Influence of Molecular Weight and Grafting Density of PEG on the Surface Properties of Polyurethanes and Their Effect on the Viability and Morphology of Fibroblasts and Osteoblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polyurethane Substrates Preparation
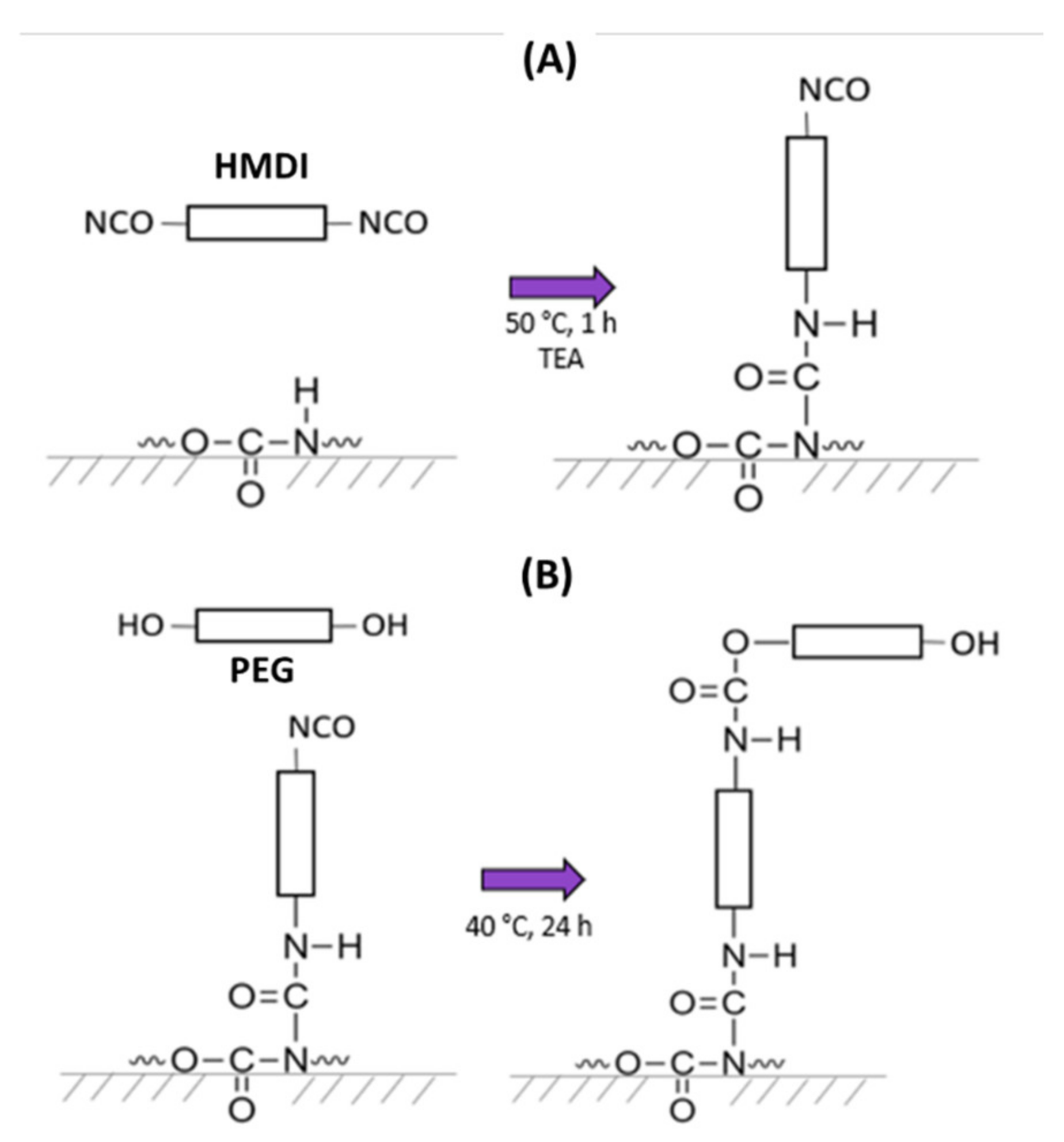
2.3. PEG Grafting Modification of PU Substrates
2.4. Physicochemical Characterization
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.2. Thermogravimetric Analysis (TGA)
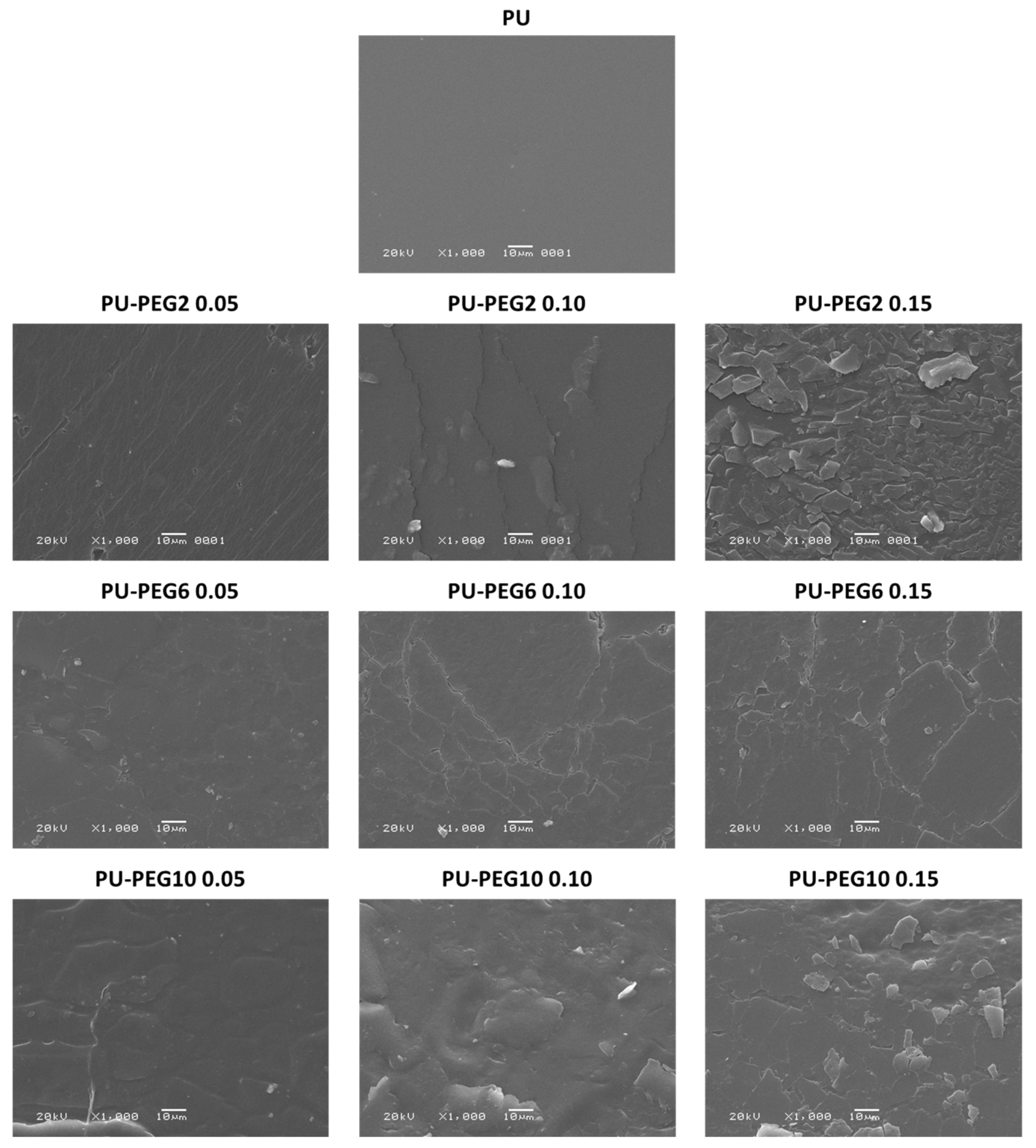
2.4.3. Scanning Electron Microscopy (SEM)
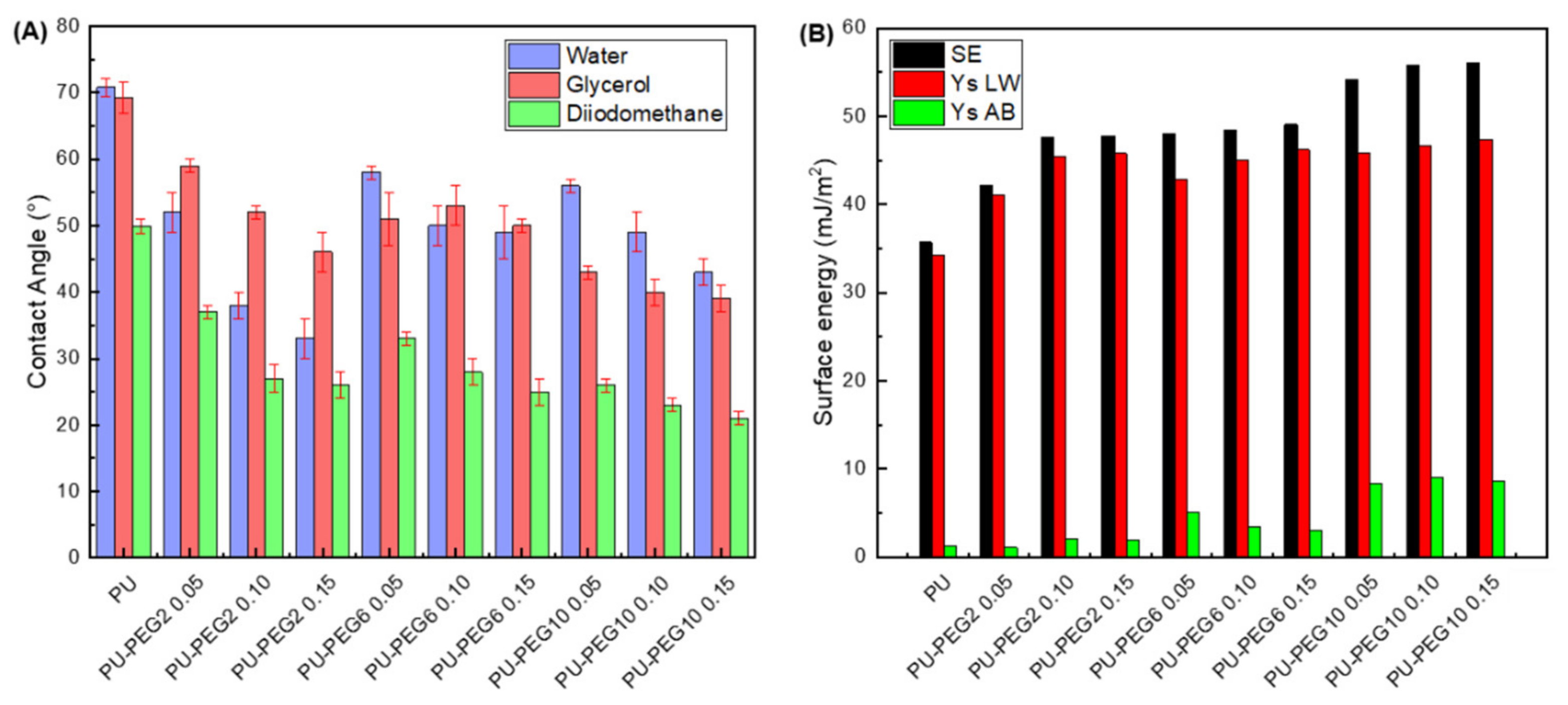
2.4.4. Contact Angle and Surface Free Energy Measurements
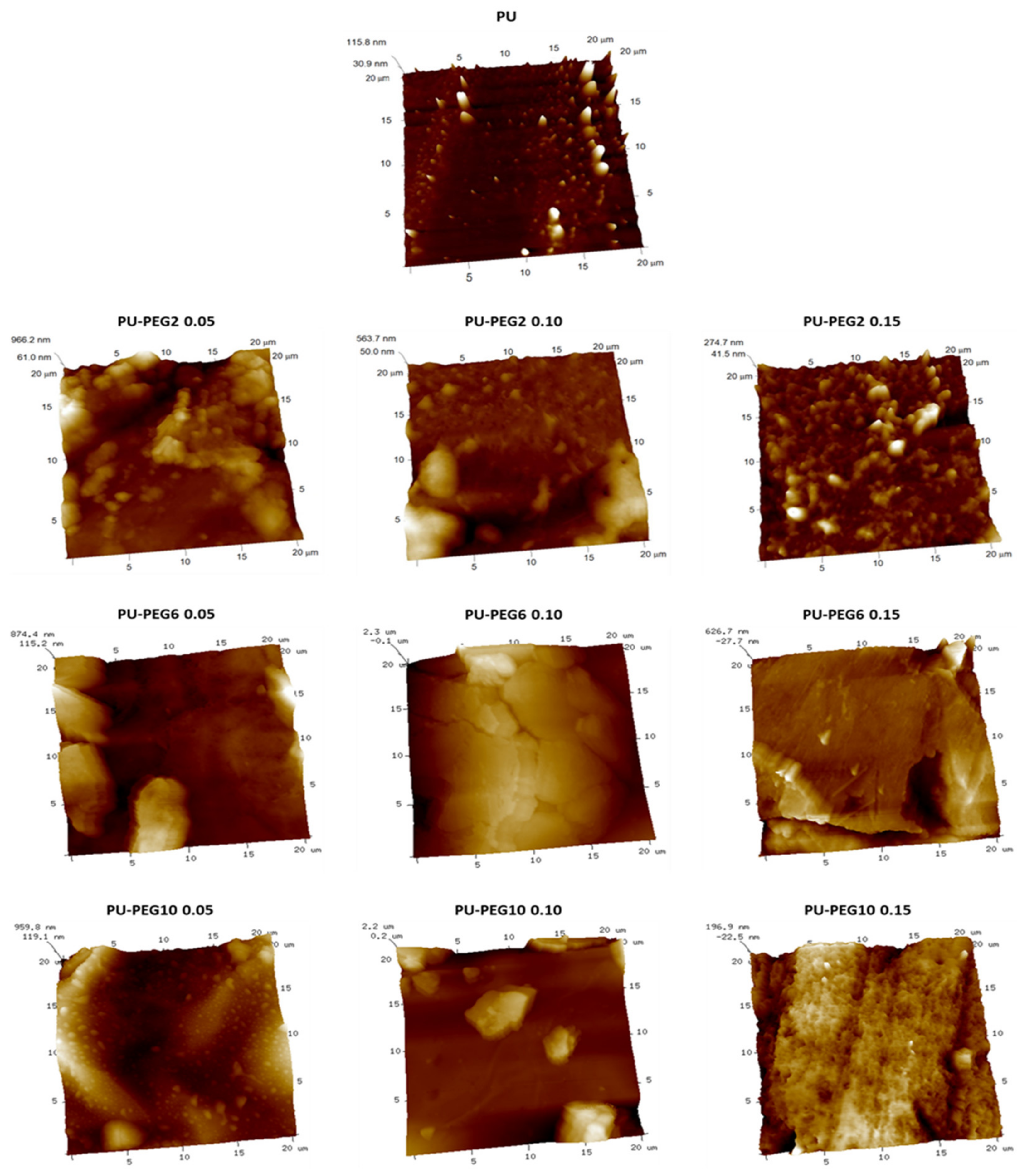
2.4.5. Atomic Force Microscopy (AFM)
2.5. Cellular Studies
2.5.1. Cell Culture
2.5.2. Cell Viability
2.5.3. Crystal Violet Staining
2.6. Statistical Analysis
3. Results
3.1. Spectroscopic Analysis
3.2. Thermogravimetric Analysis
3.3. Surface Topography
3.4. Contact Angles and Surface Free Energy
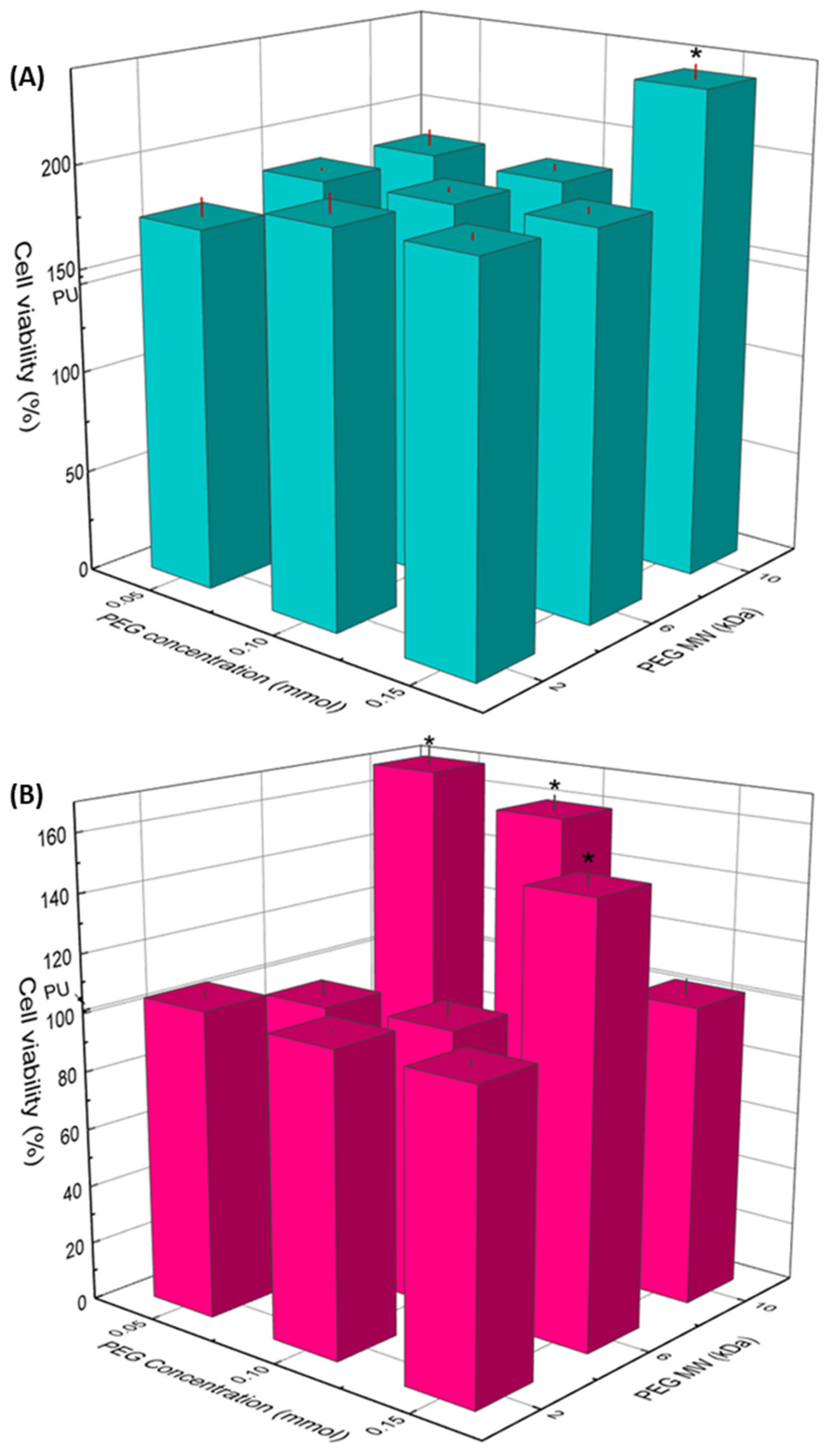
3.5. Cell Viability and Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, C.C.; Mi, F.L.; Hsu, S.H.; Don, T.M. Structure Characterizations and Protein Resistance of Chitosan Membranes Selectively Crosslinked by Poly(Ethylene Glycol) Dimethacrylate. Cellulose 2014, 21, 1431–1444. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, G.; Wei, Z.; Sang, L.; Qi, M. Hemocompatibility Evaluation of Polyurethane Film with Surface-Grafted Poly(Ethylene Glycol) and Carboxymethyl-Chitosan. J. Appl. Polym. Sci. 2013, 127, 308–315. [Google Scholar] [CrossRef]
- Bozuyuk, U.; Dogan, N.O.; Kizilel, S. Deep Insight into PEGylation of Bioadhesive Chitosan Nanoparticles: Sensitivity Study for the Key Parameters Through Artificial Neural Network Model. ACS Appl. Mater. Interfaces 2018, 10, 33945–33955. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, C. Polyurethane for Biomedical Applications: A Review of Recent Developments. In The Design and Manufacture of Medical Devices; Davim, J.P., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 115–151. [Google Scholar]
- Jung, J.-H.; Choi, C.-H.; Chung, S.; Chung, Y.-M.; Lee, C.-S. Microfluidic Synthesis of a Cell Adhesive Janus Polyurethane Microfiber. Lab Chip 2009, 9, 2596. [Google Scholar] [CrossRef] [PubMed]
- Lehle, K.; Stock, M.; Schmid, T.; Schopka, S.; Straub, R.H.; Schmid, C. Cell-Type Specific Evaluation of Biocompatibility of Commercially Available Polyurethanes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 90B, 312–318. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Guo, D.; Gu, N. A Facile Preparation of Poly(Ethylene Oxide)-Modified Medical Polyurethane to Improve Hemocompatibility. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 34–42. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, T.; Jiang, X.; Gu, N. The Surface Modification of Medical Polyurethane to Improve the Hydrophilicity and Lubricity: The Effect of Pretreatment. J. Appl. Polym. Sci. 2009, 116, 1284–1290. [Google Scholar] [CrossRef]
- Noorisafa, F.; Razmjou, A.; Emami, N.; Low, Z.; Korayem, H.; Kajani, A.A. Surface Modification of Polyurethane via Creating a Biocompatible Superhydrophilic Nanostructured Layer: Role of Surface Chemistry and Structure. J. Exp. Nanosci. 2016, 11, 1087–1109. [Google Scholar] [CrossRef]
- Shih, T.-Y.; Yang, J.-D.; Chen, Y.-H.; Hong, C.-W.; Yang, M.-J.; Chen, J.-H. Development of Peg-Containing Brush Copolymer: Their Effect on Resistance to Protein Adsorption Behaviors. Biomed. Eng. Appl. Basis Commun. 2013, 25, 1340008. [Google Scholar] [CrossRef]
- Røn, T.; Javakhishvili, I.; Jeong, S.; Jankova, K.; Lee, S. Low Friction Thermoplastic Polyurethane Coatings Imparted by Surface Segregation of Amphiphilic Block Copolymers. Colloid Interface Sci. Commun. 2021, 44, 100477. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, S.; Wu, L. Self-Repairing Nonfouling Polyurethane Coatings via 3D-Grafting of PEG-b-PHEMA-b-PMPC Copolymer. RSC Adv. 2015, 5, 104907–104914. [Google Scholar] [CrossRef]
- Jung, I.K.; Bae, J.W.; Choi, W.S.; Choi, J.H.; Park, K.D. Surface Graft Polymerization of Poly(Ethylene Glycol) Methacrylate onto Polyurethane via Thiol–Ene Reaction: Preparation and Characterizations. J. Biomater. Sci. Polym. Ed. 2009, 20, 1473–1482. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.D.; Pyo, K.B.; Park, S.Y.; Lee, H. Catechol-Grafted Poly(Ethylene Glycol) for PEGylation on Versatile Substrates. Langmuir 2010, 26, 3790–3793. [Google Scholar] [CrossRef]
- Wattendorf, U.; Merkle, H.P. PEGylation as a Tool for the Biomedical Engineering of Surface Modified Microparticles. J. Pharm. Sci. 2008, 97, 4655–4669. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Feng, X.; Zou, H.; Xu, W.; Zhuang, X. Poly(l-Glutamic Acid)-Cisplatin Nanoformulations with Detachable PEGylation for Prolonged Circulation Half-Life and Enhanced Cell Internalization. Bioact. Mater. 2021, 6, 2688–2697. [Google Scholar] [CrossRef]
- Wattendorf, U.; Koch, M.C.; Walter, E.; Vörös, J.; Textor, M.; Merkle, H.P. Phagocytosis of Poly(L-Lysine)-Graft-Poly (Ethylene Glycol) Coated Microspheres by Antigen Presenting Cells: Impact of Grafting Ratio and Poly (Ethylene Glycol) Chain Length on Cellular Recognition. Biointerphases 2006, 1, 123–133. [Google Scholar] [CrossRef]
- Xu, X.; Tang, J.; Han, Y.; Wang, W.; Chen, H.; Lin, Q. Surface PEGylation of Intraocular Lens for PCO Prevention: An in Vivo Evaluation. J. Biomater. Appl. 2016, 31, 68–76. [Google Scholar] [CrossRef]
- Tsai, W.; Chen, Y.; Chien, H. Collaborative Cell-Resistant Properties of Polyelectrolyte Multilayer Films and Surface PEGylation on Reducing Cell Adhesion to Cytophilic Surfaces. J. Biomater. Sci. Polym. Ed. 2009, 20, 1611–1628. [Google Scholar] [CrossRef]
- Sun, M.; Deng, J.; Tang, Z.; Wu, J.; Li, D.; Chen, H.; Gao, C. A Correlation Study of Protein Adsorption and Cell Behaviors on Substrates with Different Densities of PEG Chains. Colloids Surf. B Biointerfaces 2014, 122, 134–142. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, C.; Zhang, G. Synthesis of Polyurethane-g-Poly(Ethylene Glycol) Copolymers by Macroiniferter and Their Protein Resistance. Polym. Chem. 2011, 2, 1409. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, W.; Wang, Z.; Wang, Z.; Xie, Q.; Niu, H.; Guo, H.; Yuan, Y.; Liu, C. PEGylated Poly(Glycerol Sebacate)-Modified Calcium Phosphate Scaffolds with Desirable Mechanical Behavior and Enhanced Osteogenic Capacity. Acta Biomater. 2016, 44, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Wang, Z.; Zhang, J.; Zhu, J.; Ma, Y.; Yang, Z.; Yuan, Y. Optimized Synthesis of Biodegradable Elastomer PEGylated Poly(Glycerol Sebacate) and Their Biomedical Application. Polymers 2019, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.P.; Zeng, J.M.; Gao, S.J.; Ma, Y.X.; Shi, L.; Li, Y.; Too, H.-P.; Wang, S. Polyethylene Glycol Modified Polyethylenimine for Improved CNS Gene Transfer: Effects of PEGylation Extent. Biomaterials 2003, 24, 2351–2362. [Google Scholar] [CrossRef]
- Cai, L.; Wang, K.; Wang, S. Poly(Ethylene Glycol)-Grafted Poly(Propylene Fumarate) Networks and Parabolic Dependence of MC3T3 Cell Behavior on the Network Composition. Biomaterials 2010, 31, 4457–4466. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Shuai, X.; Unger, F.; Wittmar, M.; Xie, X.; Kissel, T. Synthesis, Characterization and Cytotoxicity of Poly(Ethylene Glycol)-Graft-Trimethyl Chitosan Block Copolymers. Biomaterials 2005, 26, 6343–6356. [Google Scholar] [CrossRef]
- Kasálková, N.; Makajová, Z.; Pařízek, M.; Slepička, P.; Kolářová, K.; Bačáková, L.; Hnatowicz, V.; Švorčík, V. Cell Adhesion and Proliferation on Plasma-Treated and Poly(Ethylene Glycol)-Grafted Polyethylene. J. Adhes. Sci. Technol. 2010, 24, 743–754. [Google Scholar] [CrossRef][Green Version]
- Alibeik, S.; Zhu, S.; Yau, J.W.; Weitz, J.I.; Brash, J.L. Modification of Polyurethane with Polyethylene Glycol–Corn Trypsin Inhibitor for Inhibition of Factor Xlla in Blood Contact. J. Biomater. Sci. Polym. Ed. 2012, 23, 1981–1993. [Google Scholar] [CrossRef]
- Dennes, T.J.; Schwartz, J. Controlling Cell Adhesion on Polyurethanes. Soft Matter 2008, 4, 86–89. [Google Scholar] [CrossRef]
- Xu, L.-C.; Siedlecki, C.A. Protein Adsorption, Platelet Adhesion, and Bacterial Adhesion to Polyethylene-Glycol-Textured Polyurethane Biomaterial Surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 668–678. [Google Scholar] [CrossRef]
- Ma, N.; Cao, J.; Li, H.; Zhang, Y.; Wang, H.; Meng, J. Surface Grafting of Zwitterionic and PEGylated Cross-Linked Polymers toward PVDF Membranes with Ultralow Protein Adsorption. Polymer 2019, 167, 1–12. [Google Scholar] [CrossRef]
- Wendels, S.; Avérous, L. Biobased Polyurethanes for Biomedical Applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef]
- Freij–Larsson, C.; Wesslén, B. Grafting of Polyurethane Surfaces with Poly(Ethylene Glycol). J. Appl. Polym. Sci. 1993, 50, 345–352. [Google Scholar] [CrossRef]
- McCafferty, E.; Wightman, J.P. Determination of the Acid-Base Properties of Metal Oxide Films and of Polymers by Contact Angle Measurements. J. Adhes. Sci. Technol. 1999, 13, 1415–1436. [Google Scholar] [CrossRef]
- Gudipati, C.S.; Finlay, J.A.; Callow, J.A.; Callow, M.E.; Wooley, K.L. The Antifouling and Fouling-Release Perfomance of Hyperbranched Fluoropolymer (HBFP)-Poly(Ethylene Glycol) (PEG) Composite Coatings Evaluated by Adsorption of Biomacromolecules and the Green Fouling Alga Ulva. Langmuir 2005, 21, 3044–3053. [Google Scholar] [CrossRef]
- Mattia, J.; Painter, P. A Comparison of Hydrogen Bonding and Order in a Polyurethane and Poly(Urethane−urea) and Their Blends with Poly(Ethylene Glycol). Macromolecules 2007, 40, 1546–1554. [Google Scholar] [CrossRef]
- Zimmerer, C.; Nagel, J.; Steiner, G.; Heinrich, G. Nondestructive Molecular Characterization of Polycarbonate–Polyvinylamine Composites after Thermally Induced Aminolysis. Macromol. Mater. Eng. 2016, 301, 648–652. [Google Scholar] [CrossRef]
- Guignot, C.; Betz, N.; Legendre, B.; Moel, A.L.; Yagoubi, N. Degradation of Segmented Poly(Etherurethane) Tecoflex® Induced by Electron Beam Irradiation: Characterization and Evaluation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2001, 185, 100–107. [Google Scholar] [CrossRef]
- Abreu-Rejón, A.D.; Herrera-Kao, W.; May-Pat, A.; Ávila-Ortega, A.; Rodríguez-Fuentes, N.; Uribe-Calderón, J.A.; Cervantes-Uc, J.M. Effect of PEG Grafting Density on Surface Properties of Polyurethane Substrata and the Viability of Osteoblast and Fibroblast Cells. J. Mater. Sci. Mater. Med. 2022, 33, 45. [Google Scholar] [CrossRef]
- Shufen, L.; Zhi, J.; Kaijun, Y.; Shuqin, Y.; Chow, W.K. Studies on the Thermal Behavior of Polyurethanes. Polym. Plast. Technol. Eng. 2006, 45, 95–108. [Google Scholar] [CrossRef]
- Good, R.J.; van Oss, C.J. The Modern Theory of Contact Angles and the Hydrogen Bond Components of Surface Energies. Mod. Approaches Wettability 1992, 1–27. [Google Scholar] [CrossRef]
- Chao, Y.C.; Su, S.K.; Lin, Y.W.; Hsu, W.T.; Huang, K.S. Interfacial Properties of Polyethylene Glycol/Vinyltriethoxysilane (PEG/VTES) Copolymers and Their Application to Stain Resistance. J. Surfactants Deterg. 2012, 15, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Galow, A.-M.; Rebl, A.; Koczan, D.; Bonk, S.M.; Baumann, W.; Gimsa, J. Increased Osteoblast Viability at Alkaline PH in Vitro Provides a New Perspective on Bone Regeneration. Biochem. Biophys. Rep. 2017, 10, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bumke, M.A.; Neri, D.; Elia, G. Modulation of Gene Expression by Extracellular PH Variations in Human Fibroblasts: A Transcriptomic and Proteomic Study. Proteomics 2003, 3, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of PH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Jian, L.; Li, B.; Liang, C.; Han, X.; Zhao, X.; Wang, D. Mg-Fe Layered Double Hydroxides Modified Titanium Enhanced the Adhesion of Human Gingival Fibroblasts through Regulation of Local PH Level. Mater. Sci. Eng. C 2021, 131, 112485. [Google Scholar] [CrossRef]
- Eagle, H. The Effect of Environmental PH on the Growth of Normal and Malignant Cells. J. Cell. Physiol. 1973, 82, 1–8. [Google Scholar] [CrossRef]
- Lie, S.O.; Schofield, B.H.; Taylor, H.A.; Doty, S.B. Structure and Function of the Lysosomes of Human Fibroblasts in Culture: Dependence on Medium PH. Pediatr. Res. 1973, 7, 13–19. [Google Scholar] [CrossRef][Green Version]
- Schreiber, R. Ca2+ Signaling, Intracellular PH and Cell Volume in Cell Proliferation. J. Membr. Biol. 2005, 205, 129. [Google Scholar] [CrossRef]
- Nitschke, R.; Riedel, A.; Ricken, S.; Leipziger, J.; Benning, N.; Fischer, K.-G.; Greger, R. The Effect of Intracellular PH on Cytosolic Ca2+ in HT29 Cells. Pflügers Arch. Eur. J. Physiol. 1996, 433, 98–108. [Google Scholar] [CrossRef]
- De Souza, C.N.; Breda, L.C.D.; Khan, M.A.; de Almeida, S.R.; Câmara, N.O.S.; Sweezey, N.; Palaniyar, N. Alkaline PH Promotes NADPH Oxidase-Independent Neutrophil Extracellular Trap Formation: A Matter of Mitochondrial Reactive Oxygen Species Generation and Citrullination and Cleavage of Histone. Front. Immunol. 2018, 8, 1849. [Google Scholar] [CrossRef]
- Santo-Domingo, J.; Demaurex, N. Calcium Uptake Mechanisms of Mitochondria. Biochim. Biophys. Acta Bioenergy 2010, 1797, 907–912. [Google Scholar] [CrossRef]
- Matsuyama, S.; Reed, J.C. Mitochondria-Dependent Apoptosis and Cellular PH Regulation. Cell Death Differ. 2000, 7, 1155–1165. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, W.; Sui, L.; Huang, Q.; Nan, Y.; Liu, J.; Ai, K. Reactive Oxygen Species-Based Nanomaterials for the Treatment of Myocardial Ischemia Reperfusion Injuries. Bioact. Mater. 2022, 7, 47–72. [Google Scholar] [CrossRef]
- Mei, H.; Laws, T.S.; Mahalik, J.P.; Li, J.; Mah, A.H.; Terlier, T.; Bonnesen, P.; Uhrig, D.; Kumar, R.; Stein, G.E.; et al. Entropy and Enthalpy Mediated Segregation of Bottlebrush Copolymers to Interfaces. Macromolecules 2019, 52, 8910–8922. [Google Scholar] [CrossRef]
- Teng, C.-Y.; Sheng, Y.-J.; Tsao, H.-K. Boundary-Induced Segregation in Nanoscale Thin Films of Athermal Polymer Blends. Soft Matter 2016, 12, 4603–4610. [Google Scholar] [CrossRef]
- Wu, D.T.; Fredrickson, G.H.; Carton, J.-P.; Ajdari, A.; Leibler, L. Distribution of Chain Ends at the Surface of a Polymer Melt: Compensation Effects and Surface Tension. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 2373–2389. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abreu-Rejón, A.D.; Herrera-Kao, W.A.; May-Pat, A.; Ávila-Ortega, A.; Rodríguez-Fuentes, N.; Uribe-Calderón, J.A.; Cervantes-Uc, J.M. Influence of Molecular Weight and Grafting Density of PEG on the Surface Properties of Polyurethanes and Their Effect on the Viability and Morphology of Fibroblasts and Osteoblasts. Polymers 2022, 14, 4912. https://doi.org/10.3390/polym14224912
Abreu-Rejón AD, Herrera-Kao WA, May-Pat A, Ávila-Ortega A, Rodríguez-Fuentes N, Uribe-Calderón JA, Cervantes-Uc JM. Influence of Molecular Weight and Grafting Density of PEG on the Surface Properties of Polyurethanes and Their Effect on the Viability and Morphology of Fibroblasts and Osteoblasts. Polymers. 2022; 14(22):4912. https://doi.org/10.3390/polym14224912
Chicago/Turabian StyleAbreu-Rejón, Antonio David, Wilberth Antonio Herrera-Kao, Alejandro May-Pat, Alejandro Ávila-Ortega, Nayeli Rodríguez-Fuentes, Jorge Alonso Uribe-Calderón, and José Manuel Cervantes-Uc. 2022. "Influence of Molecular Weight and Grafting Density of PEG on the Surface Properties of Polyurethanes and Their Effect on the Viability and Morphology of Fibroblasts and Osteoblasts" Polymers 14, no. 22: 4912. https://doi.org/10.3390/polym14224912
APA StyleAbreu-Rejón, A. D., Herrera-Kao, W. A., May-Pat, A., Ávila-Ortega, A., Rodríguez-Fuentes, N., Uribe-Calderón, J. A., & Cervantes-Uc, J. M. (2022). Influence of Molecular Weight and Grafting Density of PEG on the Surface Properties of Polyurethanes and Their Effect on the Viability and Morphology of Fibroblasts and Osteoblasts. Polymers, 14(22), 4912. https://doi.org/10.3390/polym14224912






