Thermal Stability, Flammability and Mechanical Performances of Unsaturated Polyester–Melamine Resin Blends and of Glass Fibre-Reinforced Composites Based on Them
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Casting and Curing of Resin Plaques
2.3. Composite Preparation
2.4. Characterisation of Resins
2.5. Flammability Assessment of Cast Resins and GFRCs
2.6. Mechanical Property Measurements of GFRCs
3. Results and Discussion
3.1. Curing Behaviour Study by DSC
3.2. Characterisation of Cured Resins and Resin Blends
3.2.1. Chemical Characterisation by Infrared Spectroscopy
3.2.2. Compatibility Study by DMTA
3.3. Thermal Stability of Resins and Resin Blends
Evolved Gas Analysis
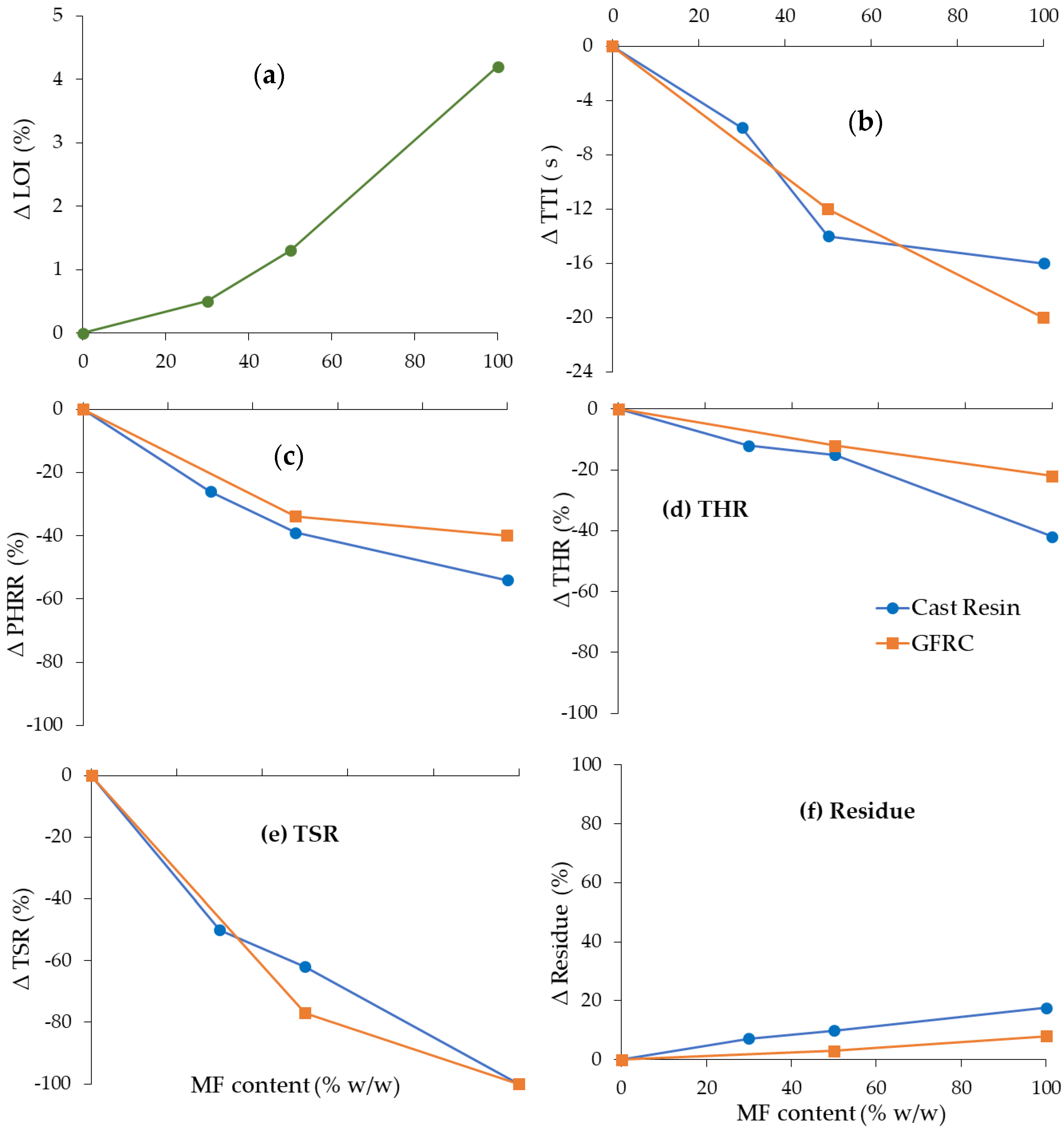
3.4. Flammability of Cast Resins and Composites
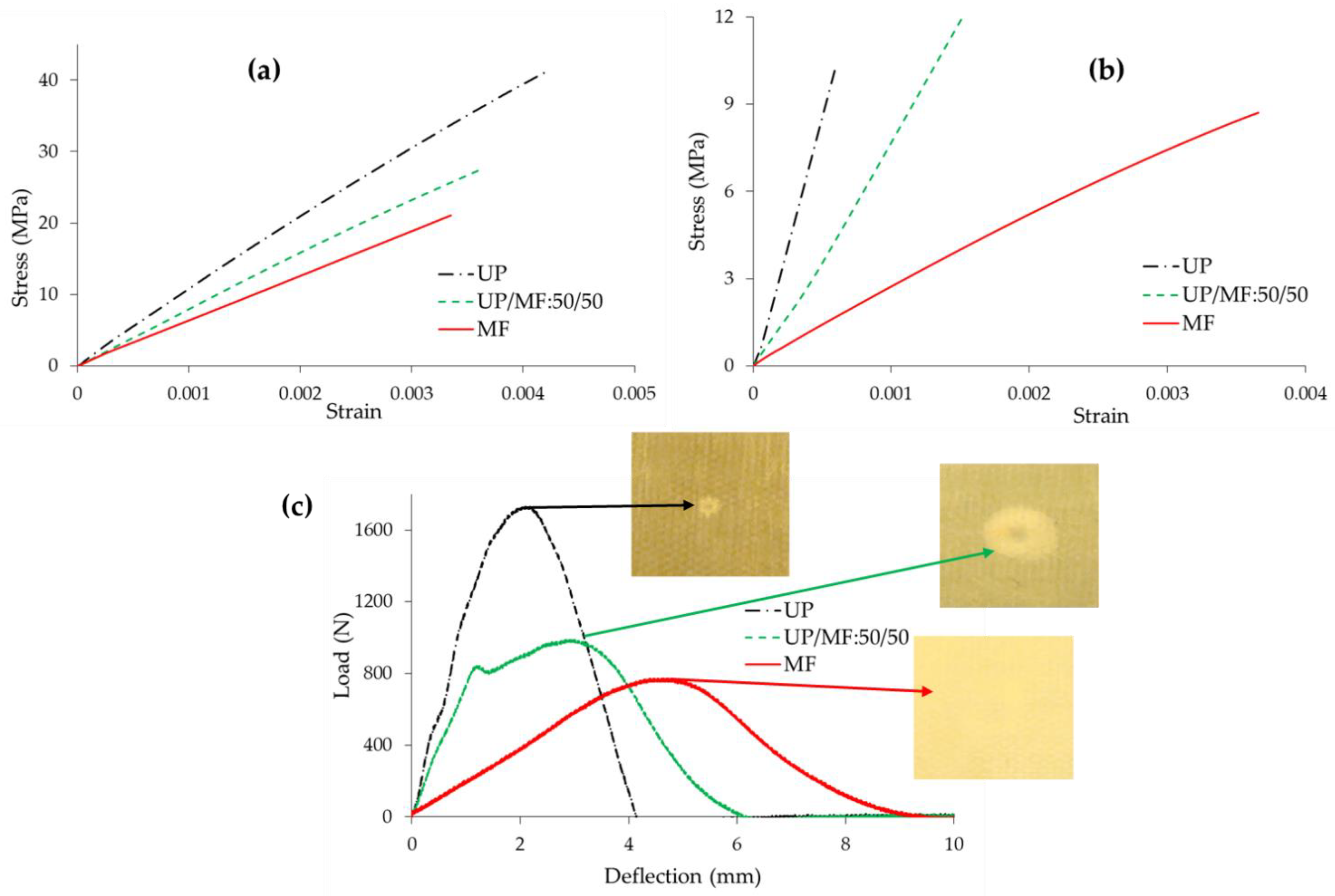
3.5. Mechanical Properties of Composite Laminates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seraji, S.M.; Song, P.; Varley, R.J.; Bourbigot, S.; Voice, D.; Wang, H. Fire-retardant unsaturated polyester thermosets: The state-of-the-art, challenges and opportunities. Chem. Eng. J. 2022, 430, 132785. [Google Scholar] [CrossRef]
- Kicko-Walczak, E.; Rymarz, G. Flame-retardant unsaturated polyester resins: An overview of past and recent developments. In Polyester; Camplibel, N.O., Ed.; IntechOpen: London, UK, 2018; Available online: https://doi.org/10.5772/intechopen.72536 (accessed on 22 September 2022).
- Kandola, B.K.; Kandare, E. Composites having improved fire resistance. In Advances in Fire Retardant Materials; Chapter 5; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing: Cambridge, UK, 2008; pp. 398–442. [Google Scholar]
- Irvine, D.J.; McCluskey, J.A.; Robinson, I.M. Fire hazards and some common polymers. Polym. Deg. Stab. 2000, 67, 383–396. [Google Scholar] [CrossRef]
- Hörold, S. Phosphorus flame retardants in thermoset resins. Polym. Deg. Stab. 1999, 64, 427–431. [Google Scholar] [CrossRef]
- Brown, J.R.; St John, N.A. Fire-retardant low-temperature-cured phenolic resins and composites. Trends Polym. Sci. 1996, 4, 416–420. Available online: https://resolver.scholarsportal.info/resolve/09664793/v04i0012/416_flprac.xml (accessed on 8 November 2022).
- Park, B.-D.; Jeong, H.-W. Cure kinetics of melamine-formaldehyde resin/clay/cellulose nanocomposites. J. Ind. Eng. Chem. 2010, 16, 375–379. [Google Scholar] [CrossRef]
- Raval, D.K.; Patel, A.J.; Narola, B.N. A Study on composites from casein modified melamine formaldehyde resin. Poly. Plast. Tech. Eng. 2006, 45, 293–299. [Google Scholar] [CrossRef]
- Dorieh, A.; Pour, M.F.; Movahed, S.G.; Pizzi, A.; Selakjani, P.P.; Kiamahalleh, M.V.; Hatefnia, H.; Shahavi, M.H.; Aghaei, R. A review of recent progress in melamine-formaldehyde resin based nanocomposites as coating materials. Prog. Org. Coat. 2022, 165, 106768. [Google Scholar] [CrossRef]
- Corcione, C.E.; Ferrari, F.; Striani, R.; Dubrulle, L.; Visconti, P.; Zammarano, M.; Greco, A. Optimizing Flame Retardancy and Durability of Melamine-Formaldehyde/Solid-Urban-Waste Composite Panels. Polymers 2021, 13, 712. [Google Scholar] [CrossRef]
- Xiong, Z.; Chen, N.; Wang, Q. Preparation and properties of melamine formaldehyde resin modified by functionalized nano-SiO2 and polyvinyl alcohol. Polym. Polym. Compos. 2021, 29, 96–106. [Google Scholar] [CrossRef]
- Merline, D.J.; Vukusic, S.; Abdala, A.A. Melamine formaldehyde: Curing studies and reaction mechanism. Polym. J. 2013, 45, 413–419. [Google Scholar] [CrossRef]
- Stoeckel, F.; Konnerth, J.; Gindl-Altmutter, W. Mechanical properties of adhesives for bonding wood—A review. Int. J. Adhes. Adhes. 2013, 45, 32–41. [Google Scholar] [CrossRef]
- Puglia, D.; Manfredi, L.B.; Vazquez, A.; Kenny, J.M. Thermal degradation and fire resistance of epoxy–amine–phenolic blends. Polym. Deg. Stab. 2001, 73, 521–527. [Google Scholar] [CrossRef]
- Cherian, A.B.; Varghese, L.A.; Thachil, E.T. Epoxy-modified, unsaturated polyester hybrid networks. Eur. Polym. J. 2007, 43, 1460–1469. [Google Scholar] [CrossRef]
- Kandola, B.K.; Ebdon, J.R. Flammability and thermal stability of unsaturated polyester resin based blends and composites. In Unsaturated Polyester Resins; Chapter 16; Thomas, S., Hosur, M., Chiraryil, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 435–469. [Google Scholar]
- Deli, D.; Kandola, B.K.; Ebdon, J.R.; Krishnan, L. Blends of unsaturated polyester and phenolic resins for application as fire-resistant matrices in fibre-reinforced composites. Part 1: Identifying compatible, co-curable resin mixtures. J. Mater. Sci. 2013, 48, 6929–6942. [Google Scholar] [CrossRef]
- Kandola, B.K.; Krishnan, L.; Ebdon, J.R. Blends of Unsaturated Polyester and Phenolic Resins for Application as Fire-Resistant Matrices in Fibre-Reinforced Composites. Part 2: Effects of Resin Structure, Compatibility and Composition on fire performance. Polym. Deg. Stab. 2015, 113, 154–167. [Google Scholar] [CrossRef]
- Kandola, B.K.; Krishnan, L.; Ebdon, J.R. Blends of unsaturated polyester and phenolic resins for application as fire-resistant matrices in fibre-reinforced composites: Effects of added flame retardants. Polym. Deg. Stab. 2014, 106, 129–137. [Google Scholar] [CrossRef]
- Kandola, B.K.; Krishnan, L.; Deli, D.; Luangtriratana, P.; Ebdon, J.R. Fire and mechanical properties of a novel free-radically cured phenolic resin based on a methacrylate-functional novolac and of its blends with an unsaturated polyester resin. RSC Adv. 2015, 5, 33772–33785. [Google Scholar] [CrossRef]
- Kandola, B.K.; Ebdon, J.R.; Luangtriratana, P.; Krishnan, L. Novel flame retardant thermoset resin blends derived from a free-radically cured vinylbenzylated phenolic novolac and an unsaturated polyester for marine composites. Polym. Deg. Stab. 2016, 127, 56–64. [Google Scholar] [CrossRef]
- Kandola, B.K.; Krishnan, L.; Ebdon, J.R.; Myler, P. Structure-property relationships in structural glass fibre reinforced composites containing unsaturated polyester-phenolic resin matrix blends. Comp. Part B 2020, 182, 107607. [Google Scholar] [CrossRef]
- Xu, W.; Yu, C.; Zhao, X.; Xu, J.; Jiang, M. Melamine formaldehyde/polyvinyl alcohol composite fiber: Structures and properties controlled by reaction-induced phase separation. J. Appl. Polym. Sci. 2015, 133, 42198–42204. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K.; Price, D. The potential of metal oxalates as novel flame retardants and synergists for engineering polymers. Polym. Degrad. Stab. 2014, 110, 290–297. [Google Scholar] [CrossRef]
- Kandola, B.K.; Horrocks, A.R.; Horrocks, S. Evidence of interaction in flame-retardant fibre-intumescent combinations by thermal analytical techniques. Thermochim. Acta 1997, 294, 113–125. [Google Scholar] [CrossRef]
- Kandelbauer, A.; Wuzella, G.; Mahendran, A.; Taudes, I.; Widsten, P. Using isoconversional kinetic analysis of liquid melamine-formaldehyde resin curing to predict laminate surface properties. J. Appl. Polym. Sci. 2009, 113, 2649–2660. [Google Scholar] [CrossRef]
- Dawbarn, M.; Ebdon, J.R.; Hewitt, S.J. Examination of some melamine-formaldehyde adducts by 13C nuclear magnetic resonance. Polymer 1978, 19, 1309–1312. [Google Scholar] [CrossRef]
- Ebdon, J.R.; Hunt, B.J.; O’Rourke, W.T.S.; Parkin, J. Characterisation of some melamine-formaldehyde condensates and some cured resins by 1H, 13C and 15N N.M.R. spectroscopy. Brit. Polym. J. 1988, 20, 327–334. [Google Scholar] [CrossRef]
- Hagstrand, P.O.; Klason, C.; Svensson, L.; Lundmark, S. Rheokinetical behaviour of melamine-formaldehyde resins. Polym. Eng. Sci. 1999, 39, 2019–2029. [Google Scholar] [CrossRef]
- Stark, W. Investigation of curing behaviour of melamine/phenolic (MP) thermosets. Polym. Test. 2010, 29, 723–728. [Google Scholar] [CrossRef]
- Baraka, A.; Hall, P.J.; Heslop, M.J. Preparation and characterization of melamine-formaldehyde-DTPA chelating resin and its use as an adsorbent for heavy metals removal from waste water. React. Funct. Polym. 2007, 67, 585–600. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Luo, S.; Li, S. Preparation and property analysis of melamine formaldehyde foam. Adv. Mater. Phys. Chem. 2012, 2, 63–67. [Google Scholar] [CrossRef]
- Cai, X.; Riedl, B.; Wan, H.; Zhang, S.Y.; Wang, X.M. A study on the curing and viscoelastic characteristics of melamine–urea–formaldehyde resin in the presence of aluminium silicate nanoclays. Comp. Part A 2010, 41, 604–611. [Google Scholar] [CrossRef]
- Levchik, S.V. Thermal degradation of thermosetting polymers. In Plastics Flammability Handbook, 3rd ed.; Troitzsch, J., Ed.; Hanser: Munich, Germany, 2004; p. 86. [Google Scholar]
- Ahamad, T.; Alshehri, S.M. Thermal degradation and evolved gas analysis: A polymeric blend of urea formaldehyde (UF) and epoxy (DGEBA) resin. Arab. J. Chem. 2014, 7, 1140–1147. [Google Scholar] [CrossRef]
- Devallencourt, C.; Saiter, J.M.; Fafet, A.; Ubrich, E. Thermogravimetry/Fourier transform infrared coupling investigations to study the thermal stability of melamine formaldehyde resin. Thermochim. Acta 1995, 259, 143–151. [Google Scholar] [CrossRef]
- Chiu, H.-T.; Chiu, S.-H.; Jeng, R.-E.; Chung, J.-S. A study of the combustion and fire-retardance behaviour of unsaturated polyester/phenolic resin blends. Polym. Deg. Stab. 2000, 70, 505–514. [Google Scholar] [CrossRef]
- Sumner, M.J.; Sankarapandian, M.; McGrath, J.E.; Riffle, S.; Sorathia, U. Flame retardant novolac–bisphthalonitrile structural thermosets. Polymer 2002, 43, 5069–5076. [Google Scholar] [CrossRef]

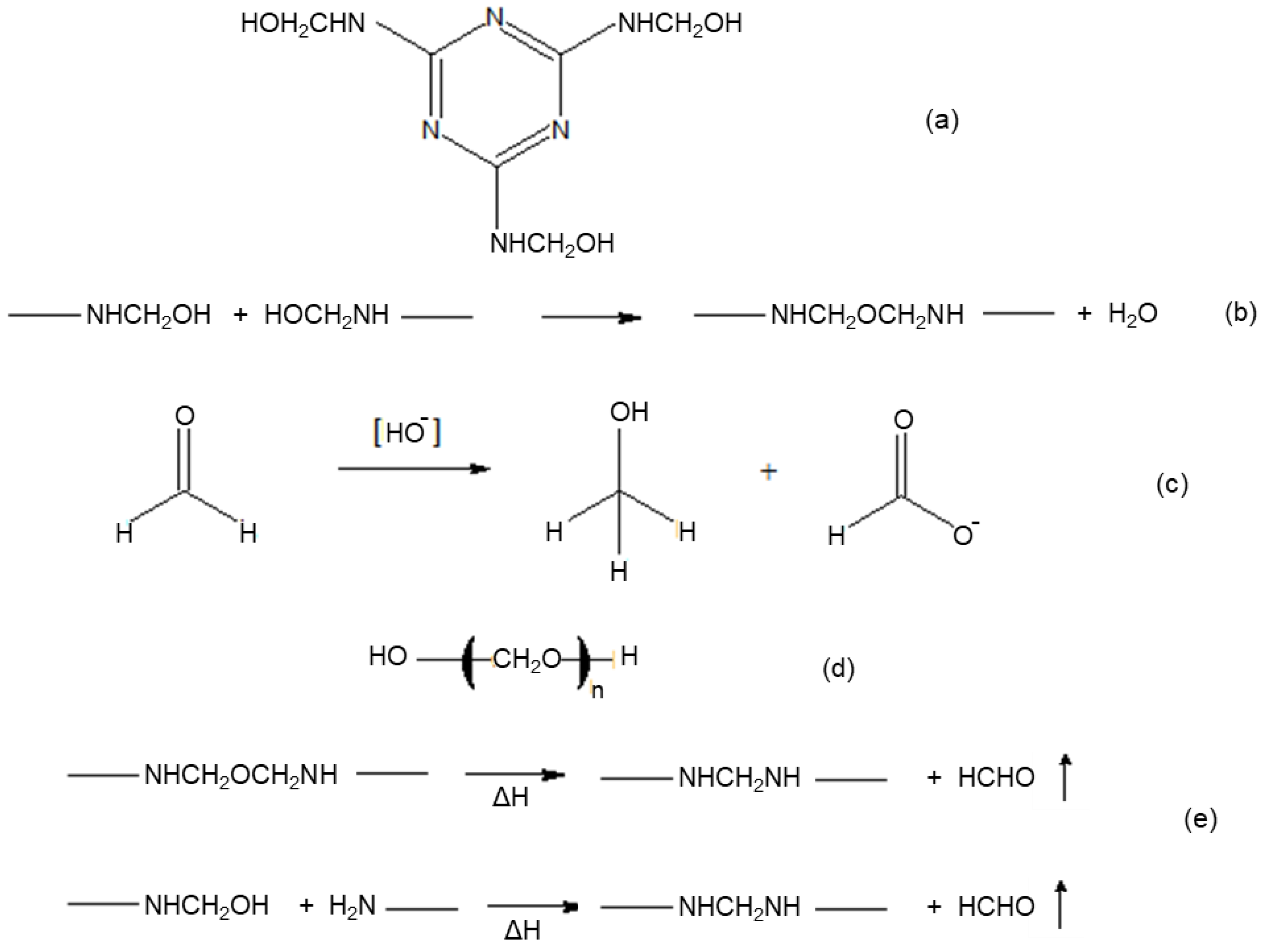






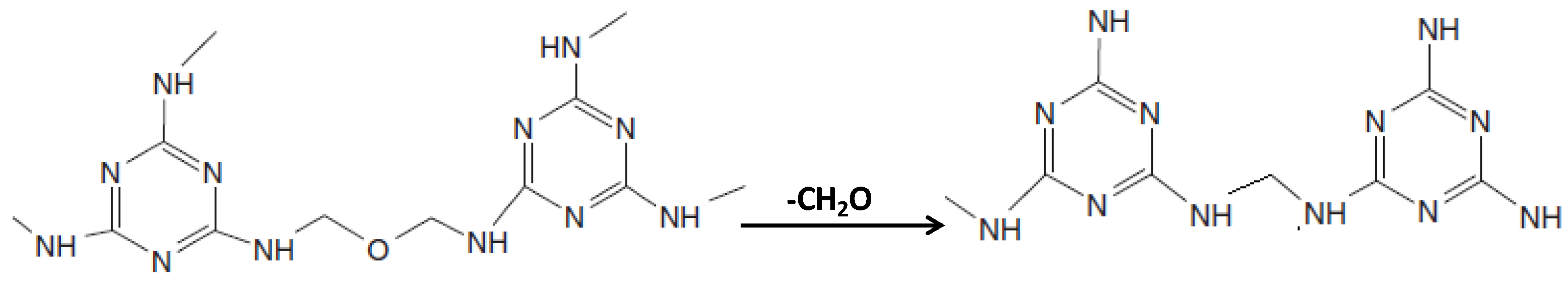
| Resin | Curing Conditions |
|---|---|
| UP | RT 24 h, 80 °C 6 h |
| MF | RT 24 h, 50 °C 24 h, 80 °C 12 h |
| UP/MF:70/30 | RT 24 h, 80 °C 24 h |
| UP/MF:50/50 | RT 24 h, 80 °C 24 h |
| Uncured Sample | Exothermic Peak (C, Figure 1) (°C) | Endothermic (°C) | Heat of Reaction (Exotherm) (J/g) | ||||
|---|---|---|---|---|---|---|---|
| Peak1 (A, Figure 1) | Peak 2 (B, Figure 1) | ||||||
| Tonset | Tpeak | Tonset | Tpeak | Tonset | Tpeak | ||
| UP | 30 | 82 | - | - | - | 282 | |
| SD-MF | - | - | 30 | 75 | 132 | 166 | - |
| MF * | - | - | 30 | 134 | 159 | 185 | - |
| UP/MF:70/30 | 30 | 74 | - | - | 137 | 167 | 197 |
| UP/MF:50/50 | 30 | 80 | - | - | 134 | 207 | 88 |
| Sample | Tg (°C) | Storage Modulus at 40 °C (MPa) |
|---|---|---|
| UP | 94 | 2122 |
| UP/MF:70/30 | 80 | 913 |
| UP/MF:50/50 | 77 | 1044 |
| Sample | Air | Nitrogen | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp Range (°C) | Mass Loss (%) | DTG Max (°C) | DTA Max (°C) (Exo) | CY (%) | Temp Range (°C) | Mass Loss (%) | DTG Max (°C) | DTA Max (°C) (Exo) | CY (%) | ||
| UP | RT–183 | 0.9 | RT–183 | 0.9 | |||||||
| 183–435 | 93.1 | 373 | 352(En.S):404 | 0.4 | 183–462 | 94.8 | 383 | 369 | 4.3 | ||
| 435–566 | 5.6 | 532 | 533 | ||||||||
| MF | RT–110 | 6.3 | RT–110 | 2.3 | |||||||
| 110–288 | 39.4 | 173,218 | 218 (En.S) | 110–287 | 31.7 | 246 | 250 | ||||
| 288–382 | 25.7 | 356 | 378 (En.S) | 13.1 | 287–383 | 36 | 358 | 367 | 21.5 | ||
| 382–639 | 26.0 | 597 | 593 | 383–524 | 9 | 472 | 474 | ||||
| UP/MF:70/30 | RT–206 | 5.1 | RT–276 | 14.5 | 220 | ||||||
| 206–437 | 78.4 | 363 | 384 (En.S) | 4.4 | 276–463 | 74.9 | 366 | 377 | 9.0 | ||
| 437–605 | 15.6 | 559 | 556 | ||||||||
| UP/MF:50/50 | RT–206 | 9.9 | RT–276 | 22 | 221 | ||||||
| 206–475 | 73.9 | 363 | 376 (En) | 7.4 | 276–453 | 63 | 373 | 383 | 10.9 | ||
| 475–627 | 16 | 576,621 | 573,623 | ||||||||
| Sample | Gas Evolved (FTIR Peak((cm−1) × 10−2)) | |||||||
|---|---|---|---|---|---|---|---|---|
| H2O | CH3OH | Ali.Hyd. Carbons | CO2 | Phthalic Anhydride | Styrene | NH3 | HNCO | |
| (3750) | (1035) | (2925) | (2360) | (1866) | (700) | (965) | (2284) | |
| UP | 0.3 | 0.89 | 1.49 | 1.91 | 2.68 | 2.53 | 0 | 0 |
| UP/MF:70/30 | 0.64 0.71 | 2.74 1.78 | 1.94 1.90 | 1.33 1.90 | 1.43 1.88 | 1.12 1.77 | 1.69 1.53 | 0.94 0.89 |
| UP/MF:50/50 | 1.1 0.98 | 3.04 2.38 | 2.3 2.18 | 1.03 1.89 | 0.72 1.34 | 1.00 1.27 | 2.72 2.56 | 1.58 1.49 |
| MF | 1.65 | 3.86 | 2.86 | 1.87 | 0 | 0.00 | 5.11 | 2.97 |
| Sample | LOI (%) | Cone Calorimetric Results at at 50 kW/m2 Heat Flux | |||||
|---|---|---|---|---|---|---|---|
| TTI (s) | FO (s) | PHRR (kW/m²) | THR (MJ/m2) | TSR (m²/m²) | Residue (%) | ||
| UP | 17.9 ± 0.1 | 36 | 188 | 1110 | 83 | 4813 | 1.3 |
| UP/MF:70/30 | 18.4 ± 0.2 (19.2) | 30 | 192 | 822 | 73 | 2385 | 8.4 |
| UP/MF:50/50 | 19.2 ± 0.1 (20.0) | 22 | 196 | 674 | 71 | 1832 | 11.1 |
| MF | 22.1 ± 0.2 | 20 | 198 | 513 | 48 | 0 | 18.9 |
| Sample | Mass Fraction (%) | Cone Calorimetric Results at 50 kW/m2 Heat Flux | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Glass | Resin | TTI (s) | FO (s) | PHRR (kW/m²) | THR (MJ/m2) | TSR (m²/ m²) | Residue (%) | ||
| UP | 57 | 43 | 34 | 128 | 599 | 33 | 1640 | 57 | |
| UP/MF:50/50 | 58 | 42 | 22 | 127 | 395 | 29 | 380 | 60 | |
| MF | 55 | 45 | 14 | 121 | 361 | 26 | 0 | 65 | |
| Sample | Thick. (mm) | FVF | Tensile Modulus (GPa) | Flexural Modulus (GPa) | Impact Modulus (GPa) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Original | Normalised * | Original | Normalised * | ||||||
| UP | 2.4 | 35.2 | 11.1 ± 0.6 | 12.6 | 15.8 ± 0.6 | 18 | 19.6 ± 0.0 | ||
| UP/MF:50/50 | 2.5 | 39.0 | 8.2 ± 0.2 | 8.4 | 8.5 ± 0.4 | 8.7 | 10.1 ± 0.1 | ||
| MF | 2.5 | 38.2 | 6.4 ± 0.3 | 6.7 | 3.5 ± 0.2 | 3.7 | 2.7 ± 0.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnan, L.; Kandola, B.K.; Deli, D.; Ebdon, J.R. Thermal Stability, Flammability and Mechanical Performances of Unsaturated Polyester–Melamine Resin Blends and of Glass Fibre-Reinforced Composites Based on Them. Polymers 2022, 14, 4885. https://doi.org/10.3390/polym14224885
Krishnan L, Kandola BK, Deli D, Ebdon JR. Thermal Stability, Flammability and Mechanical Performances of Unsaturated Polyester–Melamine Resin Blends and of Glass Fibre-Reinforced Composites Based on Them. Polymers. 2022; 14(22):4885. https://doi.org/10.3390/polym14224885
Chicago/Turabian StyleKrishnan, Latha, Baljinder K. Kandola, Dario Deli, and John R. Ebdon. 2022. "Thermal Stability, Flammability and Mechanical Performances of Unsaturated Polyester–Melamine Resin Blends and of Glass Fibre-Reinforced Composites Based on Them" Polymers 14, no. 22: 4885. https://doi.org/10.3390/polym14224885
APA StyleKrishnan, L., Kandola, B. K., Deli, D., & Ebdon, J. R. (2022). Thermal Stability, Flammability and Mechanical Performances of Unsaturated Polyester–Melamine Resin Blends and of Glass Fibre-Reinforced Composites Based on Them. Polymers, 14(22), 4885. https://doi.org/10.3390/polym14224885








