Colloidal Crystal Films with Narrow Reflection Bands by Hot-Pressing of Polymer-Grafted Silica Particles
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
3.1. Synthesis and Characterization of SiP-POA
3.2. Fabrication and Reflection Properties of SiP-POA Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pieranski, P. Colloidal Crystals. Contemp. Phys. 1983, 24, 25–73. [Google Scholar] [CrossRef]
- Fudouzi, H.; Xia, Y. Colloidal Crystals with Tunable Colors and Their Use as Photonic Papers. Langmuir 2003, 19, 9653–9660. [Google Scholar] [CrossRef]
- Furumi, S.; Fudouzi, H.; Sawada, T. Self-Organized Colloidal Crystals for Photonics and Laser Applications. Laser Photon. Rev. 2010, 4, 205–220. [Google Scholar] [CrossRef]
- Kaneda, T.; Seki, Y.; Iwata, N.; Furumi, S. Fabrication of Colloidal Crystal Gel Film Using Poly(N-vinylcaprolactam). J. Photopolym. Sci. Technol. 2021, 34, 543–548. [Google Scholar] [CrossRef]
- Kawa, T.; Shibata, Y.; Iwata, N.; Furumi, S. Suspensions of Polymer Hydrogel Microparticles with Highly Sensitive Detectability of Glucose. J. Photopolym. Sci. Technol. 2021, 34, 555–559. [Google Scholar] [CrossRef]
- Meseguer, F. Colloidal Crystals as Photonic Crystals. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 270–271, 1–7. [Google Scholar] [CrossRef]
- Joannopoulos, J.D.; Villeneuve, P.R.; Fan, S. Photonic Crystals: Putting a New Twist on Light. Nature 1997, 386, 143–149. [Google Scholar] [CrossRef]
- John, S. Strong Localization of Photons in Certain Disordered Dielectric Superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef]
- Richel, A.; Johnson, N.P.; McComb, D.W. Observation of Bragg Reflection in Photonic Crystals Synthesized from Air Spheres in a Titania Matrix. Appl. Phys. Lett. 2000, 76, 1816–1818. [Google Scholar] [CrossRef]
- Fudouzi, H. Tunable Structural Color in Organisms and Photonic Materials for Design of Bioinspired Materials. Sci. Technol. Adv. Mater. 2011, 12, 064704. [Google Scholar] [CrossRef]
- Dziomkina, N.V.; Vancso, G.J. Colloidal Crystal Assembly on Topologically Patterned Templates. Soft Matter 2005, 1, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Shibata, Y.; Furumi, S. Synthesis of Monodispersed Silica Microparticles in a Microreactor for Well-Organized Colloidal Photonic Crystals. J. Photopolym. Sci. Technol. 2020, 33, 473–477. [Google Scholar] [CrossRef]
- Furumi, S. Recent Advances in Polymer Colloidal Crystal Lasers. Nanoscale 2012, 4, 5564–5571. [Google Scholar] [CrossRef] [PubMed]
- Okubo, T. Polymer Colloidal Crystals. Prog. Polym. Sci. 1993, 18, 481–517. [Google Scholar] [CrossRef]
- Ohno, K.; Morinaga, T.; Koh, K.; Tsujii, Y.; Fukuda, T. Synthesis of Monodisperse Silica Particles Coated with Well-Defined, High-Density Polymer Brushes by Surface-Initiated Atom Transfer Radical Polymerization. Macromolecules 2005, 38, 2137–2142. [Google Scholar] [CrossRef]
- Ohno, K.; Morinaga, T.; Takeno, S.; Tsujii, Y.; Fukuda, T. Suspensions of Silica Particles Grafted with Concentrated Polymer Brush: A New Family of Colloidal Crystals. Macromolecules 2006, 39, 1245–1249. [Google Scholar] [CrossRef]
- Morinaga, T.; Ohno, K.; Tsujii, Y.; Fukuda, T. Structural Analysis of “Semisoft” Colloidal Crystals by Confocal Laser Scanning Microscopy. Macromolecules 2008, 41, 3620–3626. [Google Scholar] [CrossRef]
- Ohno, K. Colloidal Crystals Formed by Polymer Brush-Afforded Fine Particles. Polym. Chem. 2010, 1, 1545–1551. [Google Scholar] [CrossRef]
- Ohno, K.; Mizuta, Y. Structural Color Materials Using Polymer-Brush-Decorated Hybrid Particles. ACS Appl. Polym. Mater. 2020, 2, 368–375. [Google Scholar] [CrossRef]
- Monguzzi, A.; Mauri, M.; Bianchi, A.; Dibbanti, M.K.; Simonutti, R.; Meinardi, F. Solid-State Sensitized Upconversion in Polyacrylate Elastomers. J. Phys. Chem. C 2016, 120, 2609–2614. [Google Scholar] [CrossRef]
- Huang, C.; Tassone, T.; Woodberry, K.; Sunday, D.; Green, D.L. Impact of ATRP Initiator Spacer Length on Grafting Poly(methyl methacrylate) from Silica Nanoparticles. Langmuir 2009, 25, 13351–13360. [Google Scholar] [CrossRef] [PubMed]
- Nomura, A.; Okayasu, K.; Ohno, K.; Fukuda, T.; Tsujii, Y. Lubrication Mechanism of Concentrated Polymer Brushes in Solvents: Effect of Solvent Quality and Thereby Swelling State. Macromolecules 2011, 44, 5013–5019. [Google Scholar] [CrossRef]
- Choi, J.; Hui, C.M.; Pietrasik, J.; Dong, H.; Matyjaszewski, K.; Bockstaller, M.R. Toughening Fragile Matter: Mechanical Properties of Particle Solids Assembled from Polymer-Grafted Hybrid Particles Synthesized by ATRP. Soft Matter 2012, 8, 4072–4082. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Tsai, H.-T. Complementary Multiple Hydrogen-Bonding Interactions Increase the Glass Transition Temperatures to PMMA Copolymer Mixtures. Macromolecules 2009, 42, 4701–4711. [Google Scholar] [CrossRef]
- Fetters, L.J.; Lohse, D.J.; Colby, R.H. Chain Dimensions and Entanglement Spacings. In Physical Properties of Polymers Handbook, 2nd ed.; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 447–454. [Google Scholar]
- Choi, J.; Hui, C.M.; Schmitt, M.; Pietrasik, J.; Margel, S.; Matyjazsewski, K.; Bockstaller, M.R. Effect of Polymer-Graft Modification on the Order Formation in Particle Assembly Structures. Langmuir 2013, 29, 6452–6459. [Google Scholar] [CrossRef] [PubMed]
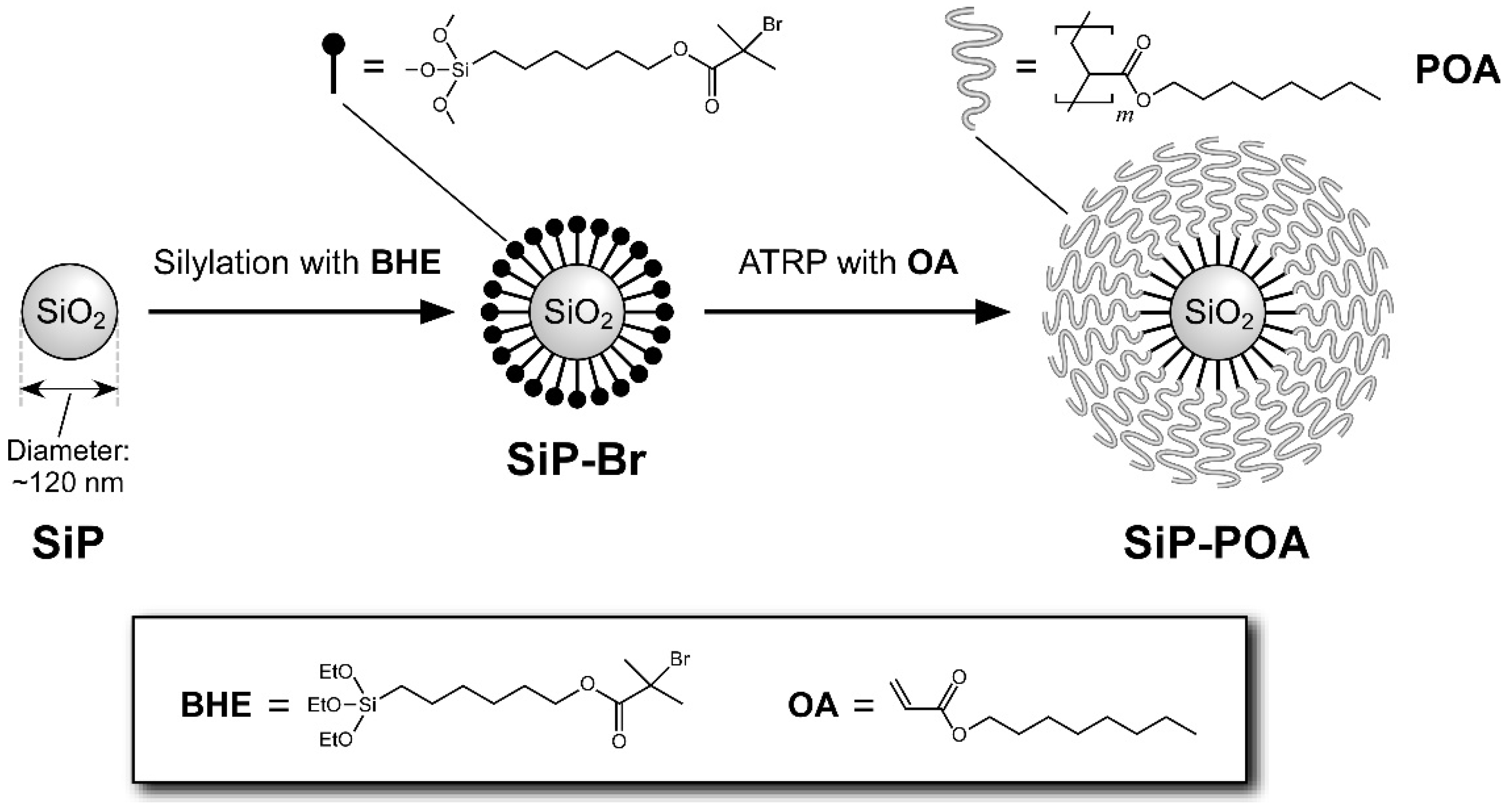
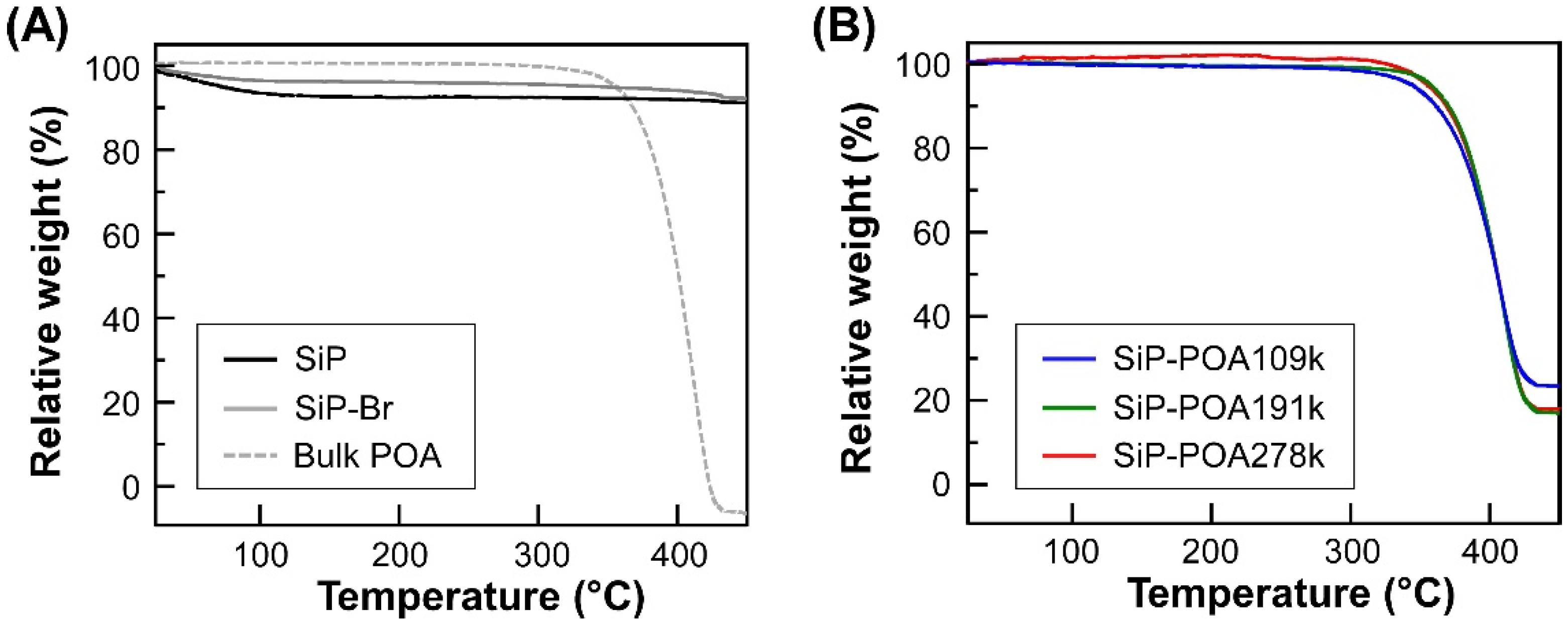
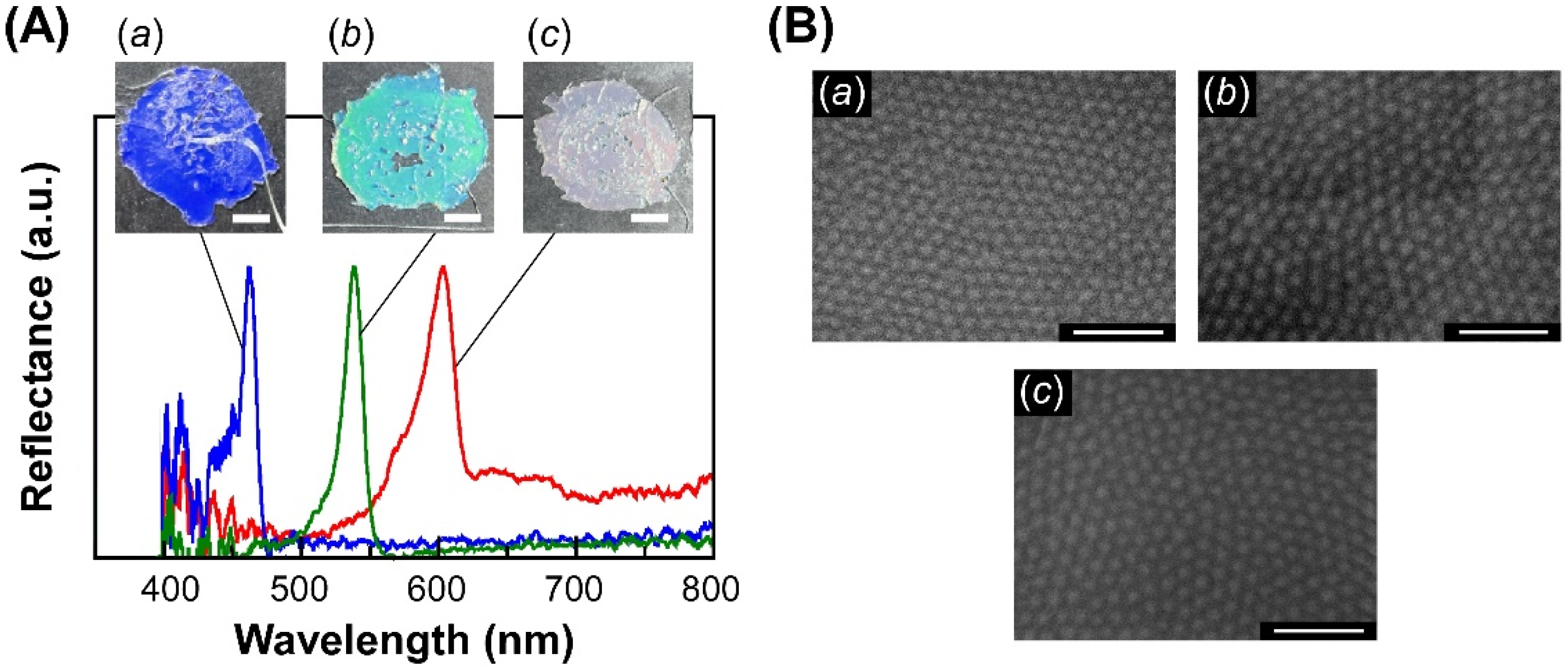
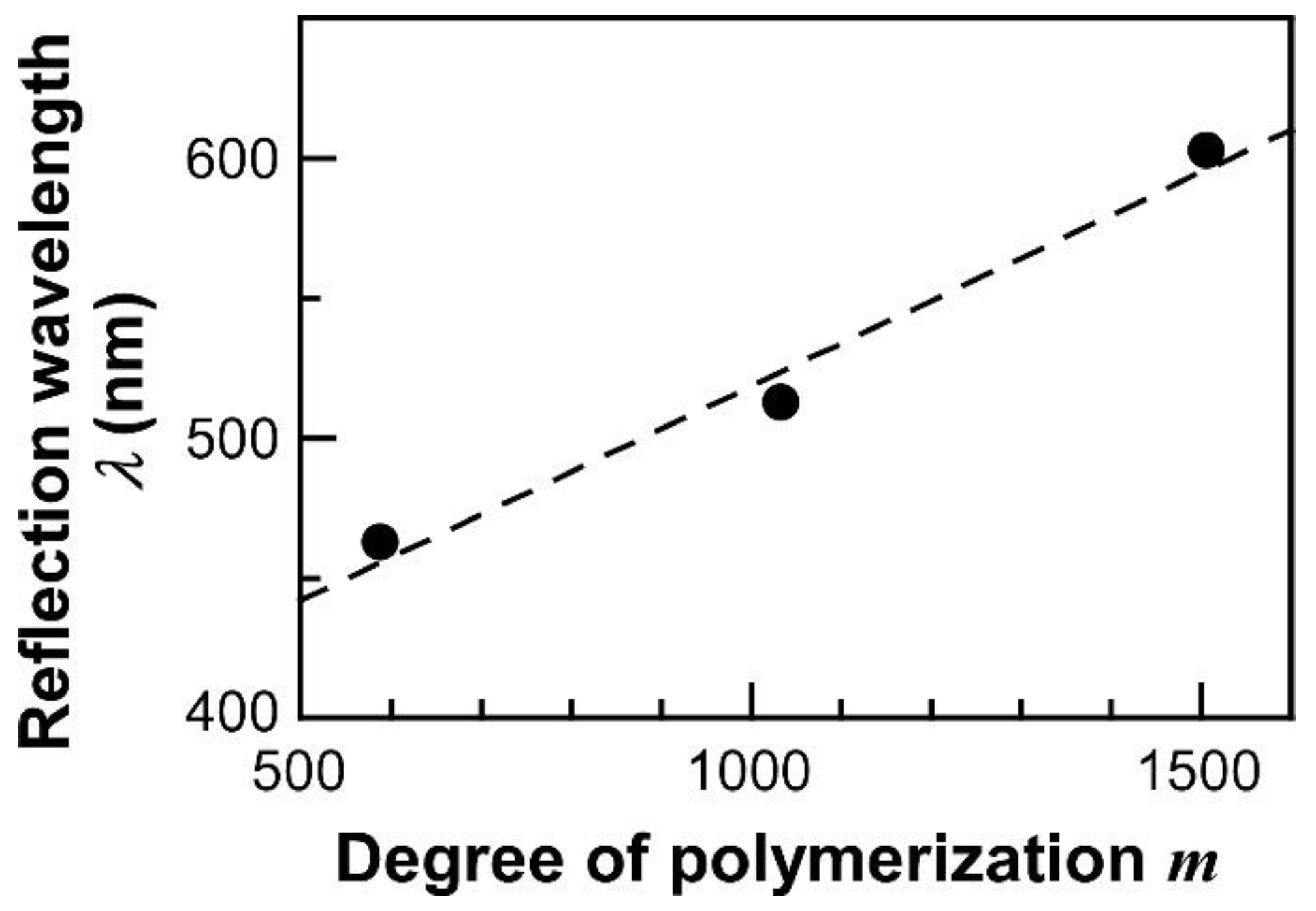
| Sample Code | EBIB (mg) | Me6TREN (mg) | Cu(I)Br (mg) | Cu(II)Br (mg) | Polymerization Time (h) |
|---|---|---|---|---|---|
| SiP-POA109k | 1.97 | 28.0 | 13.6 | 6.80 | 2.5 |
| SiP-POA191k | 1.97 | 28.0 | 13.1 | 7.10 | 5.3 |
| SiP-POA278k | 1.97 | 28.0 | 16.8 | 6.90 | 3.5 |
| Sample Code | Weight Fraction of POA φw (%) (1) | Mn (×105) (2) | Mw/Mn (2) | Graft Density σ (Chains/nm2) |
|---|---|---|---|---|
| SiP-POA109k | 76.9 | 1.09 | 1.16 | 0.81 |
| SiP-POA191k | 83.2 | 1.91 | 1.14 | 0.69 |
| SiP-POA278k | 82.5 | 2.78 | 1.10 | 0.45 |
| Sample | Reflection Colors of CC Films | ||
|---|---|---|---|
| Blue | Green | Red | |
| SiP-PMMAs (w/o carbon black nanoparticles) (1) | ~67 nm | ~160 nm | ~190 nm |
| SiP-PMMAs (w/carbon black nanoparticles) (1) | ~30 nm | ~56 nm | ~63 nm |
| SiP-POAs (w/o carbon black nanoparticles) (2) | ~13 nm | ~17 nm | ~23 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuura, S.; Obara, M.; Iwata, N.; Furumi, S. Colloidal Crystal Films with Narrow Reflection Bands by Hot-Pressing of Polymer-Grafted Silica Particles. Polymers 2022, 14, 5157. https://doi.org/10.3390/polym14235157
Matsuura S, Obara M, Iwata N, Furumi S. Colloidal Crystal Films with Narrow Reflection Bands by Hot-Pressing of Polymer-Grafted Silica Particles. Polymers. 2022; 14(23):5157. https://doi.org/10.3390/polym14235157
Chicago/Turabian StyleMatsuura, Sawa, Mami Obara, Naoto Iwata, and Seiichi Furumi. 2022. "Colloidal Crystal Films with Narrow Reflection Bands by Hot-Pressing of Polymer-Grafted Silica Particles" Polymers 14, no. 23: 5157. https://doi.org/10.3390/polym14235157
APA StyleMatsuura, S., Obara, M., Iwata, N., & Furumi, S. (2022). Colloidal Crystal Films with Narrow Reflection Bands by Hot-Pressing of Polymer-Grafted Silica Particles. Polymers, 14(23), 5157. https://doi.org/10.3390/polym14235157







