Novel Polyelectrolyte Complex Membranes Containing Carboxymethyl Cellulose–Gelatin for Pervaporation Dehydration of Azeotropic Bioethanol for Biofuel
(This article belongs to the Section Polymer Applications)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
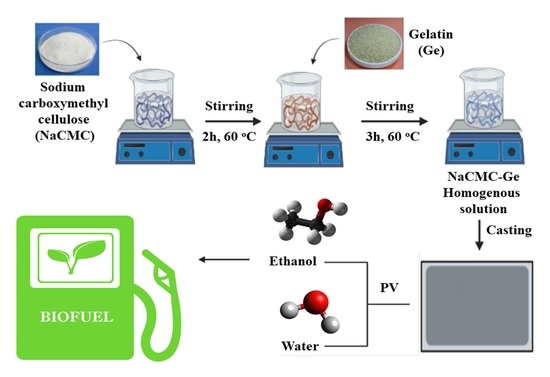
2.2. Fabrication of PECMs
2.3. Characterization of PECMs
2.4. Degree of Swelling (DS)
2.5. Pervaporation Experiments
3. Results
3.1. Characterization of PECMs
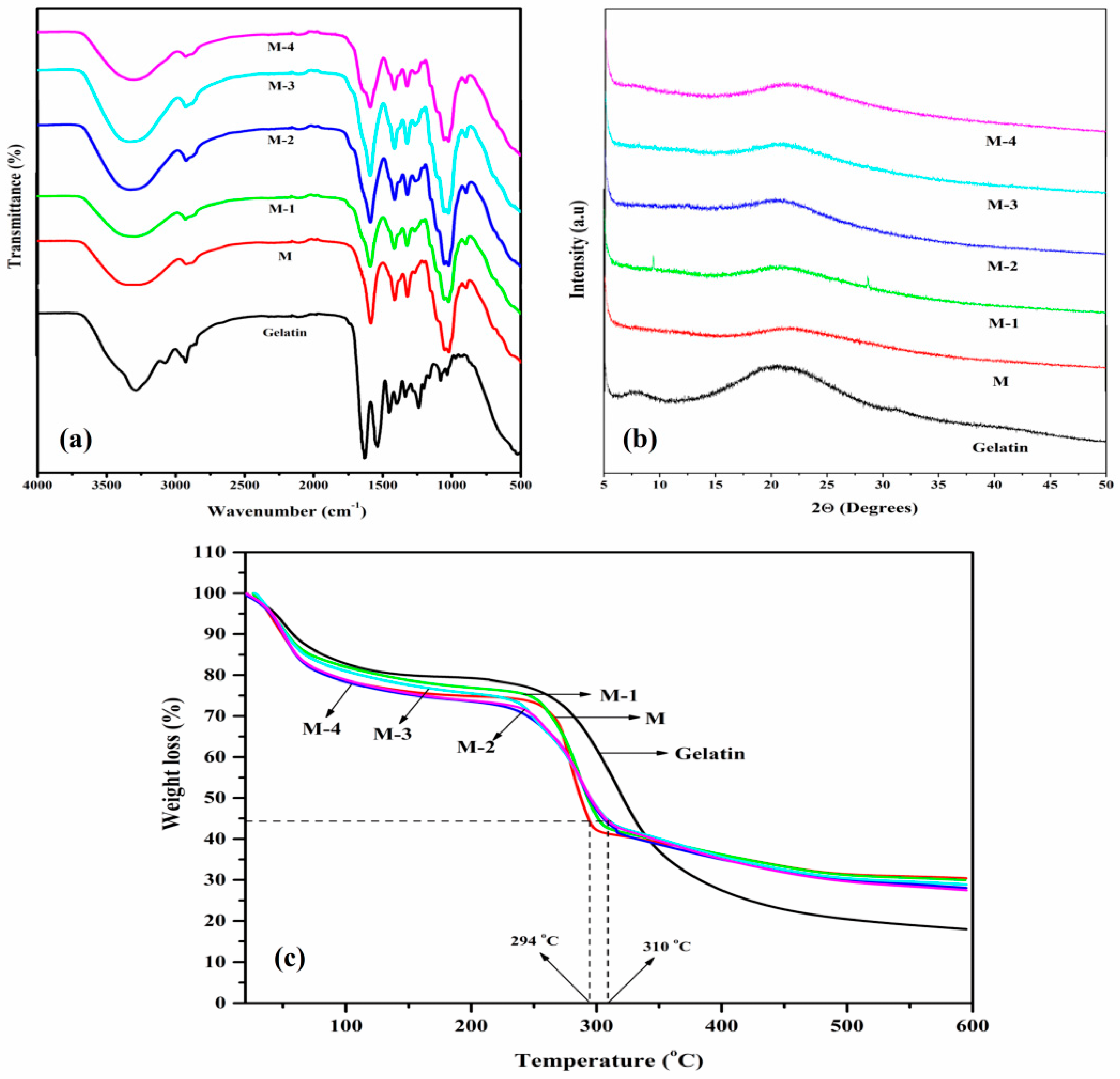
3.1.1. FTIR Analysis
3.1.2. WAXD Analysis
3.1.3. TGA Analysis
3.1.4. SEM Analysis
3.1.5. Tensile Strength
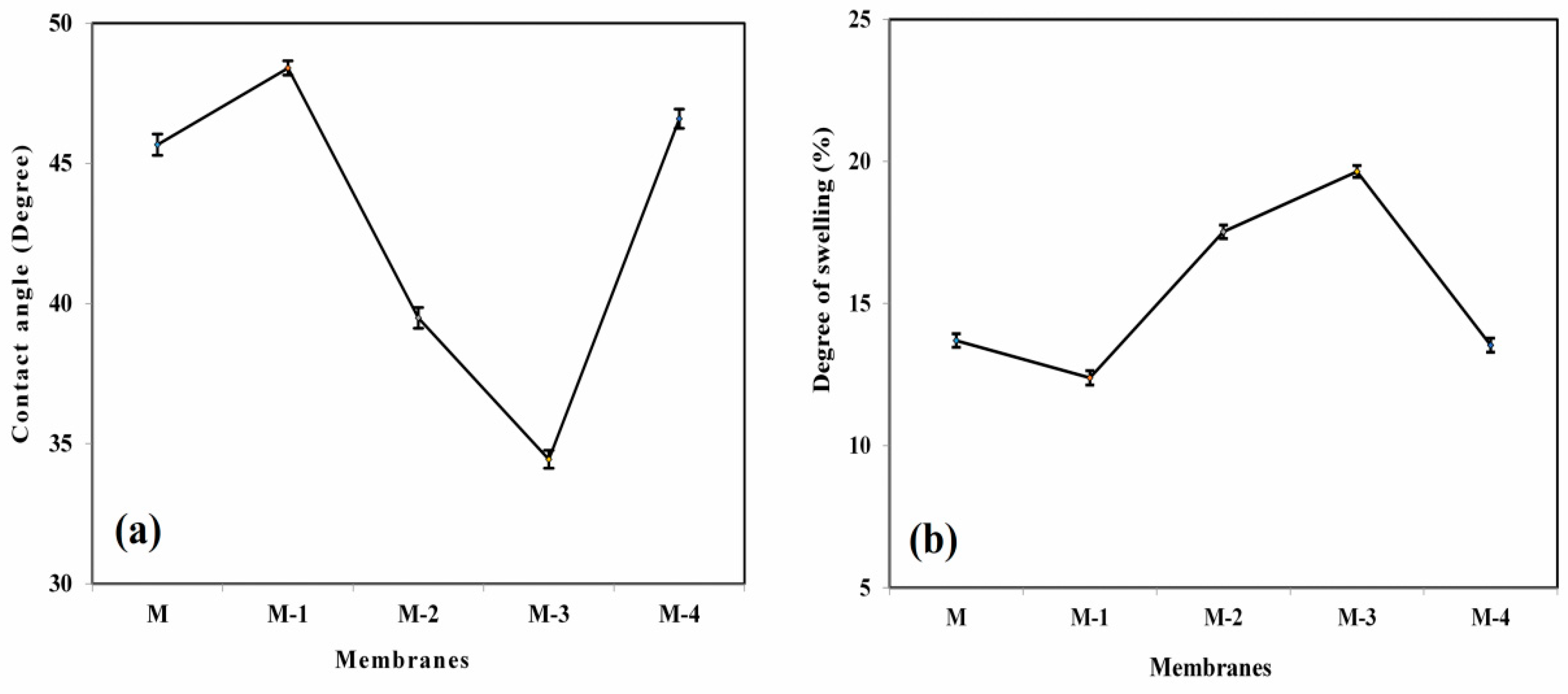
3.1.6. Contact Angle Analysis
3.2. Influence of Ge Content on Membrane Swelling
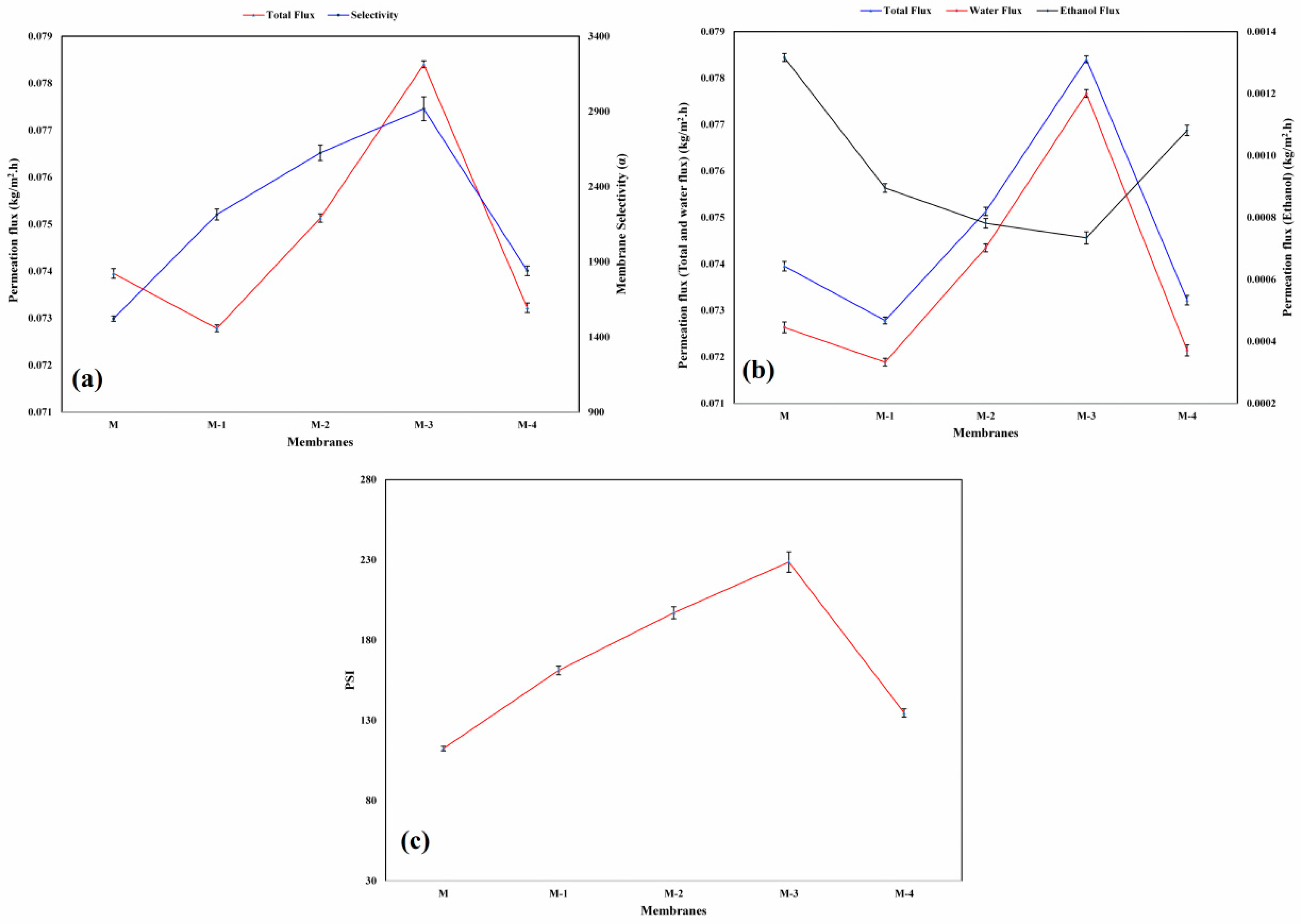
3.3. Inflence of Ge Content on PV
3.4. Influence of Ge Content on PSI
3.5. Comparison of PV Performance with the Literature
3.6. Diffusion Coefficient
3.7. Effect of Temperature on Membrane Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Mw | Molecular weight |
| A | Effective membrane area (m2) |
| DS | Degree of swelling (%) |
| Do | Pre-exponential factor for diffusion |
| ED | Activation energy for diffusion (kJ/mol) |
| EDw | Activation energy for diffusion of water (kJ/mol) |
| EDET | Activation energy for diffusion of bioethanol (kJ/mol) |
| Ep | Activation energy for permeation (kJ/mol) |
| Epw | Activation energy for permeation of water (kJ/mol) |
| EpET | Activation energy for permeation of bioethanol (kJ/mol) |
| Ex | Activation energy for permeation or diffusion (kJ/mol) |
| ET | Bioethanol |
| ΔHs | Heat of sorption (kJ/mol) |
| J | Total flux (kg/m2h) |
| Jo | Pre-exponential factor for permeation |
| PSI | Pervaporation separation index |
| P and F | Mass percent of permeate and feed |
| R | Gas constant |
| t | Permeation time (h) |
| T | Temperature (K) |
| W | Mass of permeate (kg) |
| Ws and Wd | Mass of the swollen and dry membranes |
| Greek letters | |
| δ | Membrane thickness (50 μm) |
| αsep | Separation factor |
References
- Sandesh, K.; Ujwal, P. Trends and perspectives of liquid biofuel-process and industrial viability. Energy Convers. Manag. 2021, 10, 100075. [Google Scholar] [CrossRef]
- Peng, P.; Lan, Y.; Liang, L.; Jia, K. Membranes for bioethanol production by pervaporation. Biotechnol. Biofuels 2021, 14, 10. [Google Scholar] [CrossRef]
- Khalid, A.; Aslam, M.; Qyyum, M.A.; Faisal, A.; Khan, A.L.; Ahmed, F.; Lee, M.; Kim, J.; Jang, N.; Chang, I.S.; et al. Membrane separation processes for dehydration of bioethanol from fermentation broths: Recent developments, challenges, and prospects. Renew. Sustain. Energy Rev. 2019, 105, 427–443. [Google Scholar] [CrossRef]
- Rahimalimamaghani, A.; Pacheco Tanaka, D.; Llosa Tanco, M.; Neira D’Angelo, F.; Gallucci, F. New hydrophilic carbon molecular sieve membranes for bioethanol dehydration via pervaporation. Chem. Eng. J. 2022, 435, 134891. [Google Scholar] [CrossRef]
- Karimi, S.; Karri, R.R.; Tavakkoli Yaraki, M.; Koduru, J.R. Processes and separation technologies for the production of fuel-grade bioethanol: A review. Environ. Chem. Lett. 2021, 19, 2873–2890. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; Fíla, V.; Drioli, E.; Figoli, A. Mixed matrix membranes (MMMs) for ethanol purification through pervaporation: Current state of the art. Rev. Chem. Eng. 2019, 35, 565–590. [Google Scholar] [CrossRef]
- Ehsan, M.; Razzaq, H.; Razzaque, S.; Bibi, A.; Yaqub, A. Recent advances in sodium alginate-based membranes for dehydration of aqueous ethanol through pervaporation. J. Polym. Sci. 2022, 60, 2435–2453. [Google Scholar] [CrossRef]
- Rajawat, A.; Sundarrajan, S.; Ramakrishna, S. Progress on Silica Pervaporation Membranes in Solvent Dehydration and Solvent Recovery Processes. Materials 2020, 13, 3354. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ma, J.; Li, S.; Guan, J.; Jiang, B.; Wang, L.; Li, J.; Wang, X.; Chen, L. Magnetic copper-based metal organic framework as an effective and recyclable adsorbent for removal of two fluoroquinolone antibiotics from aqueous solutions. J. Colloid Interface Sci. 2018, 528, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, A.M.; Kariduraganavar, M.Y. Development of novel membranes for PV separation of water–isopropanol mixtures using poly (vinyl alcohol) and gelatin. J. Membr. Sci. 2013, 438, 8–11. [Google Scholar] [CrossRef]
- Semenova, S.I.; Ohya, H.; Soontarapa, K. Hydrophilic membranes for pervaporation: An analytical review. Desalination 1997, 110, 251–286. [Google Scholar] [CrossRef]
- Jyothi, M.S.; Reddy, K.R.; Soontarapa, K.; Naveen, S.; Raghu, A.V.; Kulkarni, R.V.; Suhas, D.P.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Membranes for dehydration of alcohols via pervaporation. J. Environ. Manag. 2019, 242, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, L.; Matsuura, T.; Chung, T.S.; Goh, S.H. Investigation of the fundamental differences between polyamide-imide (PAI) and polyetherimide (PEI) membranes for isopropanol dehydration via pervaporation. J. Membr. Sci. 2008, 318, 217–226. [Google Scholar] [CrossRef]
- Amnuaypanich, S.; Kongchana, N. Natural Rubber/Poly (acrylic acid) Semi-Interpenetrating Polymer Network Membranes for the Pervaporation of Water–Ethanol Mixtures. J. Appl. Polym. Sci. 2009, 114, 3501–3509. [Google Scholar] [CrossRef]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Pal Singh Chauhan, N.; Jadoun, S.; Dehghani Soltani, M.; Zarrintaj, P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef] [PubMed]
- Achari, D.D.; Hegde, S.N.; Pattanashetti, N.A.; Kamble, R.R.; Kariduraganavar, M.Y. Development of zeolite-A incorporated PVA/CS nanofibrous composite membranes using the electrospinning technique for pervaporation dehydration of water/tert-butanol. New J. Chem. 2021, 45, 3981–3996. [Google Scholar] [CrossRef]
- Teleky, B.E.; Mitrea, L.; Plamada, D.; Nemes, S.A.; Călinoiu, L.F.; Pascuta, M.S.; Varvara, R.A.; Szabo, K.; Vajda, P.; Szekely, C.; et al. Development of Pectin and Poly (vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants 2022, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
- Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Lahderanta, E.; Solovyev, N.; Penkova, A. Modification Approaches to Enhance Dehydration Properties of Sodium Alginate-Based Pervaporation Membranes. Membranes 2021, 11, 255. [Google Scholar] [CrossRef]
- Jiraratananon, R.; Chanachai, A.; Huang, R.; Uttapap, D. Pervaporation dehydration of ethanol–water mixtures with chitosan /hydroxyethylcellulose (CS/HEC) composite membrane. I. Effect of operating conditions. J. Membr. Sci. 2002, 195, 143–151. [Google Scholar] [CrossRef]
- Rachipudi, P.S.; Kittur, A.A.; Sajjan, A.M.; Kariduraganavar, M.Y. Synthesis and characterization of hybrid membranes using chitosan and 2-(3, 4-epoxycyclohexyl) ethyltrimethoxysilane for pervaporation dehydration of isopropanol. J. Membr. Sci. 2013, 441, 83–92. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Liu, J.; He, F.; Gunn, T.M.; Zhao, D.; Roberts, C.B. Precise seed-mediated growth and size-controlled synthesis of palladium nanoparticles using a green chemistry approach. Langmuir 2009, 25, 7116–7128. [Google Scholar] [CrossRef] [PubMed]
- Rogers, Y.E.; Kamitakahara, H.; Yoshinaga, A.; Takano, T. Radially oriented cellulose triacetate chains on gold nanoparticles. Cellulose 2010, 17, 923–936. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, J.; Li, O.; Guo, Y.; Zhang, L. Preparation and characterization of novel quaternized cellulose nanoparticles as protein carriers. Macromol. Biosci. 2009, 9, 857–863. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhao, D.; Liu, J.; Roberts, C.B. Stabilization of Fe–Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichloroethylene in soil and groundwater. Ind. Eng. Chem. Res. 2007, 46, 29–34. [Google Scholar] [CrossRef]
- Palem, R.R.; Rao, K.M.; Shimoga, G.; Saratale, R.G.; Shinde, S.K.; Ghodake, G.S.; Lee, S. Physicochemical characterization, drug release, and biocompatibility evaluation of carboxymethyl cellulose-based hydrogels reinforced with sepiolite nanoclay. Int. J. Biol. Macromol. 2021, 178, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Sivudu, K.S.; Mohan, Y.M.; Sreedhar, B.; Raju, K.M. Controlled silver nanoparticles synthesis in semi-hydrogel networks of poly (acrylamide) and carbohydrates: A rational methodology for antibacterial application. Carbohydr. Polym. 2009, 75, 463–471. [Google Scholar] [CrossRef]
- Zhong, X.; Dongye, Z.; Gang, P. Rapid and controlled transformation of nitrate in water and brine by stabilized iron nanoparticles. J. Nanoparticle Res. 2009, 11, 807–819. [Google Scholar]
- El Sayed, A.M.; El Gamal, S.; Morsi, W.M.; Mohammed, G. Effect of PVA and copper oxide nanoparticles on the structural, optical, and electrical properties of carboxymethyl cellulose films. J Mater. Sci. 2015, 50, 4717–4728. [Google Scholar] [CrossRef]
- Kim, S.G.; Lee, K.S.; Lee, K.H. Pervaporation separation of sodium alginate/chitosan polyelectrolyte complex composite membrane for the separation of water/alcohol mixtures: Characterization of the permeation behaviour with molecular modeling techniques. J. Appl. Polym. Sci. 2007, 103, 2634–2641. [Google Scholar] [CrossRef]
- Zhao, Q.; An, Q.F.; Ji, Y.; Qian, J.; Gao, C. Polyelectrolyte complex membranes for pervaporation, nanofiltration and fuel cell applications. J. Membr. Sci. 2011, 379, 19–45. [Google Scholar] [CrossRef]
- Thunemann, A.F.; Muller, M.; Dautzenberg, H.; Joanny, J.F.; Luwen, H. Polyelectrolyte complexes. Adv. Polym. Sci. 2004, 166, 113–171. [Google Scholar]
- Liu, Y.; Zhu, M.; Zhao, Q.; An, Q.; Qian, J.; Lee, K.; Lai, J. The chemical cross-linking of polyelectrolyte complex colloidal particles and the pervaporation performance of their membranes. J. Membr. Sci. 2011, 385, 132–140. [Google Scholar] [CrossRef]
- Soradech, S.; Nunthanid, J.; Limmatvapirat, S.; Luangtana-anan, M. An approach for the enhancement of the mechanical properties and film coating efficiency of shellac by the formation of composite films based on shellac and gelatin. J. Food Eng. 2012, 108, 94–102. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Higuchi, A.; Ishikawa, M.; Guiver, M.D.; Robertson, G.P. Vapor permeation of aqueous 2-propanol solutions through gelatin/torlon((R)) poly(amide imide) blended membranes. J. Membr. Sci. 2004, 243, 89–95. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; An, Q.; Zhao, Q.; Lee, K.; Qian, J.; Gao, C. Homogenous polyelectrolyte complex membranes incorporated with strong ion-pairs with high pervaporation performance for dehydration of ethanol. J. Membr. Sci. 2013, 435, 71–79. [Google Scholar] [CrossRef]
- Zhao, Q.; Qian, J.; An, Q.; Gao, C.; Gui, Z.; Jin, H. Synthesis and characterization of soluble chitosan/sodium carboxymethyl cellulose polyelectrolyte complexes and the pervaporation dehydration of their homogeneous membranes. J. Membr. Sci. 2009, 333, 68–78. [Google Scholar] [CrossRef]
- Wang, X.; An, Q.; Liu, T.; Zhao, Q.; Hung, W.; Lee, K.; Gao, C. Novel polyelectrolyte complex membranes containing free sulfate groups with improved pervaporation dehydration of ethanol. J. Membr. Sci. 2014, 452, 73–81. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Naik, M.L.; Kulkarni, A.S.; Rudgi, U.; Ashwini, M.; Shirnalli, G.G.; Sharanappa, A.; Kalahal, P.B. Preparation and characterization of PVA-Ge/PEG-400 biodegradable plastic blend films for packaging applications. Chem. Data Collect. 2020, 26, 100338. [Google Scholar] [CrossRef]
- Achari, D.D.; Heggannavar, G.B.; Kariduraganavar, M.Y. Modification of highly brittle polystyrene sulfonic acid-co-maleic acid cross-linked sodium alginate membrane into flexible membranes by the incorporation of dibutyl phthalate as a plasticizer for pervaporation separation. J. Appl. Polym. Sci. 2020, 46, 49431. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Premakshi, H.G.; Kariduraganavar, M.Y. Synthesis and characterization of GTMAC grafted chitosan membranes for the dehydration of low water content isopropanol by pervaporation. J. Ind. Eng. Chem. 2015, 25, 151–161. [Google Scholar] [CrossRef]
- Kalahal, P.B.; Kulkarni, A.S.; Sajjan, A.M.; Khan, T.M.Y.; Badruddin, I.A.; Kamangar, S.; Banapurmath, N.R.; Ayachit, N.H.; Naik, M.L.; Marakatti, V.S. Fabrication and physicochemical study of B2SA grafted poly(vinyl Alcohol)–graphene hybrid membranes for dehydration of bioethanol by pervaporation. Membranes 2021, 11, 110. [Google Scholar] [CrossRef]
- Namboodiri, V.V.; Ponangi, R.; Vane, L.M. A novel hydrophilic polymer membrane for the dehydration of organic solvents. Eur. Polym. J. 2006, 42, 3390–3393. [Google Scholar] [CrossRef]
- Rao, K.K.; Subha, M.C.S.; Sairam, M.; Mallikarjuna, N.N.; Aminabhavi, T. Blend membranes of chitosan and poly (vinyl alcohol) In pervaporation dehydration of isopropanol and tetrahydrofuran. J. Appl. Polym. Sci. 2006, 103, 1918–1926. [Google Scholar] [CrossRef]
- Suhas, D.P.; Raghu, A.V.; Jeong, H.M.; Aminabhavi, T.M. Graphene-loaded sodium alginate nanocomposite membranes with enhanced isopropanol dehydration performance via a pervaporation technique. RSC Adv. 2013, 3, 17120–17130. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Badry, R.; Ezzat, H.A.; El-Khodary, S.; Morsy, M.; Elhaes, H.; Nada, N.; Ibrahim, M. Spectroscopic and thermal analyses for the effect of acetic acid on the plasticized sodium carboxymethyl cellulose. J. Mol. Struct. 2021, 1224, 129013. [Google Scholar] [CrossRef]
- Fan, L.; Du, Y.; Huang, R.; Wang, Q.; Wang, X.; Zhang, L. Preparation and characterization of alginate/gelatin blend fibers. J. Appl. Polym. Sci. 2005, 96, 1625–1629. [Google Scholar] [CrossRef]
- Bigi, A.; Panzavolta, S.; Rubini, K. Relationship between triple-helix content mechanical properties of gelatin films. Biomaterials 2004, 25, 5675–5680. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, X.; Lou, T. Preparation of chitosan/gelatin composite foam with ternary solvents of dioxane/acetic acid/water and its water absorption capacity. Polym. Bull. 2020, 77, 5227–5244. [Google Scholar] [CrossRef]
- Zhao, Q.; Qian, J.; An, Q.; Gui, Z.; Jin, H.; Yin, M. Pervaporation dehydration of isopropanol using homogeneous polyelectrolyte complex membranes of poly(diallyl dimethylammonium chloride)/sodium carboxymethyl cellulose. J. Membr. Sci. 2009, 329, 175–182. [Google Scholar] [CrossRef]
- Acharia, D.; Rachipudi, P.; Naik, S.; Karuppannan, R.; Kariduraganavar, M. Polyelectrolyte complex membranes made of chitosan—PSSAMA for pervaporation separation of industrially important azeotropic mixtures. J. Ind. Eng. Chem. 2019, 78, 383–395. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, S.; Lu, W.; Chen, N.; Zhu, D.; Li, Y. Preparation and characterization of enzymatically cross-linked gelatin/cellulose nanocrystal composite hydrogels. RSC Adv. 2021, 11, 10794–10803. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Sajjan, A.M.; Ashwini, M.; Banapurmath, N.R.; Ayachit, N.H.; Shirnalli, G.G. Novel fabrication of PSSAMA_Na capped silver nanoparticle embedded sodium alginate membranes for pervaporative dehydration of bioethanol. RSC Adv. 2020, 10, 22645–22655. [Google Scholar] [CrossRef]
- Chiang, W.Y.; Lin, Y.H. Properties of modified poly (vinyl alcohol) membranes prepared by the grafting of new polyelectrolyte copolymers for water–ethanol mixture separation. J. Appl. Polym. Sci. 2002, 86, 2854–2859. [Google Scholar] [CrossRef]
- Shieh, J.J.; Huang, R.Y. Pervaporation with chitosan membranes II. Blend membranes of chitosan and polyacrylic acid and comparison of homogeneous and composite membrane based on polyelectrolyte complexes of chitosan and polyacrylic acid for the separation of ethanol-water mixtures. J Membr. Sci. 1997, 127, 185–202. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Pal, R.; Moon, G.Y. Pervaporation dehydration of aqueous ethanol and isopropanol mixtures through alginate/chitosan two ply composite membranes supported by poly (vinylidene fluoride) porous membrane. J Membr. Sci. 2000, 167, 275–289. [Google Scholar] [CrossRef]
- Van Veen, H.M.; Van Delft, Y.C.; Engelen, C.W.R.; Pex, P.P.A.C. Dewatering of organics by pervaporation with silica membranes. Sep. Purif. Technol. 2001, 22, 361–366. [Google Scholar] [CrossRef]
- Ishikawa, T. Membranes for dehydrating aqueous ethanol by pervaporation. Kem. Enjiniyaringu 1984, 29, 19–25. [Google Scholar]
- Nguyen, Q.T.; Le Blanc, L.; Neel, J. Preparation of membranes from polyacrylonitrile—Polyvinylpyrrolidone blends and the study of their behaviour in the pervaporation of water—Organic liquid mixtures. J Membr. Sci. 1985, 22, 245–255. [Google Scholar] [CrossRef]
- Uragami, T.; Morikawa, T. Studies on syntheses and permeabilities of special polymer membranes, 70. Permeation and separation characteristics for aqueous alcoholic solutions by evapomeation and pervaporation through polystyrene membranes. Die Makromol. Chem. Macromol. Chem. Phys. 1989, 190, 399–404. [Google Scholar] [CrossRef]
- Uragami, T.; Morikawa, T.; Okuno, H. Characteristics of permeation and separation of aqueous alcohol solutions through hydrophobic polymer membranes. Polym. J. 1989, 30, 1117–1122. [Google Scholar] [CrossRef]
- Uragami, T.; Saito, M. Studies on syntheses and permeabilities of special polymer membranes. 68. Analysis of permeation and separation characteristics and new technique for separation of aqueous alcoholic solutions through alginic acid membranes. Sep. Sci. Technol. 1989, 24, 541–554. [Google Scholar] [CrossRef]
- Uragami, T.; Matsugi, H.; Miyata, T. Pervaporation characteristics of organic−inorganic hybrid membranes composed of poly (vinyl alcohol-co-acrylic acid) and tetraethoxysilane for water/ethanol separation. Macromolecules 2005, 38, 8440–8446. [Google Scholar] [CrossRef]
- Uragami, T.; Takigawa, K. Permeation and separation characteristics of ethanol-water mixtures through chitosan derivative membranes by pervaporation and evapomeation. Polymer 1990, 31, 668–672. [Google Scholar] [CrossRef]
- Praptowidodo, V.S. Influence of swelling on water transport through PVA-based membrane. J. Mol. Struct. 2005, 739, 207–212. [Google Scholar] [CrossRef]
- Rafik, M.; Mas, A.; Guimon, M.F.; Guimon, C.; Elharfi, A.; Schue, F. Plasma-modified poly (vinyl alcohol) membranes for the dehydrationof ethanol. Polym. Int. 2003, 52, 1222–1229. [Google Scholar] [CrossRef]
- Bolto, B.; Hoang, M.; Xie, Z. A review of membrane selection for the dehydration of aqueous ethanol by pervaporation. Chem. Eng. Process. 2011, 50, 227–235. [Google Scholar] [CrossRef]
- Yamasaki, A.; Iwatsubo, T.; Masuoka, T.; Mizoguchi, K. Pervaporation of ethanol/water through a poly (vinyl alcohol)/cyclodextrin (PVA/CD) membrane. J. Membr. Sci. 1994, 89, 111–117. [Google Scholar] [CrossRef]
- Rachipudi, P.S.; Kariduraganavar, M.Y.; Kittur, A.A.; Sajjan, A.M. Synthesis and characterization of sulfonated-poly(vinyl alcohol) membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2011, 383, 224–234. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Yeom, C.K. Pervaporation separation of aqueous mixtures using cross-linked polyvinyl alcohol membranes. III. Permeation of acetic acid–water mixtures. J Membr. Sci. 1991, 58, 33–47. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Badi, S.M.; Sajjan, A.M.; Banapurmath, N.R.; Kariduraganavar, M.Y.; Shettar, A.S. Preparation and characterization of B2SA grafted hybrid poly (vinyl alcohol) membranes for pervaporation separation of water-isopropanol mixtures. Chem. Data Collect. 2019, 22, 100245. [Google Scholar] [CrossRef]
- Weinkauf, D.H.; Paul, D.R. Effects of structural order on barrier properties. In Barrier Polymers and Structures; ACS Symposium Series; Koros, W.J., Ed.; ACS: Washington, DC, USA, 1990; Volume 423, pp. 61–91. [Google Scholar]





| Membrane | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| M | 22.570 ± 2.60 | 3.745 ± 1.71 |
| M-1 | 28.640 ± 3.18 | 3.322 ± 1.96 |
| M-2 | 30.672 ± 3.27 | 4.021 ± 2.83 |
| M-3 | 31.031 ± 2.19 | 4.529 ± 2.14 |
| M-4 | 31.925 ± 2.40 | 4.695 ± 1.20 |
| Temp. °C | Pw/l (GPU) | ||||
| M | M-1 | M-2 | M-3 | M-4 | |
| 30 | 2017.63 ± 3.20 | 1996.85 ± 2.28 | 2065.23 ± 2.35 | 2157.46 ± 2.34 | 2003.86 ± 3.32 |
| 40 | 2054.88 ± 2.60 | 2040.59 ± 0.94 | 2092.53 ± 3.00 | 2199.01 ± 3.03 | 2032.82 ± 28.98 |
| 50 | 2097.63 ± 1.91 | 2080.73 ± 2.28 | 2113.18 ± 3.17 | 2238.27 ± 3.25 | 2038.54 ± 28.35 |
| Temp. °C | PE/l (GPU) | ||||
| M | M-1 | M-2 | M-3 | M-4 | |
| 30 | 46.28 ± 0.45 | 31.48 ± 0.49 | 27.47 ± 0.54 | 25.82 ± 0.68 | 38.02 ± 0.59 |
| 40 | 52.90 ± 0.37 | 38.80 ± 0.80 | 44.99 ± 0.51 | 33.52 ± 0.54 | 50.01 ± 0.27 |
| 50 | 59.08 ± 0.90 | 51.01 ± 0.50 | 54.13 ± 0.64 | 47.53 ± 0.51 | 64.00 ± 1.03 |
| Temp. °C | Piw (Barrer) (104) | ||||
| M | M-1 | M-2 | M-3 | M-4 | |
| 30 | 8.07 ± 0.012 | 7.99 ± 0.009 | 8.26 ± 0.009 | 8.63 ± 0.009 | 8.02 ± 0.013 |
| 40 | 8.22 ± 0.010 | 8.16 ± 0.003 | 8.37 ± 0.012 | 8.37 ± 0.012 | 8.13 ± 0.115 |
| 50 | 8.39 ± 0.007 | 8.32 ± 0.009 | 8.45 ± 0.012 | 8.95 ± 0.013 | 8.15 ± 0.113 |
| Temp. °C | PiE (Barrer) (103) | ||||
| M | M-1 | M-2 | M-3 | M-4 | |
| 30 | 1.85 ± 0.018 | 1.26 ± 0.019 | 1.10 ± 0.021 | 1.03 ± 0.027 | 1.52 ± 0.023 |
| 40 | 2.12 ± 0.015 | 1.55 ± 0.032 | 1.80 ± 0.020 | 1.34 ± 0.021 | 2.00 ± 0.011 |
| 50 | 2.36 ± 0.036 | 2.04 ± 0.020 | 2.17 ± 0.025 | 1.90 ± 0.020 | 2.56 ± 0.041 |
| Membranes | Temp. (°C) | Permeation Flux (J) (kg/m2h) | Separation Selectivity (αsep) | Ref. |
|---|---|---|---|---|
| Na-Alg/3.0% Ag_Nps-PSSAMA_Na | 30 | 0.134 | 1140 | [54] |
| PVA/PSStSA-co-MA | 30 | 0.43 | 190 | [55] |
| Chitosan/PAA | 30 | 0.033 | 2216 | [56] |
| PVDF/Chitosan-Alginate | 50 | 0.095 | 202 | [57] |
| ECN silica membrane | 70 | 1.6 | 350 | [58] |
| Nafion-H+ | 70 | 5 | 2.5 | [59] |
| Nafion-Na+ | 70 | 0.5 | 5 | [59] |
| Nafion-K+ | 70 | 0.2 | 9.8 | [59] |
| PAN–PVP | 20 | 2.2 | 3.2 | [60] |
| Polystyrene | 40 | 0.005 | 101 | [61] |
| PVC | 40 | 0.003 | 63 | [62] |
| Alginic acid | 40 | 0.048 | 8.8 | [63] |
| Chitosan | 40 | 0.004 | 2208 | [64] |
| Chitosan acetate salt | 40 | 0.002 | 2556 | [65] |
| Chitosan/GA | 40 | 0.007 | 202 | [65] |
| Cationic PVA/GA | 40 | 0.089 | 709 | [66] |
| Anionic PVA/GA | 40 | 0.086 | 837 | [66] |
| PVA/GA | 40 | 0.189 | 335 | [66] |
| PVA/GA acrylic acid | 40 | 0.135 | 14 | [67] |
| Unmodified PVA | 40 | 0.091 | 15 | [67] |
| Cellulose acetate | 60 | 0.2 | 5.9 | [68] |
| Teflon-g-PVP | 25 | 2.2 | 2.9 | [68] |
| NaCMC (M) | 30 | 0.073 | 1521 | Present work |
| NaCMC/5 mass% Ge (M-1) | 30 | 0.072 | 2214 | Present work |
| NaCMC/10 mass% Ge (M-2) | 30 | 0.075 | 2624 | Present work |
| NaCMC/15 mass% Ge (M-3) | 30 | 0.078 | 2917 | Present work |
| NaCMC/20 mass% Ge (M-4) | 30 | 0.073 | 1839 | Present work |
| Temp. °C | Dw (10−11 m2/s) | ||||
| M | M-1 | M-2 | M-3 | M-4 | |
| 30 | 2.31 ± 0.0036 | 2.28 ± 0.0026 | 2.36 ± 0.0026 | 2.47 ± 0.0026 | 2.29 ± 0.0037 |
| 40 | 2.35 ± 0.0029 | 2.33 ± 0.0010 | 2.39 ± 0.0034 | 2.51 ± 0.0034 | 2.32 ± 0.0033 |
| 50 | 2.40 ± 0.0021 | 2.38 ± 0.0026 | 2.42 ± 0.0036 | 2.56 ± 0.0037 | 2.33 ± 0.0032 |
| Temp. °C | DE (10−14 m2/s) | ||||
| M | M-1 | M-2 | M-3 | M-4 | |
| 30 | 1.92 ± 0.018 | 1.30 ± 0.020 | 1.14 ± 0.022 | 1.07 ± 0.028 | 1.58 ± 0.024 |
| 40 | 2.19 ± 0.015 | 1.61 ± 0.033 | 1.86 ± 0.021 | 1.39 ± 0.022 | 2.07 ± 0.011 |
| 50 | 2.45 ± 0.037 | 2.11 ± 0.021 | 2.24 ± 0.026 | 1.97 ± 0.021 | 2.65 ± 0.043 |
| Membrane | Temperature (°C) | J × 10−2 (kg/m2h) | αsep. | Pi/l (GPU) |
| 30 | 7.3950 ± 0.00010 | 1521.52 ± 17.40 | 2017.63 ± 3.20 | |
| M | 40 | 7.5480 ± 0.00008 | 1355.66 ± 10.57 | 2054.88 ± 2.60 |
| 50 | 7.7195 ± 0.00009 | 1239.28 ± 17.70 | 2097.63 ± 1.91 | |
| 30 | 7.2781 ± 0.00008 | 2214.40 ± 36.45 | 1996.85 ± 2.28 | |
| M-1 | 40 | 7.4564 ± 0.00003 | 1835.87 ± 37.78 | 2040.59 ± 0.94 |
| 50 | 7.6357 ± 0.00008 | 1423.66 ± 15.27 | 2080.73 ± 2.28 | |
| 30 | 7.5129 ± 0.00008 | 2624.18 ± 51.00 | 2065.23 ± 2.35 | |
| M-2 | 40 | 7.6610 ± 0.00010 | 1623.57 ± 19.77 | 2092.53 ± 3.00 |
| 50 | 7.7613 ± 0.00011 | 1362.73 ± 17.59 | 2113.18 ± 3.17 | |
| 30 | 7.8403 ± 0.00007 | 2917.42 ± 79.56 | 2157.46 ± 2.34 | |
| M-3 | 40 | 8.0117 ± 0.00010 | 2289.79 ± 38.95 | 2199.01 ± 3.03 |
| 50 | 8.1929 ± 0.00010 | 1643.59 ± 20.25 | 2238.27 ± 3.25 | |
| 30 | 7.3220 ± 0.00010 | 1839.93 ± 31.93 | 2003.86 ± 3.32 | |
| M-4 | 40 | 7.4603 ± 0.00104 | 1418.65 ± 19.13 | 2032.82 ± 28.98 |
| 50 | 7.5207 ± 0.00104 | 1111.80 ± 9.41 | 2038.54 ± 28.35 |
| Source of Variation | Degree of Freedom (df) | Sum of Squares (SS) | Mean Squares (MS) | F | p-Value | Fcrit |
|---|---|---|---|---|---|---|
| Total Permeation Flux | ||||||
| Type of Membrane (M) | 4 | 0.000245 | 6.13 × 10−5 | 366.7553 | 5.3 × 10−25 | 2.689628 |
| Temperature (T) | 2 | 5.5 × 10−5 | 2.75 × 10−5 | 164.5891 | 6.72 × 10−17 | 3.31583 |
| Interaction (M*T) | 8 | 4.14 × 10−6 | 5.18 × 10−7 | 3.098072 | 0.011378 | 2.266163 |
| Error | 30 | 5.01 × 10−6 | 1.67 × 10−7 | |||
| Selectivity | ||||||
| Type of Membrane (M) | 4 | 4,795,879 | 1,198,970 | 1080.586 | 5.99 × 10−32 | 2.689628 |
| Temperature (T) | 2 | 5,714,707 | 2,857,354 | 2575.224 | 2.76 × 10−34 | 3.31583 |
| Interaction (M*T) | 8 | 1,242,278 | 155,284.7 | 139.9522 | 1.34 × 10−21 | 2.266163 |
| Error | 30 | 33,286.67 | 1109.556 | |||
| Parameters (kJ mol−1) | M | M-1 | M-2 | M-3 | M-4 |
|---|---|---|---|---|---|
| EP | 1.75 | 1.95 | 1.33 | 1.79 | 1.09 |
| Epw | 1.58 | 1.67 | 0.93 | 1.50 | 0.70 |
| EPE | 9.94 | 19.61 | 27.71 | 24.78 | 21.19 |
| ED | 1.59 | 1.69 | 0.95 | 1.51 | 0.72 |
| EDw | 1.58 | 1.67 | 0.93 | 1.50 | 0.70 |
| EDE | 9.94 | 19.61 | 27.71 | 24.78 | 21.19 |
| ∆HS | 0.16 | 0.26 | 0.37 | 0.28 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalahal, P.B.; Sajjan, A.M.; Yunus Khan, T.M.; Rajhi, A.A.; Achappa, S.; Banapurmath, N.R.; M, A.; Duhduh, A.A. Novel Polyelectrolyte Complex Membranes Containing Carboxymethyl Cellulose–Gelatin for Pervaporation Dehydration of Azeotropic Bioethanol for Biofuel. Polymers 2022, 14, 5114. https://doi.org/10.3390/polym14235114
Kalahal PB, Sajjan AM, Yunus Khan TM, Rajhi AA, Achappa S, Banapurmath NR, M A, Duhduh AA. Novel Polyelectrolyte Complex Membranes Containing Carboxymethyl Cellulose–Gelatin for Pervaporation Dehydration of Azeotropic Bioethanol for Biofuel. Polymers. 2022; 14(23):5114. https://doi.org/10.3390/polym14235114
Chicago/Turabian StyleKalahal, Prakash B., Ashok M. Sajjan, T. M. Yunus Khan, Ali A. Rajhi, Sharanappa Achappa, Nagaraj R. Banapurmath, Ashwini M, and Alaauldeen A. Duhduh. 2022. "Novel Polyelectrolyte Complex Membranes Containing Carboxymethyl Cellulose–Gelatin for Pervaporation Dehydration of Azeotropic Bioethanol for Biofuel" Polymers 14, no. 23: 5114. https://doi.org/10.3390/polym14235114
APA StyleKalahal, P. B., Sajjan, A. M., Yunus Khan, T. M., Rajhi, A. A., Achappa, S., Banapurmath, N. R., M, A., & Duhduh, A. A. (2022). Novel Polyelectrolyte Complex Membranes Containing Carboxymethyl Cellulose–Gelatin for Pervaporation Dehydration of Azeotropic Bioethanol for Biofuel. Polymers, 14(23), 5114. https://doi.org/10.3390/polym14235114