Preparation and Corrosion Performance of PPy/Silane Film on AZ31 Magnesium Alloy via One-Step Cyclic Voltammetry
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. The Infrared Spectrum Analysis of PPy/Silane Film
3.2. Formation Mechanism of PPy/Silane Composite Film on Mg Alloy Surface
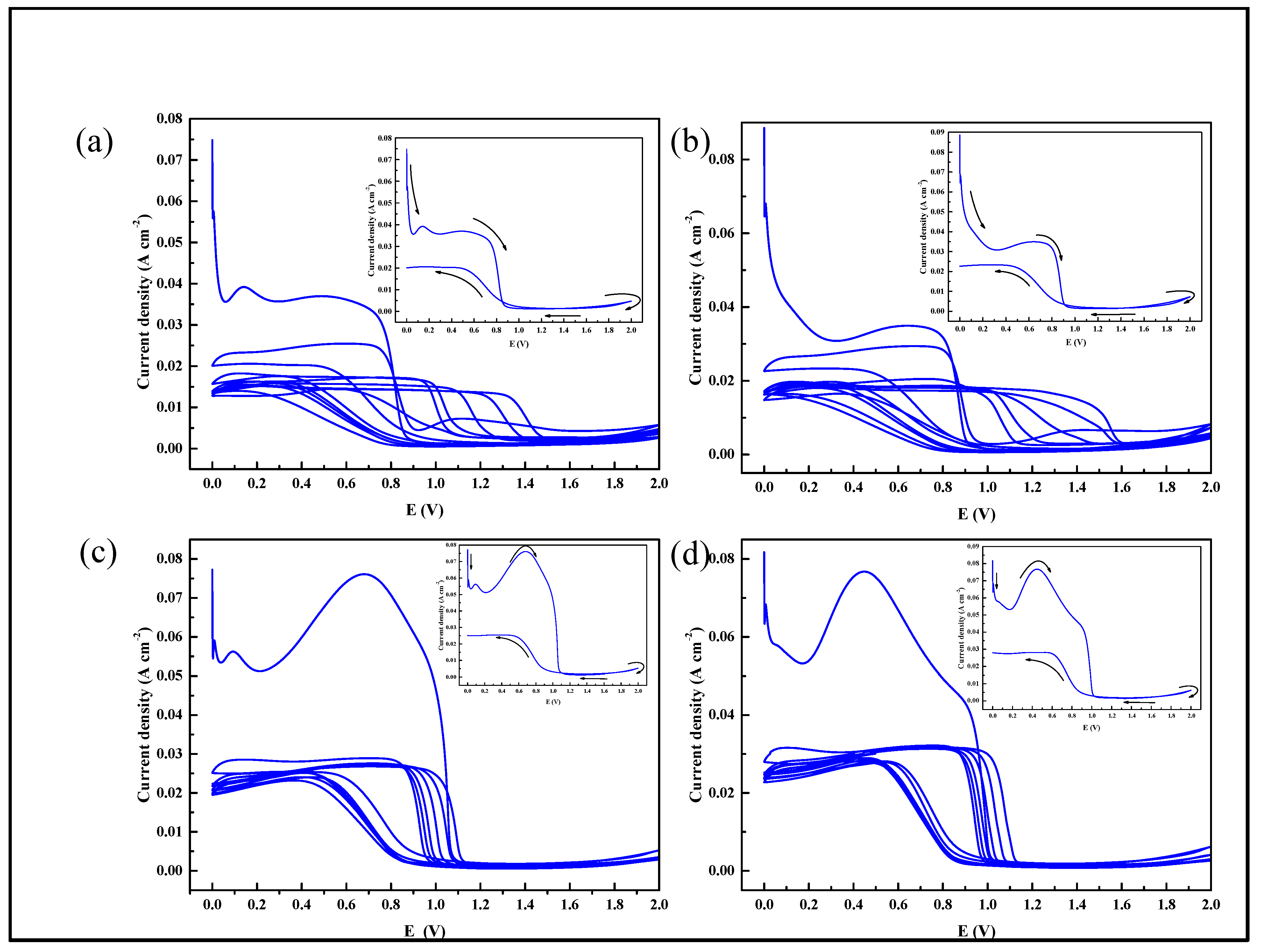
3.3. Composite Film Prepared by Cyclic Voltammetry
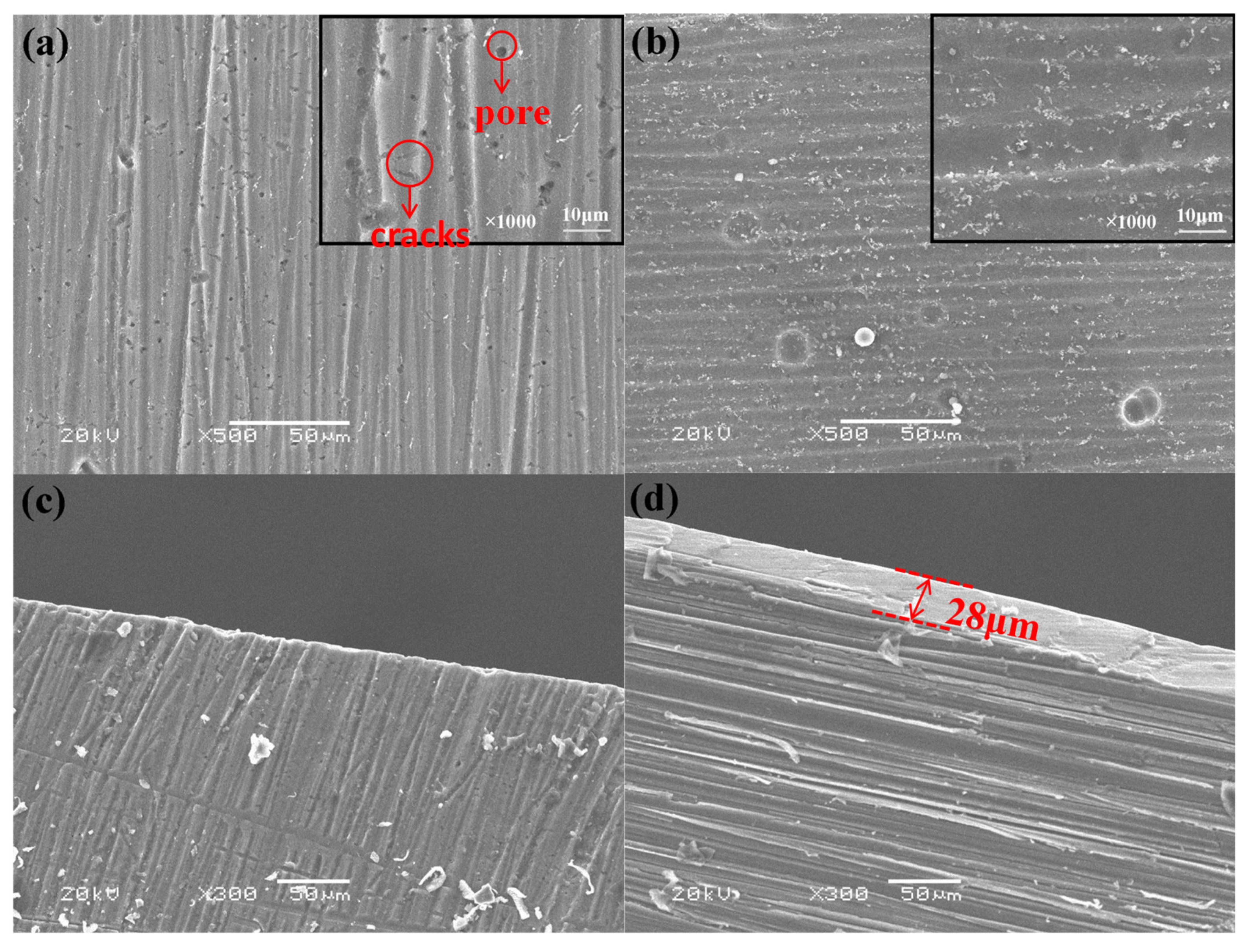
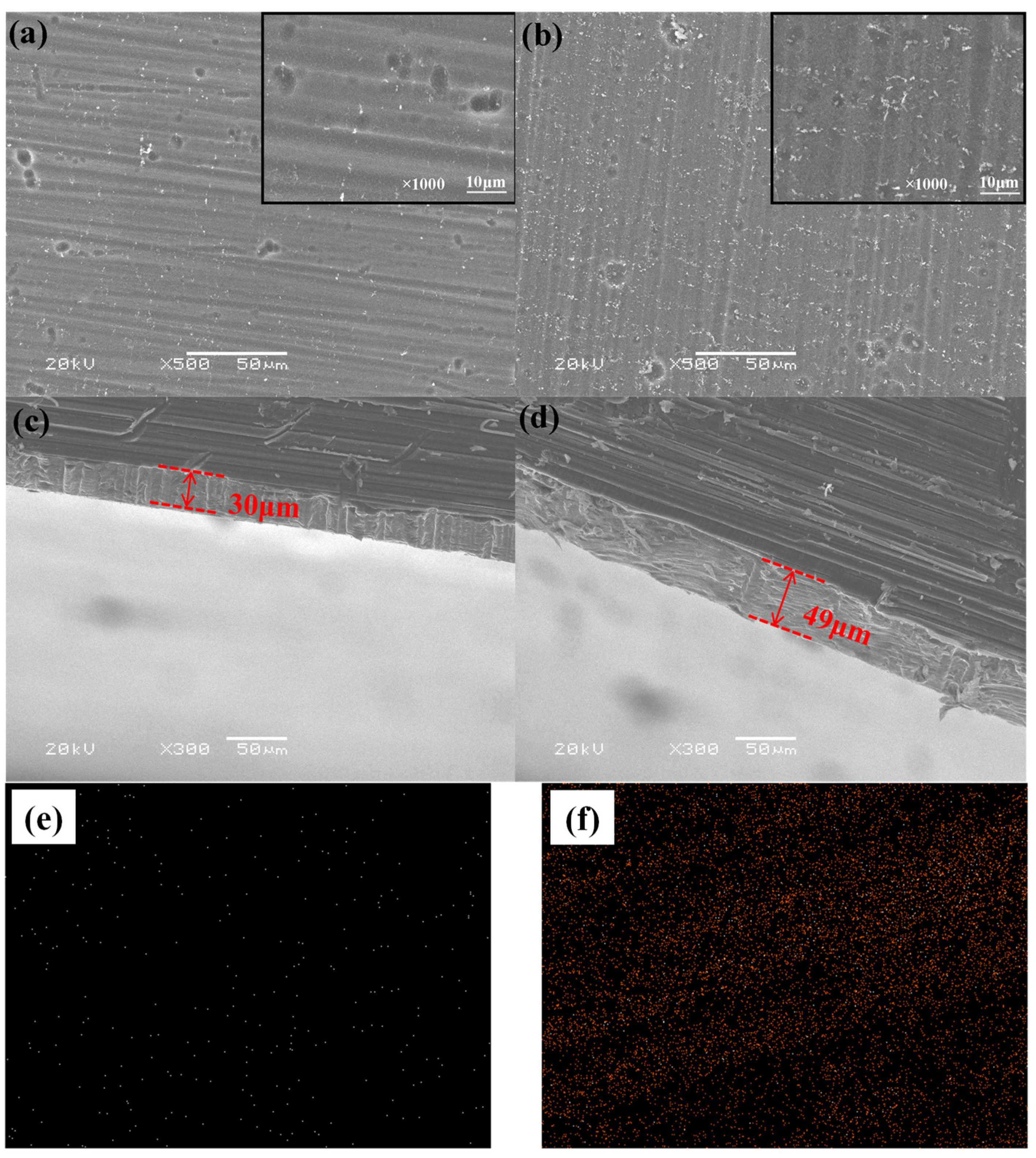
3.4. The Influence of Volume Fraction of Silane Coupling Agent on the Morphology of Composite Film
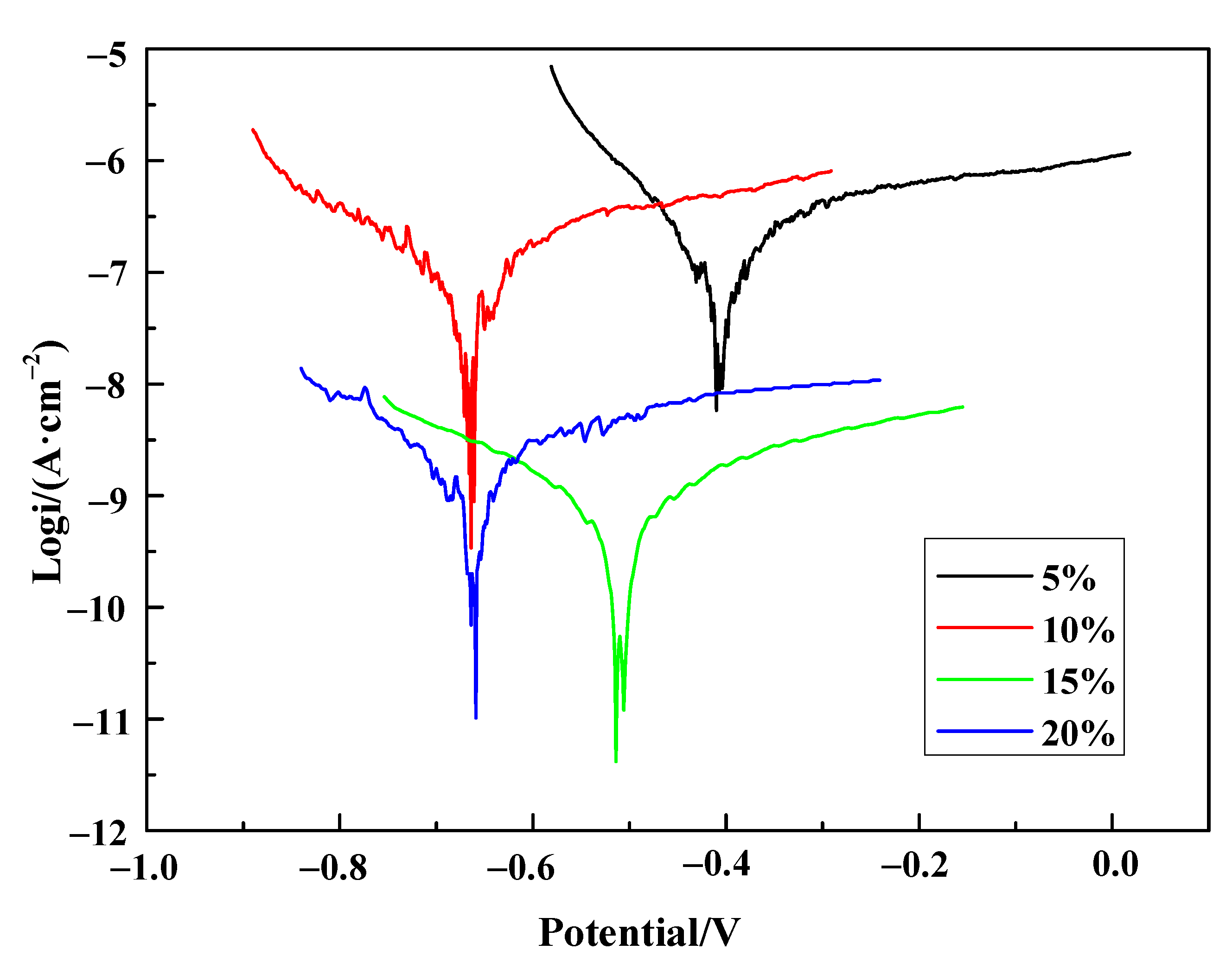
3.5. The Electro-Chemical Influence of Silane Coupling Agent on Composite Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asif, A.; Farasat, L.; Akhlaq, A.; Fakhera, L.; Anaum, N.; Aqif, A.; Saadat, A.; Ihteshm, R. Hydrothermal deposition of high strength calcium phosphate coatings on magnesium alloy for biomedical applications. Surf. Coat. Technol. 2019, 357, 716–727. [Google Scholar] [CrossRef]
- Chuayan, Z.; Jun, Z.; Shiyu, Z.; Zhenlin, W. Comparison of calcium phosphate coatings on AZ31 and fluoride-treated AZ31 alloy prepared by hydrothermal method and their electrochemical corrosion behaviour. Mater. Chem. Phys. 2018, 220, 395–401. [Google Scholar] [CrossRef]
- Lingyu, L.; Lanyue, C.; Rongchang, Z.; Shuoqi, L.; Xiaobo, C.; Yufeng, Z.; Mbobby, K. Advances in functionalized polymer coatings on biodegradable magnesium alloys—A review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Rita, M.; Abdul, R.S.; Prvan, K.K.; Kantesh, B. Mechanical, tribological and anti-corrosive properties of polyaniline/graphene coated Mg-9Li-7Al-1Sn and Mg-9Li-5Al-3Sn-1Zn alloys. J. Mater. Sci. Technol. 2019, 35, 1767–1778. [Google Scholar] [CrossRef]
- Xingguo, F.; Chao, Z.; Xiang, L.; Yiji, Z.; Tong, W.; Yu, Z.; Xuhui, Z.; Yuchao, D.; Mai, W. The influence of hydrofluoric acid doped polyaniline on the protective performance of a mg-rich epoxy coating on AZ91D magnesium alloy. Prog. Org. Coat. 2020, 141, 105550. [Google Scholar] [CrossRef]
- Jothi, V.; Adesina, A.Y.; Kumar, A.M.; Rahman, M.M.; Ram, J.S.N. Enhancing the biodegradability and surface protective performance of AZ31 Mg alloy using polypyrrole/gelatin composite coatings with anodized Mg surface. Surf. Coat. Technol. 2020, 381, 125139. [Google Scholar] [CrossRef]
- Niedbała, J. Structure, morphology and corrosion resistance of Ni-Mo plus PTh composite coatings. Bull. Mater. Sci. 2015, 38, 695–699. [Google Scholar] [CrossRef][Green Version]
- Maryam, H.; Mahdi, Y.; Ahmad, K.; Mohsen, S.; Reza, N. Electrochemical behavior of polypyrrole-coated AZ31 alloymodified by fluoride anions. J. Solid State Electrochem. 2017, 21, 777–785. [Google Scholar] [CrossRef]
- Martinez, A.L.; Brugnoni, L.I.; Flamini, D.O.; Saidman, S.B. Immobilization of Zn species in a polypyrrole matrix to prevent corrosion and microbial growth on Ti-6Al-4V alloy for biomedical applications. Prog. Org. Coat. 2020, 144, 105650. [Google Scholar] [CrossRef]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. Corrosion behaviour of polypyrrole-coated WE43 Mg alloy in a modifiedsimulated body fluid solution. Corros. Sci. 2018, 133, 261–275. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mukherjee, D.C.; Gohil, J.M.; Kumar, Y.; Kundu, S. Preparation, characterization and performance of conducting polypyrrole composites based on polysulfone. Desalination 2008, 225, 366–372. [Google Scholar] [CrossRef]
- Oscar, V.N.; Javier, G.; Nohra, G.R.; Juan, R.G.; Ana, M.M.; Jose, R.A.; Ulises, P.G. Polypyrrole Films Deposited on Carbon-Steel CS-1018 and Its Interaction with Mexican Crude Oil. Int. J. Electrochem. Sci. 2015, 10, 6378–6391. [Google Scholar]
- Metehan, C.; Turhan, M.; Weiser, M.S.; Killian, B.; Leitner, S. Electrochemical polymerization and characterization of polypyrrole on Mg–Al alloy (AZ91D). Synth. Met. 2011, 161, 360–364. [Google Scholar] [CrossRef]
- Srinivasan, A.; Ranjani, P.; Rajendran, N. Electrochemical polymerization of pyrrole over AZ31 Mg alloy for biomedical applications. Electrochim. Acta 2013, 88, 310–321. [Google Scholar] [CrossRef]
- Mei, L.; Yaqin, Y.; Liang, L.; Jiming, H.; Jianqing, Z. Electro-assisted preparation of dodecyltrimethoxysilane/TiO2 composite films for corrosion protection of AA2024-T3 (aluminum alloy). Electrochim. Acta 2010, 55, 3008–3014. [Google Scholar] [CrossRef]
- Xueming, W.; Aiju, L.; Guoli, L.; Weiqiang, W.; Yonghui, L. Studies of the Preparation of KH-560 Silane Films on Metallic Surface. Chin. Surf. Eng. 2004, 17, 27–31. (In Chinese) [Google Scholar]
- Franquet, A.; Lepen, C.; Terryn, H.; Verreecken, J. Effect of bath concentration and curing time on the structure of non-functional thin organosilane layers on aluminium. Electrochim. Acta 2003, 48, 1245–1255. [Google Scholar] [CrossRef]
- Eun, J.J.; Umashankar, M.; Do, S.H. Preparation of Gelatin-assisted Polypyrrole–Poly (3,4-ethylenedioxythiophene) Composites. Bull. Korean Chem. Soc. 2016, 37, 1789–1796. [Google Scholar] [CrossRef]
- Guoli, L.; Xueming, W.; Aiju, L.; Weiqiang, W.; Liqiang, Z. Fabrication and adhesive properties of thin organosilane films coated on low carbon steel substrates. Surf. Coat. Technol. 2007, 201, 9571–9578. [Google Scholar] [CrossRef]
- Xueming, W.; Guoli, L.; Aiju, L.; Zuoguang, Z. Influence of thermal curing on the fabrication and properties of thin organosilane films coated on low carbon steel substrates. J. Mater. Process. Technol. 2007, 186, 259–264. [Google Scholar] [CrossRef]
- Riegel, B.; Blittersdorf, S.; Kiefer, W.; Hofacker, S.; Muller, M.; Schottner, G. Kinetic investigations of hydrolysis and condensation of the glycidoxypropyltrimethoxysilaner/aminopropyltriethoxy-silane system by means of FT-Raman spectroscopy I. J. Non-Cryst. Solids 1998, 226, 76–84. [Google Scholar] [CrossRef]
- Turhan, M.C.; Weiser, M.; Jha, H.; Virtanen, S. Optimization of electrochemical polymerization parameters of polypyrrole on Mg–Al alloy (AZ91D) electrodes and corrosion performance. Electrochim. Acta 2011, 56, 5347–5354. [Google Scholar] [CrossRef]
- Jaouhari, A.E.; Jadi, S.B.; Aouzal, Z.; Bouabdallaoui, M.; Bazzaoui, E.A.; Wang, R.; Bazzaoui, M. Comparison study between corrosion protection of polypyrrole synthesized on stainless steel from phthalate and saccharinate aqueous medium. Polym. Test. 2018, 67, 302–308. [Google Scholar] [CrossRef]
- Qi, L.; Ruian, M.; An, D.; Xiaoran, Z.; Huazhen, Y.; Yongzhe, F.; Xue, Z.; Xiaoming, C. Investigation of the anticorrosion properties of graphene oxide doped thin organic anticorrosion films for hot-dip galvanized steel. Appl. Surf. Sci. 2019, 480, 646–654. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.W.; Xie, Y.; Zhang, H.H.; Zhang, Z. Preparation and anti-corrosion performance of superhydrophobic silane/graphene oxide composite coating on copper. Surf. Coat. Technol. 2021, 423, 127623. [Google Scholar] [CrossRef]
- Sougueh, C.; Lakard, S.; Husson, J.; Contal, E.; Monney, S.; Moutarlier, V.; Magnenet, C.; Lakard, B. Influence of pre-grafted pyrrole-based silane on the electrodeposition and chemical properties of polypyrrole films. Synth. Met. 2018, 246, 220–229. [Google Scholar] [CrossRef]
- Colucci, J.; Montalvo, V.; Hernandez, R.; Poullet, C.; Charlotte, P. Electrochemical oxidation potential of photocatalyst reducing agents. Electrochim. Acta 1999, 44, 2507–2514. [Google Scholar] [CrossRef]
- Petitjean, J.; Aeiyach, S.; Lacroix, J.C.; Lacaze, P.C. Ultra-fast electropolymerization of pyrrole in aqueous media on oxidizable metals in a one-step process. J. Electroanal. Chem. 1999, 478, 92–100. [Google Scholar] [CrossRef]
- González, M.B.; Saidman, S.B. Electrosynthesis of hollow polypyrrole microtubes with a rectangular cross-section. Electrochem. Commun. 2011, 13, 513–516. [Google Scholar] [CrossRef]
- Xuejun, C.; Xiuzhou, L.; Chunhai, L.; Ruisong, Y.; Xingwen, Z.; Min, G. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Chengdong, C.; Shigang, D.; Ruiqing, H.; Juan, H.; Pingli, J.; Chenqing, Y.; Ronggui, D.; Changjian, L. Insight into the anti-corrosion performance of electrodeposited silane/nano-CeO2 film on carbon steel. Surf. Coat. Technol. 2017, 326, 183–191. [Google Scholar] [CrossRef]



| Volume Fraction | 5% | 10% | 15% | 20% |
| Ratio | 1:18:1 | 1:8:1 | 3:14:3 | 1:3:1 |
| Concentration | Rs(Ω·cm−2) | CPE1(F·cm−2) | Rpore (Ω·cm−2) | CPE2(F·cm−2) | Rct(Ω·cm−2) | Zw(Ω−0.5·cm−2·S−1) |
|---|---|---|---|---|---|---|
| 5 wt% | 340 | 7.36 × 10−8 | 2.59 × 103 | 2.35 × 10−7 | 9.70 × 103 | 3.07 × 10−5 |
| 10 wt% | 790 | 6.02 × 10−9 | 2.66 × 104 | 5.89 × 10−8 | 3.41 × 103 | 1.35 × 10−5 |
| 15 wt% | 0.0138 | 1.41 × 10−10 | 2.46 × 163 | 1.26 × 10−9 | 2.87 × 106 | 5.43 × 10−7 |
| 20 wt% | 0.02442 | 5.14 × 10−11 | 9.11 × 105 | 1.69 × 10−9 | 9.11 × 105 | 1.07 × 10−7 |
| Concentration | 5% | 10% | 15% | 20% |
| Corrosion Current Density ((A·cm−2)) | 1.833 × 10−7 | 1.736 × 10−7 | 5.935 × 10−10 | 1.888 × 10−9 |
| Potential (V) | −0.410 | −0.664 | −0.576 | −0.659 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Cao, N.; Qi, Z.; Yan, Y.; Wu, R.; Wang, G. Preparation and Corrosion Performance of PPy/Silane Film on AZ31 Magnesium Alloy via One-Step Cyclic Voltammetry. Polymers 2021, 13, 3148. https://doi.org/10.3390/polym13183148
Peng C, Cao N, Qi Z, Yan Y, Wu R, Wang G. Preparation and Corrosion Performance of PPy/Silane Film on AZ31 Magnesium Alloy via One-Step Cyclic Voltammetry. Polymers. 2021; 13(18):3148. https://doi.org/10.3390/polym13183148
Chicago/Turabian StylePeng, Chuang, Nana Cao, Ziheng Qi, Yongde Yan, Ruizhi Wu, and Guixiang Wang. 2021. "Preparation and Corrosion Performance of PPy/Silane Film on AZ31 Magnesium Alloy via One-Step Cyclic Voltammetry" Polymers 13, no. 18: 3148. https://doi.org/10.3390/polym13183148
APA StylePeng, C., Cao, N., Qi, Z., Yan, Y., Wu, R., & Wang, G. (2021). Preparation and Corrosion Performance of PPy/Silane Film on AZ31 Magnesium Alloy via One-Step Cyclic Voltammetry. Polymers, 13(18), 3148. https://doi.org/10.3390/polym13183148







