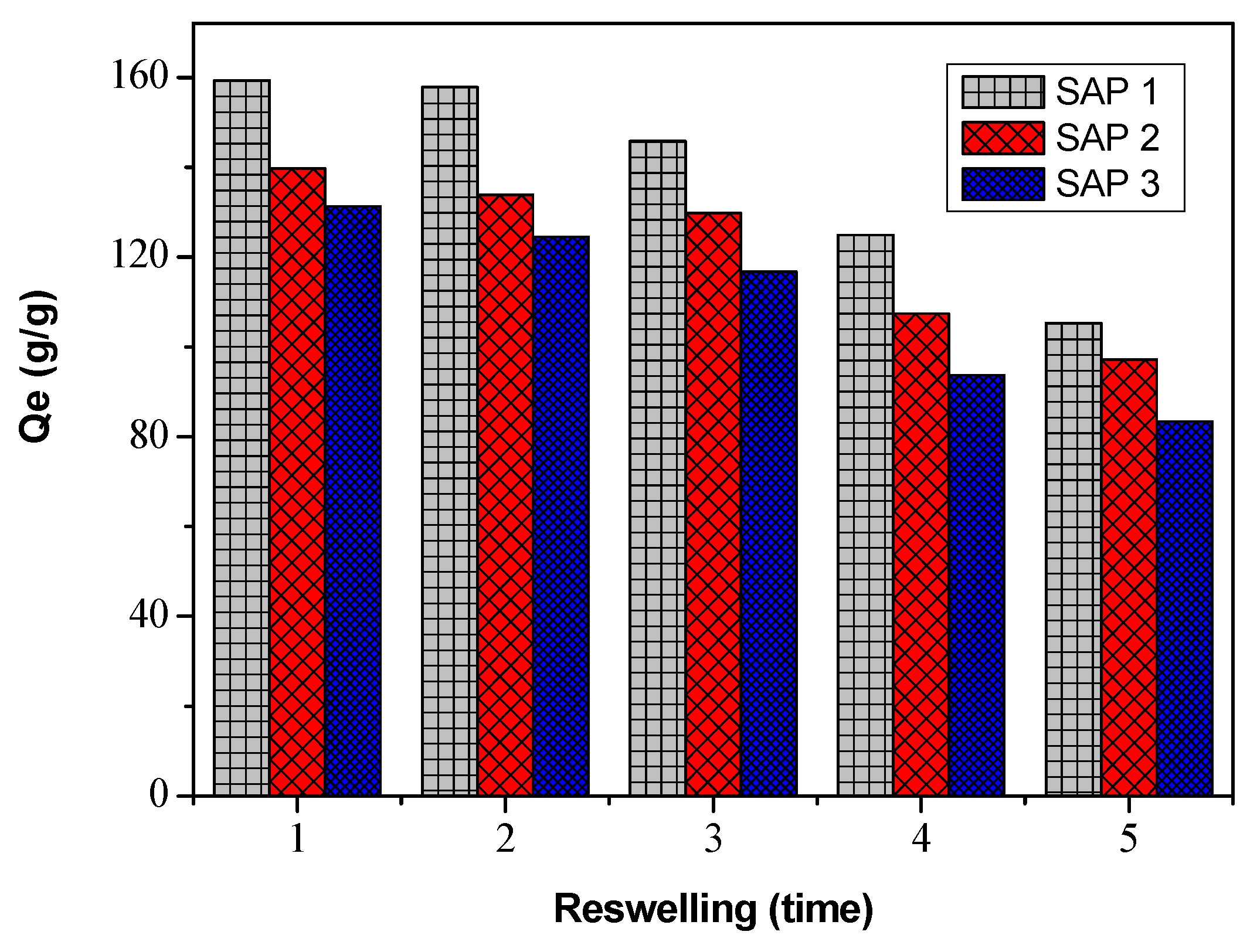
3.4. Superabsorbent Reuse
3.5. Superabsorbent Characterization
3.5.1. Scanning Electron Microscope (SEM)
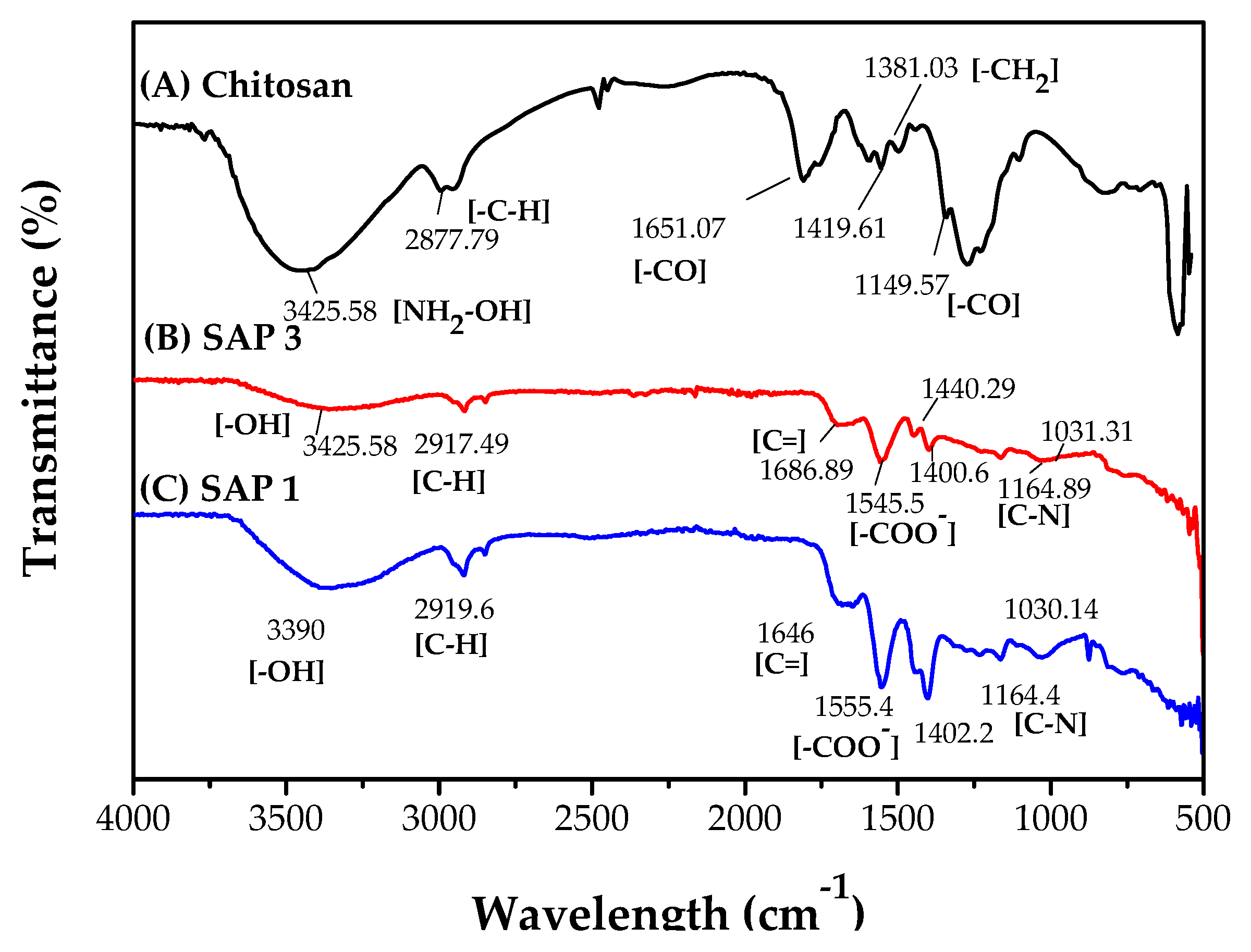
3.5.2. Fourier Transform Infrared Spectroscopy (FTIR)
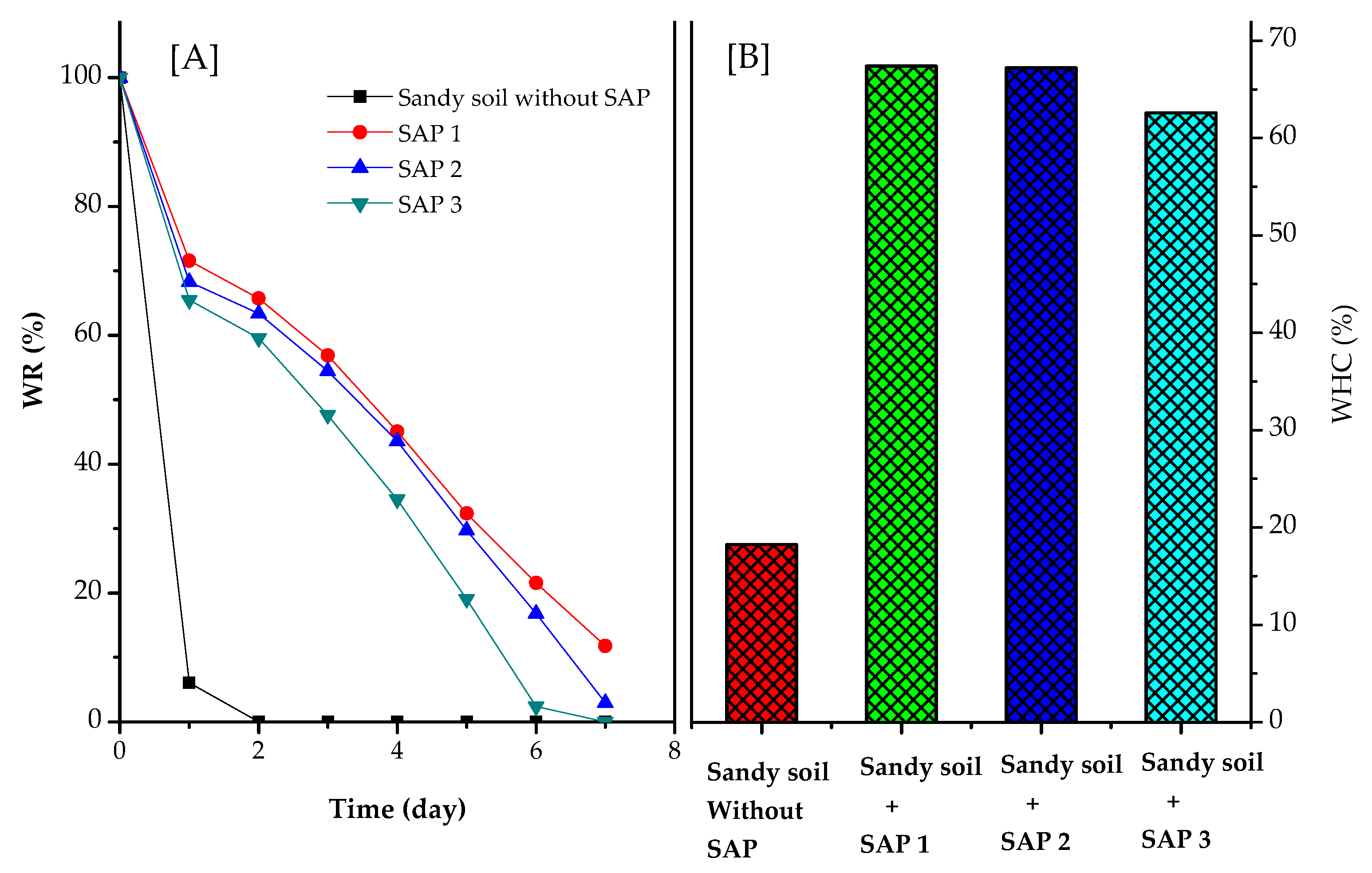
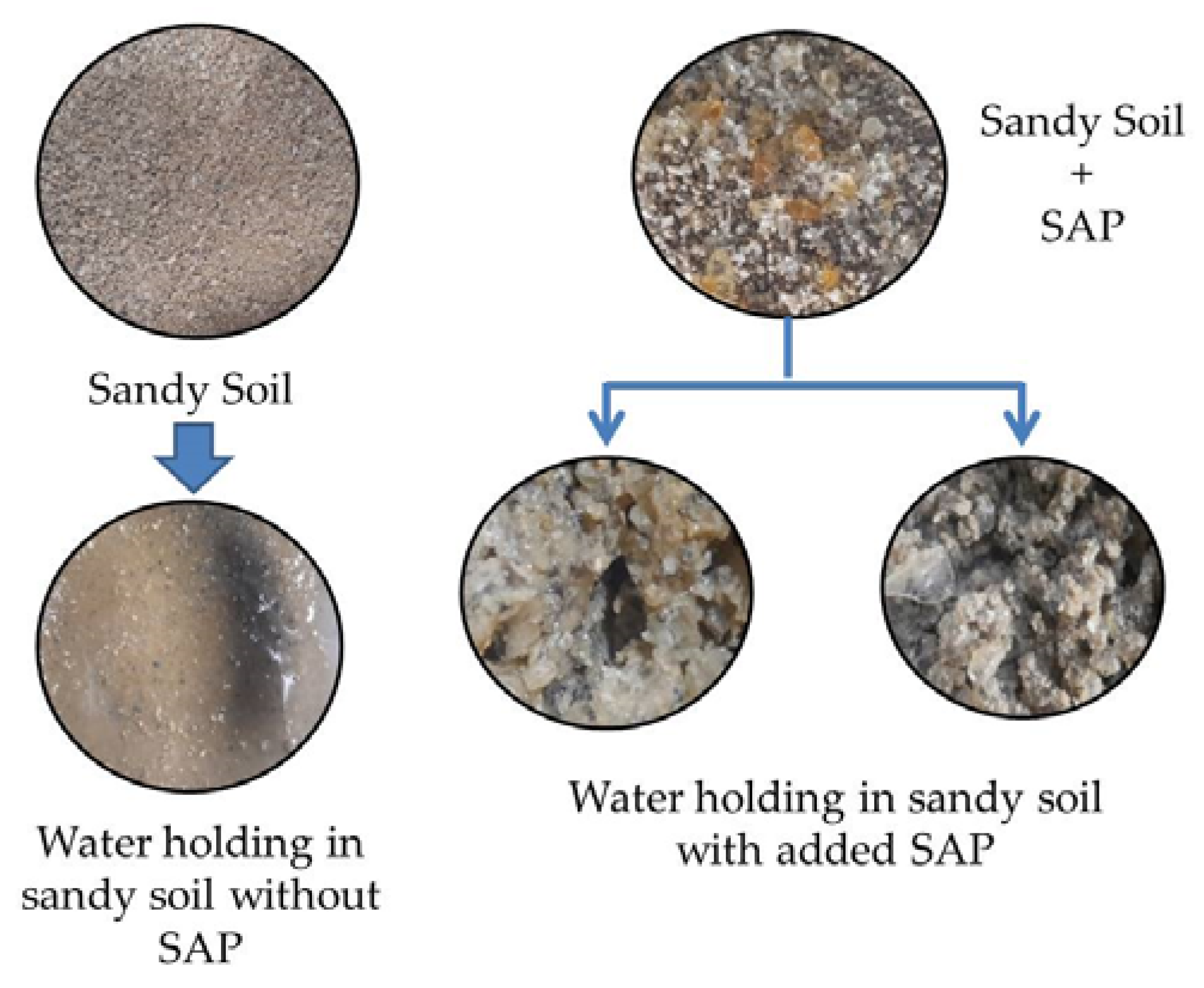
3.6. Water-Holding Capacity and Water Retention of Sandy Soils
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herawati, A.; Syamsiyah, J.; Mujiyo; Rochmadtulloh, M.; Susila, A.A.; Romadhon, M.R. Mycorrhizae and a Soil Ameliorant on Improving the Characteristics of Sandy Soil. Sains Tanah 2021, 12, 73–80. [Google Scholar] [CrossRef]
- Huang, J.; Hartemink, A.E. Soil and Environmental Issues in Sandy Soils. Earth-Sci. Rev. 2020, 208, 103295. [Google Scholar] [CrossRef]
- Mohana Raju, K.; Padmanabha Raju, M.; Murali Mohan, Y. Synthesis of Superabsorbent Copolymers as Water Manageable Materials. Polym. Int. 2003, 52, 768–772. [Google Scholar] [CrossRef]
- Abdulhameed, A.; Mbuvi, H.M.; Changamu, E.O. Synthesis of Cellulose-Based Superabsorbent Hydrogel from Rice Husk Using a Microwave. Am. J. Mater. Sci. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent Hydrogels Based on Cellulose for Smart Swelling and Controllable Delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Miljković, V.; Gajić, I.; Nikolić, L. Waste Materials as a Resource for Production of Cmc Superabsorbent Hydrogel for Sustainable Agriculture. Polymers 2021, 13, 4115. [Google Scholar] [CrossRef]
- Feng, D.; Bai, B.; Wang, H.; Suo, Y. Novel Fabrication of Biodegradable Superabsorbent Microspheres with Diffusion Barrier through Thermo-Chemical Modification and Their Potential Agriculture Applications for Water Holding and Sustained Release of Fertilizer. J. Agric. Food Chem. 2017, 65, 5896–5907. [Google Scholar] [CrossRef]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D. Superabsorbent Polymers Used for Agricultural Water Retention. Polym. Test. 2021, 94, 107021. [Google Scholar] [CrossRef]
- Narayanan, A.; Kartik, R.; Sangeetha, E.; Dhamodharan, R. Super Water Absorbing Polymeric Gel from Chitosan, Citric Acid and Urea: Synthesis and Mechanism of Water Absorption. Carbohydr. Polym. 2018, 191, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Wang, S. Thermal Preparation of Chitosan-Acrylic Acid Superabsorbent: Optimization, Characteristic and Water Absorbency. Carbohydr. Polym. 2014, 113, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Borzacchiello, A.; Ambrosio, L.; Netti, P.A.; Nicolais, L.; Peniche, C.; Gallardo, A.; Roman, J.S.A.N. Chitosan-Based Hydrogels: Synthesis and Characterization. J. Mater. Sci. Mater. Med. 2001, 2, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Pourjavadi, A.; Hosseinzadeh, H.; Zohuriaan, M.J. Modified Chitosan 4. Superabsorbent Hydrogels from Poly(Acrylic Acid-Co-Acrylamide) Grafted Chitosan with Salt- and PH-Responsiveness Properties. Eur. Polym. J. 2004, 40, 1399–1407. [Google Scholar] [CrossRef]
- Singh, V.; Tiwari, A.; Tripathi, D.N.; Sanghi, R. Microwave Enhanced Synthesis of C hitosan-Graft-Polyacrylamide. Polymer 2006, 47, 254–260. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Xing, R.; Chen, X.; Liu, S.; Qin, Y.; Li, K.; Wang, X.; Li, R.; Li, P. Synthesis of Superabsorbent Polymers Based on Chitosan Derivative Graft Acrylic Acid-Co-Acrylamide and Its Property Testing. Int. J. Biol. Macromol. 2019, 132, 575–584. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Aiouaz, N.; Dairi, N.; Hadj-Hamou, A.S. Preparation of Chitosan-g-Poly(Acrylamide)/Montmorillonite Superabsorbent Polymer Composites: Studies on Swelling, Thermal, and Antibacterial Properties. J. Appl. Polym. Sci. 2014, 131, 1–14. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Deng, Y.; He, X.; Yang, X.; Chen, R.; Lei, Z. Preparation of Superabsorbent Polymer Gel Based on PVPP and Its Application in Water-Holding in Sandy Soil. J. Environ. Chem. Eng. 2021, 9, 106760. [Google Scholar] [CrossRef]
- El-Tohamy, W.A.; El-Abagy, H.M.; Ahmed, E.M.; Aggor, F.S.; Hawash, S.I. Application of Super Absorbent Hydrogel Poly(Acrylate/Acrylic Acid) for Water Conservation in Sandy Soil. TESCE 2014, 40, 1–14. [Google Scholar]
- Erizal, E.; Perkasa, D.P.; Abbas, B.; Sudirman, S.; Sulistioso, G.S. Fast Swelling Superabsorbent Hydrogels Starch Based Prepared by Gamma Radiation Techniques. Indones. J. Chem. 2014, 14, 246–252. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Zhang, C.; Cui, B.; Li, Z.; Zhao, D.; Wang, Z. Kaolin-Enhanced Superabsorbent Composites: Synthesis, Characterization and Swelling Behaviors. Polymers 2021, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, M.; Liu, Z.; Guo, Y.; Zhang, Q. Salt-Tolerant Superabsorbent Polymer with High Capacity of Water-Nutrient Retention Derived from Sulfamic Acid-Modified Starch. ACS Omega 2019, 4, 5923–5930. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gao, B.; Zimmerman, A.R.; Zheng, Y.; Lyu, H. Novel Biochar-Impregnated Calcium Alginate Beads with Improved Water Holding and Nutrient Retention Properties. J. Environ. Manag. 2018, 209, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, Y.; Wang, A. Fast Removal of Copper Ions from Aqueous Solution by Chitosan-g-Poly(Acrylic Acid)/Attapulgite Composites. J. Hazard. Mater. 2009, 168, 970–977. [Google Scholar] [CrossRef]
- Motamedi, E.; Motesharezedeh, B.; Shirinfekr, A.; Samar, S.M. Synthesis and Swelling Behavior of Environmentally Friendly Starch-Based Superabsorbent Hydrogels Reinforced with Natural Char Nano/Micro Particles. J. Environ. Chem. Eng. 2020, 8, 103583. [Google Scholar] [CrossRef]
- Wang, X.; Lü, S.; Gao, C.; Xu, X.; Wei, Y.; Bai, X.; Feng, C.; Gao, N.; Liu, M.; Wu, L. Biomass-Based Multifunctional Fertilizer System Featuring Controlled-Release Nutrient, Water-Retention and Amelioration of Soil. RSC Adv. 2014, 4, 18382–18390. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Mahdavinia, G.R. Chitosan-g-Poly(Acrylic Acid)/Kaolin Superabsorbent Composite: Synthesis and Characterization. Polym. Polym. Compos. 2006, 14, 203–211. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.M.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent Hydrogels via Graft Polymerization of Acrylic Acid from Chitosan-Cellulose Hybrid and Their Potential in Controlled Release of Soil Nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Erizal, E.; Wikanta, T. Synthesis of Polyethylene Oxide (Peo)–Chitosan Hydrogel Prepared By Gamma Radiation Technique. Indones. J. Chem. 2011, 11, 16–20. [Google Scholar] [CrossRef]
- Saleem, A.; Akhtar, N.; Minhas, M.U.; Mahmood, A.; Khan, K.U.; Abdullah, O. Highly Responsive Chitosan-Co-Poly (MAA) Nanomatrices through Cross-Linking Polymerization for Solubility Improvement. Gels 2022, 8, 196. [Google Scholar] [CrossRef]
- Sutradhar, S.C.; Khan, M.M.R.; Rahman, M.M.; Dafadar, N.C. The Synthesis of Superabsorbent Polymers from a Carboxymethylcellulose/Acrylic Acid Blend Using Gamma Radiation and Its Application in Agriculture. J. Phys. Sci. 2015, 26, 23–39. [Google Scholar]
- Wu, L.; Liu, M. Preparation and Properties of Chitosan-Coated NPK Compound Fertilizer with Controlled-Release and Water-Retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Nadiah Abdul Hamid, N.; Mohamad, N.; Yip Hing, L.; Fairuz Dimin, M.; Asyadi Azam, M.; Haneesyah Che Hassan, M.; Khairul Shahril Mustaq Ahmad, M.; Shaaban, A. The Effect of Chitosan Content to Physical and Degradation Properties of Biodegradable Urea Fertilizer. J. Sci. Innov. Res. 2013, 2, 893–902. [Google Scholar]
- Yu, J.; Zhang, H.; Li, Y.; Lu, Q.; Wang, Q.; Yang, W. Synthesis, Characterization, and Property Testing of PGS/P(AMPS-Co-AM) Superabsorbent Hydrogel Initiated by Glow-Discharge Electrolysis Plasma. Colloid Polym. Sci. 2016, 294, 257–270. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S.; Chen, Z.; Wang, M.; Cao, J.; Wang, R. Preparation and Characterization of Superabsorbent Polymers Based on Sawdust. Polymers 2019, 11, 1891. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Li, P.; Xing, R.; Liu, S.; Qin, Y.; Yu, H.; Chen, X.; Li, K. Synthesis of Chitosan Derivative Graft Acrylic Acid Superabsorbent Polymers and Its Application as Water Retaining Agent. Int. J. Biol. Macromol. 2018, 115, 754–761. [Google Scholar] [CrossRef]
- Jayanudin; Fahrurrozi, M.; Wirawan, S.K. Rochmadi Characterization and Release Kinetics of Red Ginger Oleoresin Encapsulation Based on the Effect of Glutaraldehyde Concentration as Crosslinking Agent. Res. J. Chem. Environ. 2019, 23, 15–25. [Google Scholar]
- Rodrigues, F.H.A.; Fajardo, A.R.; Pereira, A.G.B.; Ricardo, Ń.M.P.S.; Feitosa, J.P.A.; Muniz, E.C. Chitosan-Graft-Poly(Acrylic Acid)/Rice Husk Ash Based Superabsorbent Hydrogel Composite: Preparation and Characterization. J. Polym. Res. 2012, 19, 1. [Google Scholar] [CrossRef]
- Melo, B.C.; Paulino, F.A.A.; Cardoso, V.A.; Pereira, A.G.B.; Fajardo, A.R.; Rodrigues, F.H.A. Cellulose Nanowhiskers Improve the Methylene Blue Adsorption Capacity of Chitosan-g-Poly(Acrylic Acid) Hydrogel. Carbohydr. Polym. 2018, 181, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Herawati, A.; Mujiyo; Syamsiyah, J.; Baldan, S.K.; Arifin, I. Application of Soil Amendments as a Strategy for Water Holding Capacity in Sandy Soils. IOP Conf. Ser. Earth Environ. Sci. 2021, 724, 012014. [Google Scholar] [CrossRef]
- Cheng, B.; Pei, B.; Wang, Z.; Hu, Q. Advances in Chitosan-Based Superabsorbent Hydrogels. RSC Adv. 2017, 7, 42036–42046. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, W.; Liang, L.; Lei, Z. Effects of Eco-Friendly Carbohydrate-Based Superabsorbent Polymers on Seed Germination and Seedling Growth of Maize. R. Soc. Open Sci. 2018, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. Applications of Absorbent Polymers for Sustainable Plant Protection and Crop Yield. Sustainability 2021, 13, 3253. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent Hydrogels Based on Polysaccharides for Application in Agriculture as Soil Conditioner and Nutrient Carrier: A Review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Abdallah, A.M. The Effect of Hydrogel Particle Size on Water Retention Properties and Availability under Water Stress. Int. Soil Water Conserv. Res. 2019, 7, 275–285. [Google Scholar] [CrossRef]


| No | Symbol | Definition |
|---|---|---|
| 1 | SAP 1 | Acrylic acid superabsorbent grafted with chitosan concentration of 4% (w/v) |
| 2 | SAP 2 | Acrylic acid superabsorbent grafted with chitosan concentration of 2% (w/v) |
| 3 | SAP 3 | Acrylic acid superabsorbent grafted with chitosan concentration of 0.5% (w/v) |
| Formulation | Models | Absorption Capacity at Equilibrium (Qe) (g/g) | Rate Constants (k) [g/(g·min)] | R2 |
|---|---|---|---|---|
| SAP 1 | Pseudo-first-order | 149.941 | 0.04224 | 0.990 |
| Pseudo-second-order | 158.814 | 0.00043 | 0.996 | |
| SAP 2 | Pseudo-first-order | 135.591 | 0.04785 | 0.998 |
| Pseudo-second-order | 142.350 | 0.00059 | 0.993 | |
| SAP 3 | Pseudo-first-order | 120.943 | 0.03850 | 0.982 |
| Pseudo-second-order | 128.313 | 0.00044 | 0.990 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayanudin; Lestari, R.S.D.; Barleany, D.R.; Pitaloka, A.B.; Yulvianti, M.; Prasetyo, D.; Anggoro, D.V.; Ruhiatna, A. Chitosan-Graft-Poly(acrylic acid) Superabsorbent’s Water Holding in Sandy Soils and Its Application in Agriculture. Polymers 2022, 14, 5175. https://doi.org/10.3390/polym14235175
Jayanudin, Lestari RSD, Barleany DR, Pitaloka AB, Yulvianti M, Prasetyo D, Anggoro DV, Ruhiatna A. Chitosan-Graft-Poly(acrylic acid) Superabsorbent’s Water Holding in Sandy Soils and Its Application in Agriculture. Polymers. 2022; 14(23):5175. https://doi.org/10.3390/polym14235175
Chicago/Turabian StyleJayanudin, Retno S. D. Lestari, Dhena Ria Barleany, Alia Badra Pitaloka, Meri Yulvianti, Dimas Prasetyo, Dendy Vito Anggoro, and Adam Ruhiatna. 2022. "Chitosan-Graft-Poly(acrylic acid) Superabsorbent’s Water Holding in Sandy Soils and Its Application in Agriculture" Polymers 14, no. 23: 5175. https://doi.org/10.3390/polym14235175
APA StyleJayanudin, Lestari, R. S. D., Barleany, D. R., Pitaloka, A. B., Yulvianti, M., Prasetyo, D., Anggoro, D. V., & Ruhiatna, A. (2022). Chitosan-Graft-Poly(acrylic acid) Superabsorbent’s Water Holding in Sandy Soils and Its Application in Agriculture. Polymers, 14(23), 5175. https://doi.org/10.3390/polym14235175






