Functionalized Moringa oleifera Gum as pH-Responsive Nanogel for Doxorubicin Delivery: Synthesis, Kinetic Modelling and In Vitro Cytotoxicity Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
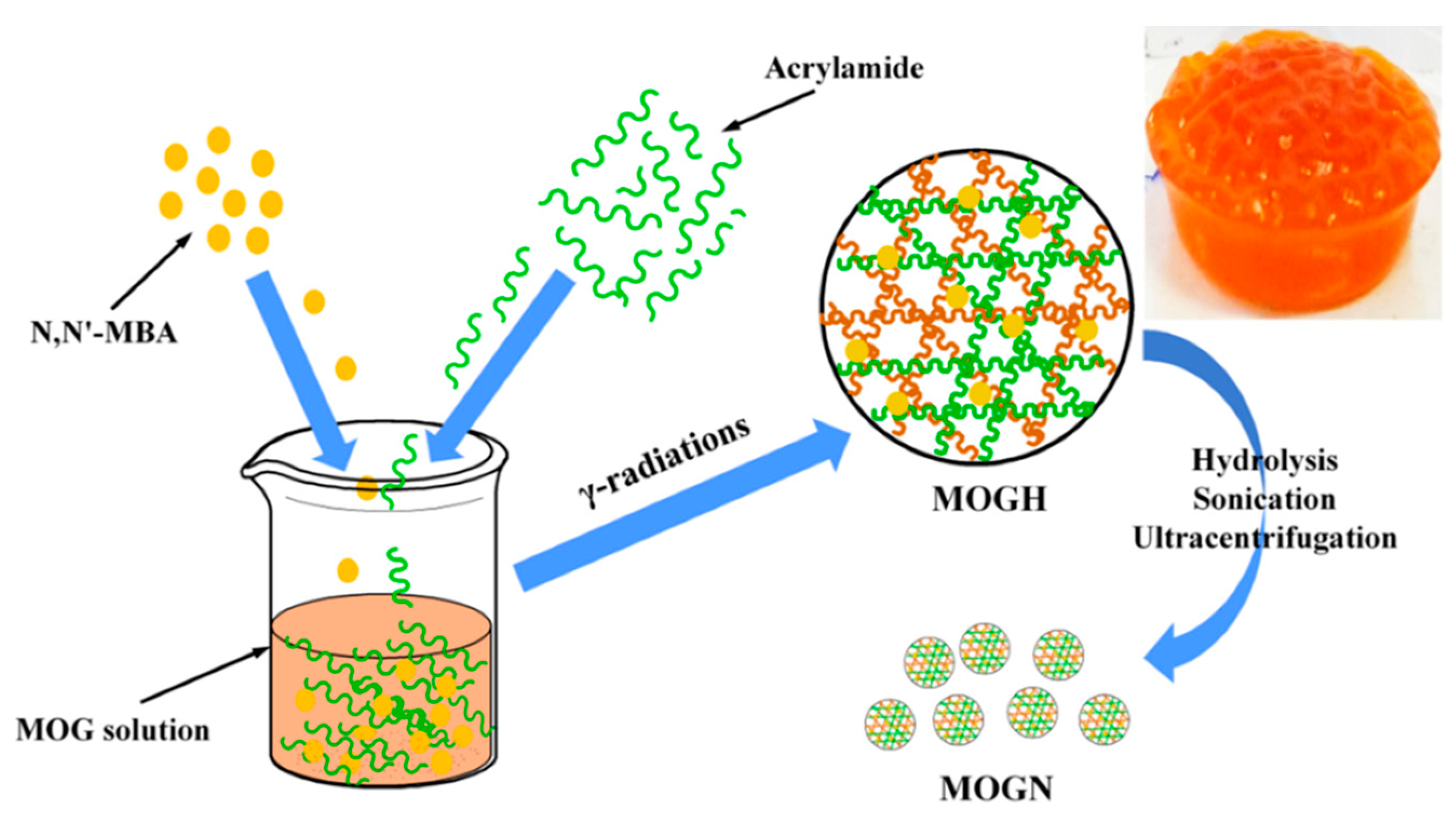
2.2. Preparation of Moringa oleifera Gum Nanogel (MOGN) and DOX-Loaded MOGN (DOX–MOGN)
2.3. Swelling Studies of Moringa oleifera Gum Nanogel (MOGN)
2.4. DOX Loading and Release Studies
2.5. Characterization Studies
2.6. Antitumor Activity Study
2.6.1. Cell Culture
2.6.2. Anti–Tumor Activity Assay
3. Results and Discussion
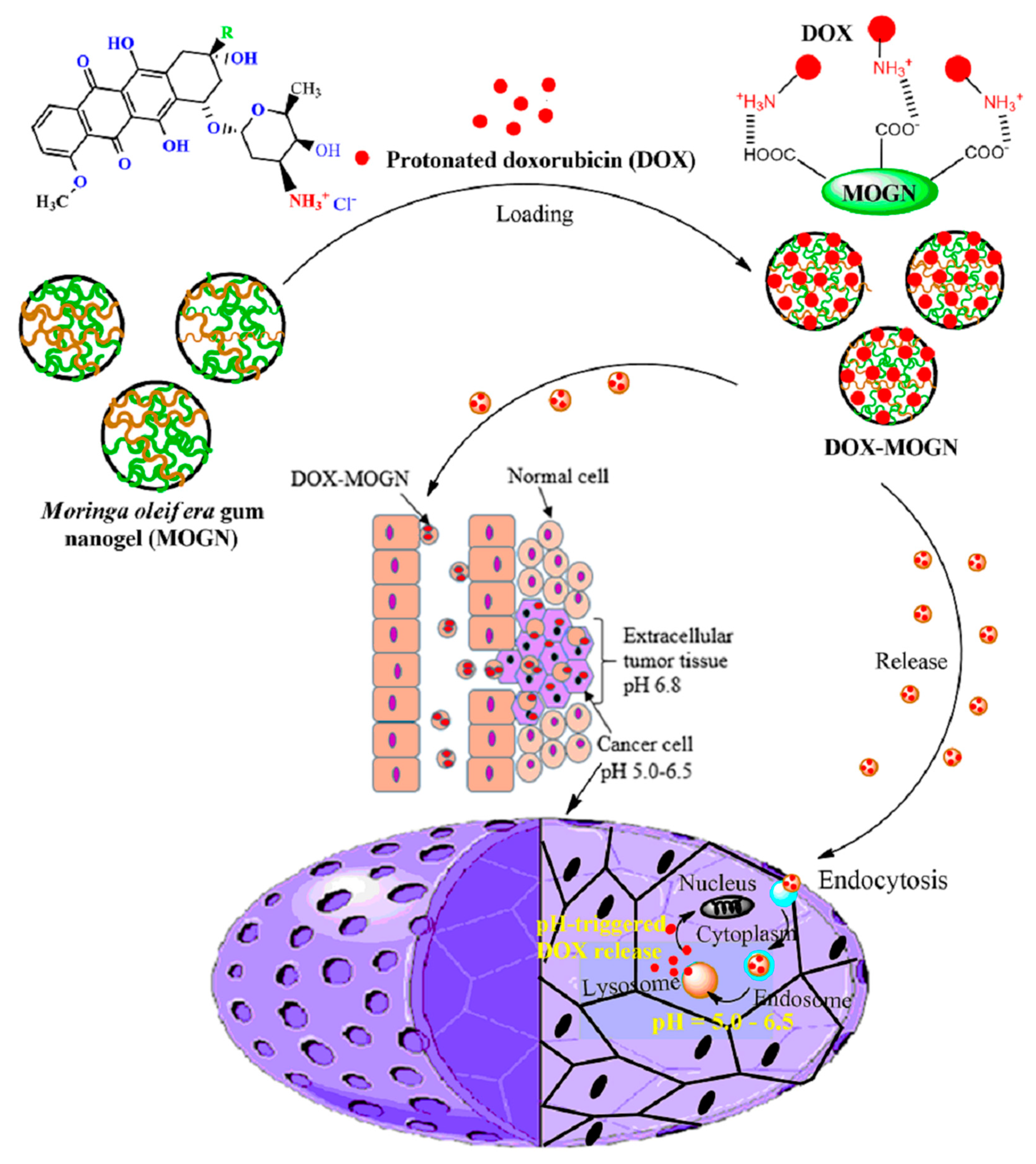
3.1. Synthesis of Moringa oleifera Gum Nanogel (MOGN)
3.2. All Characterization Studies
3.2.1. FTIR Analysis
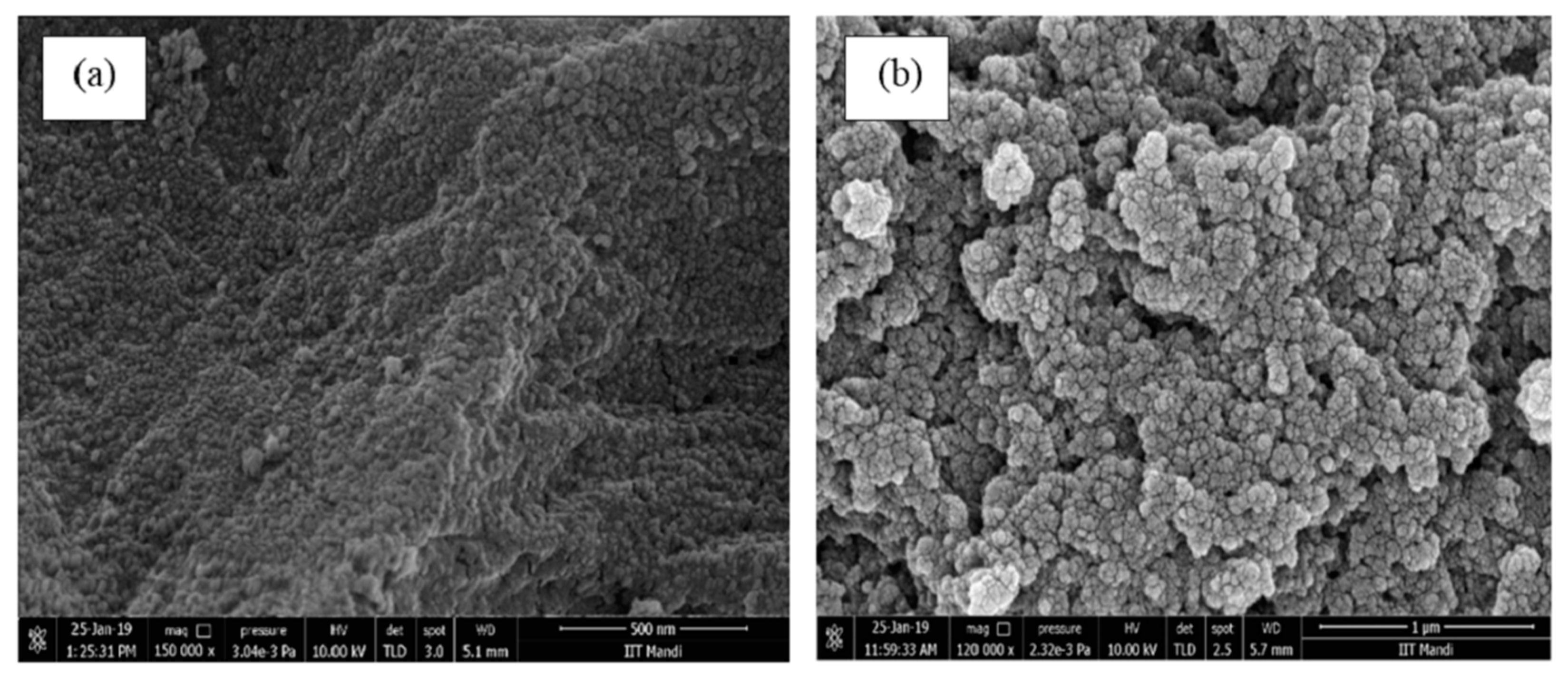
3.2.2. SEM Analysis
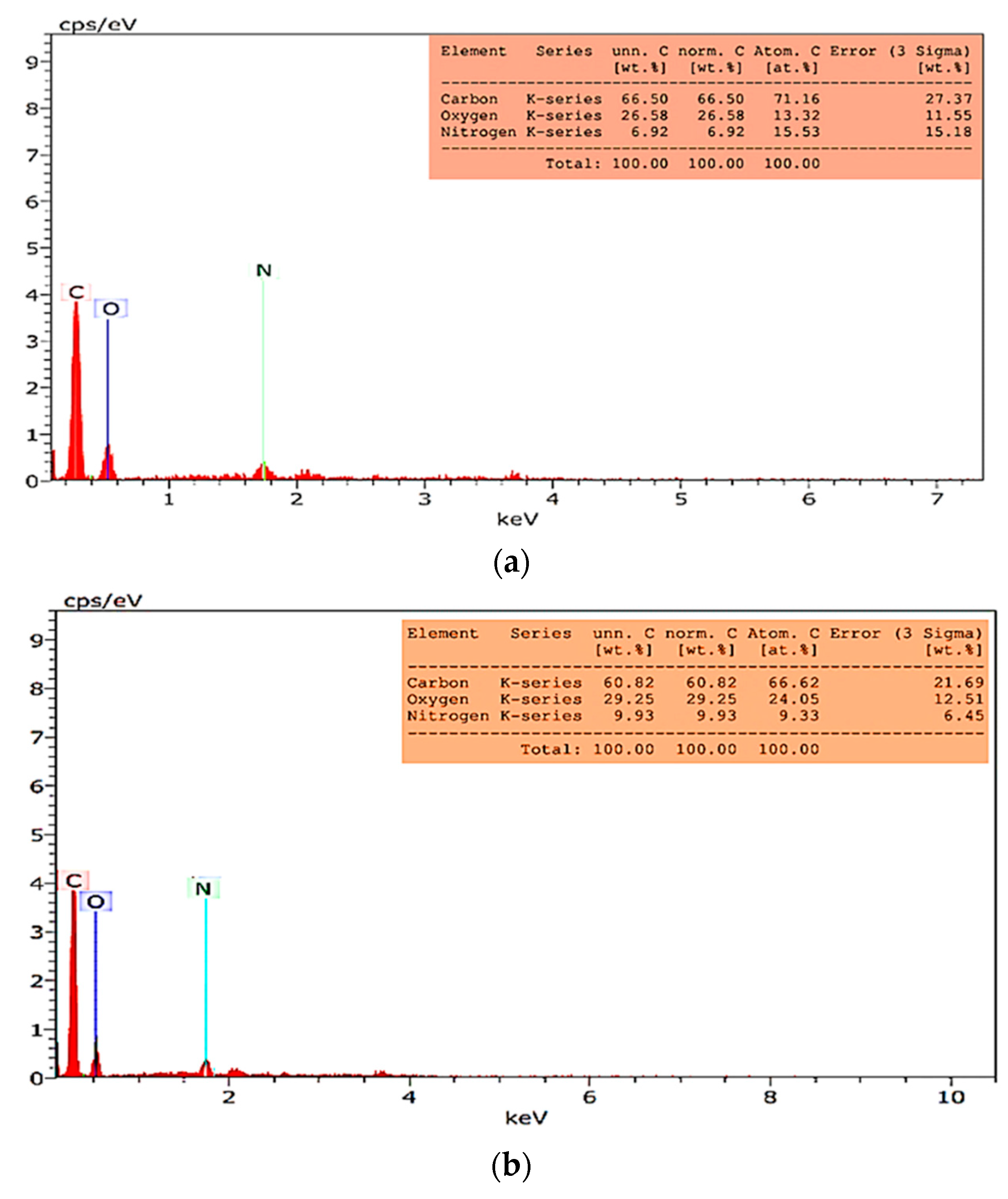
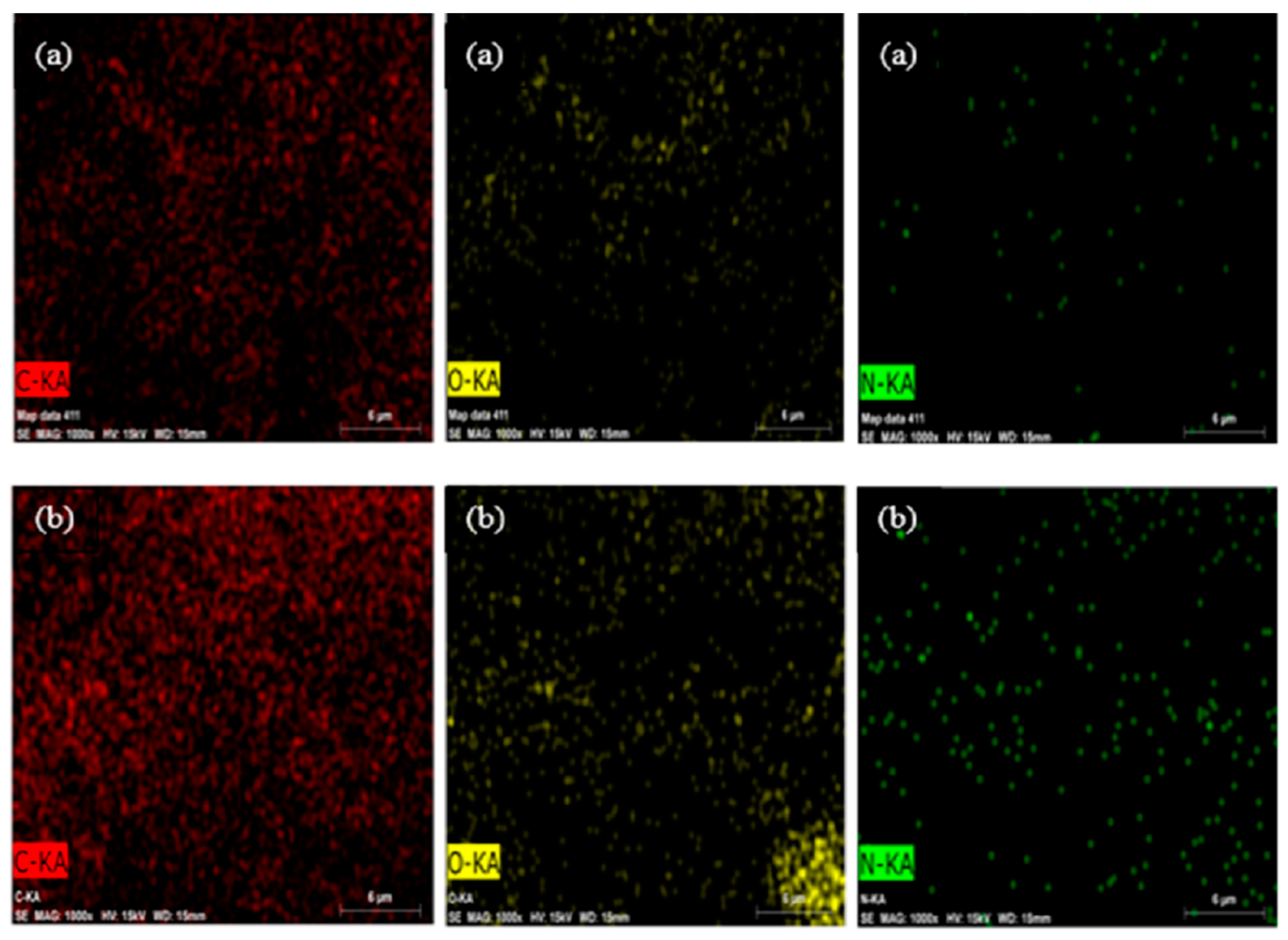
3.2.3. EDS and Elemental Mapping Analysis
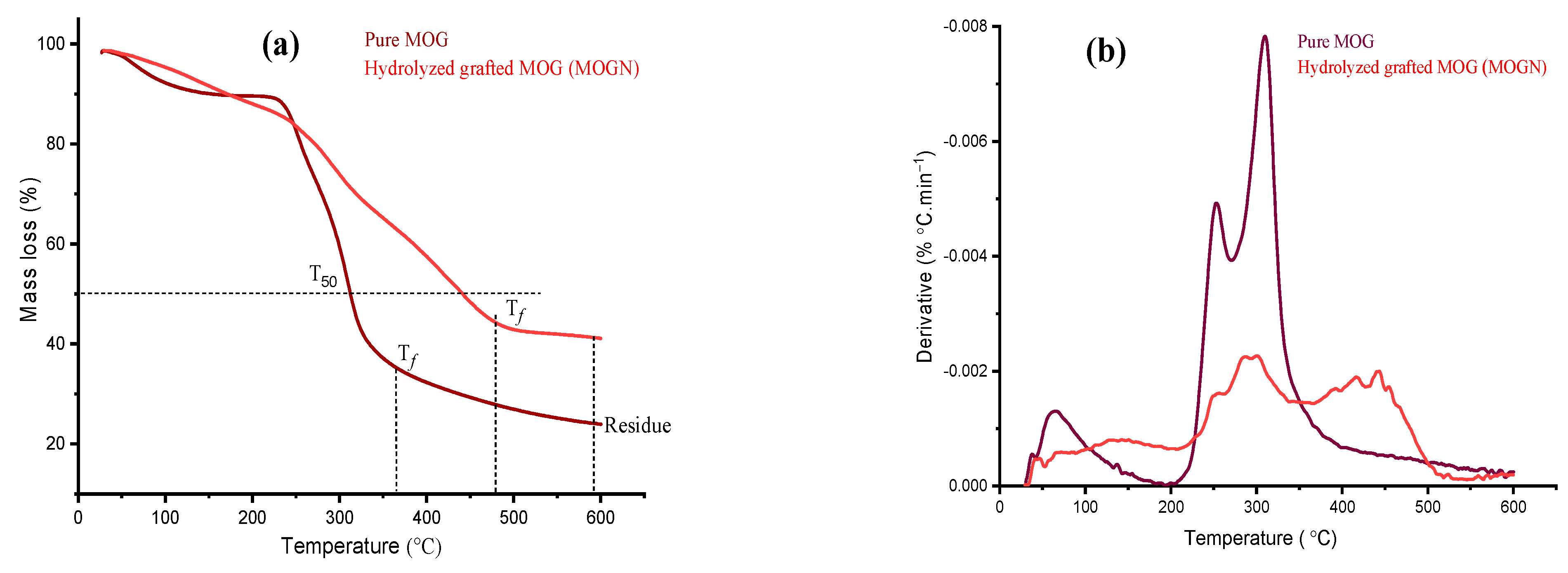
3.2.4. Thermal Analysis
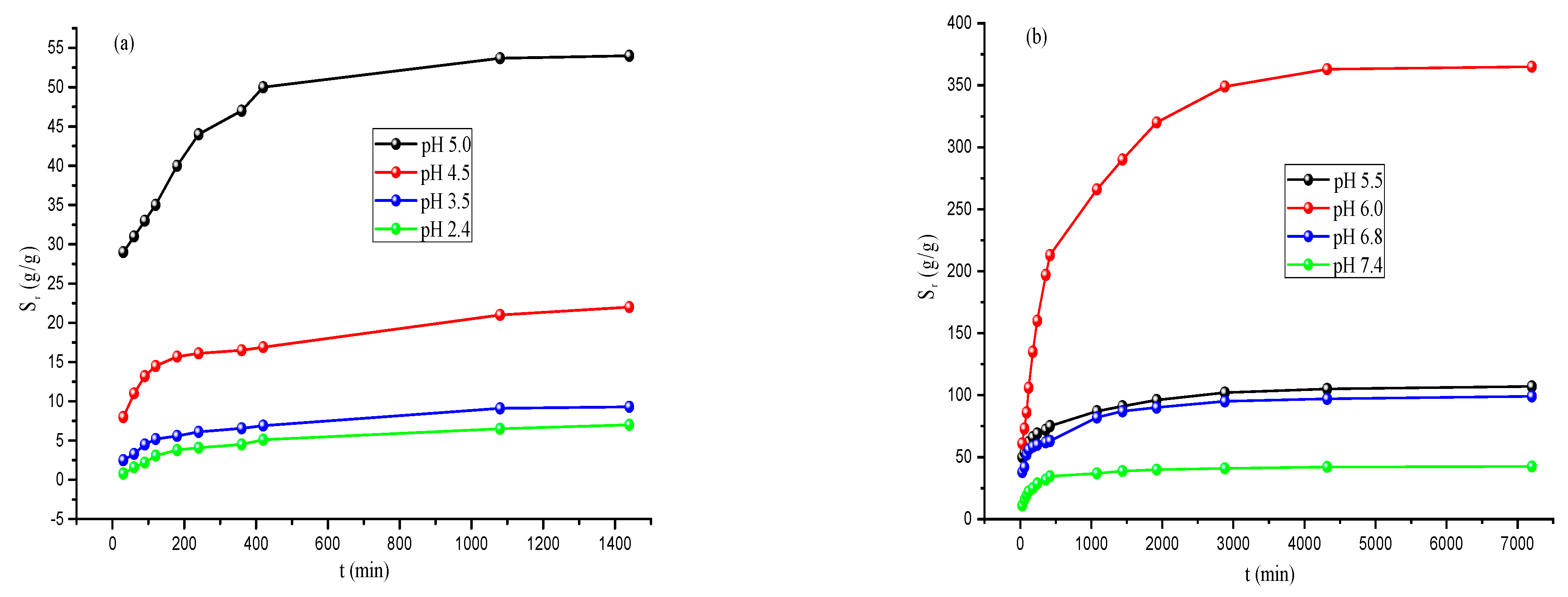
3.3. Swelling Studies of MOGN
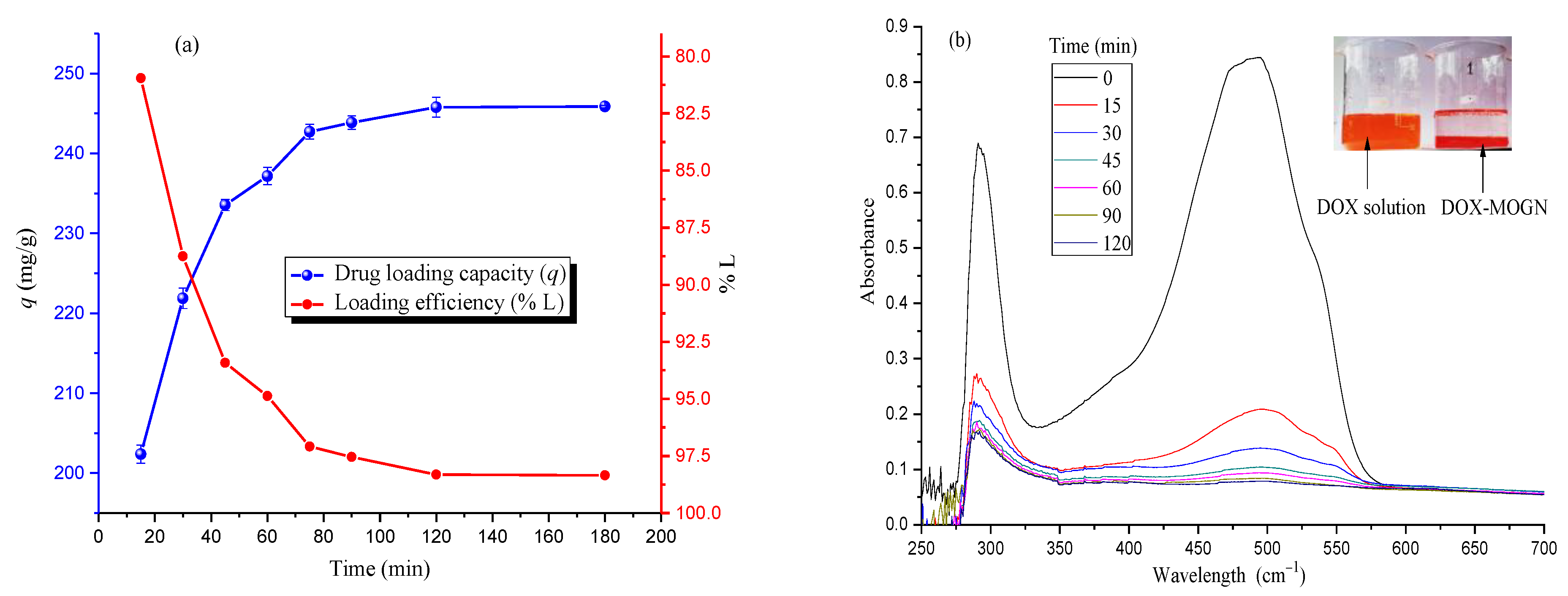
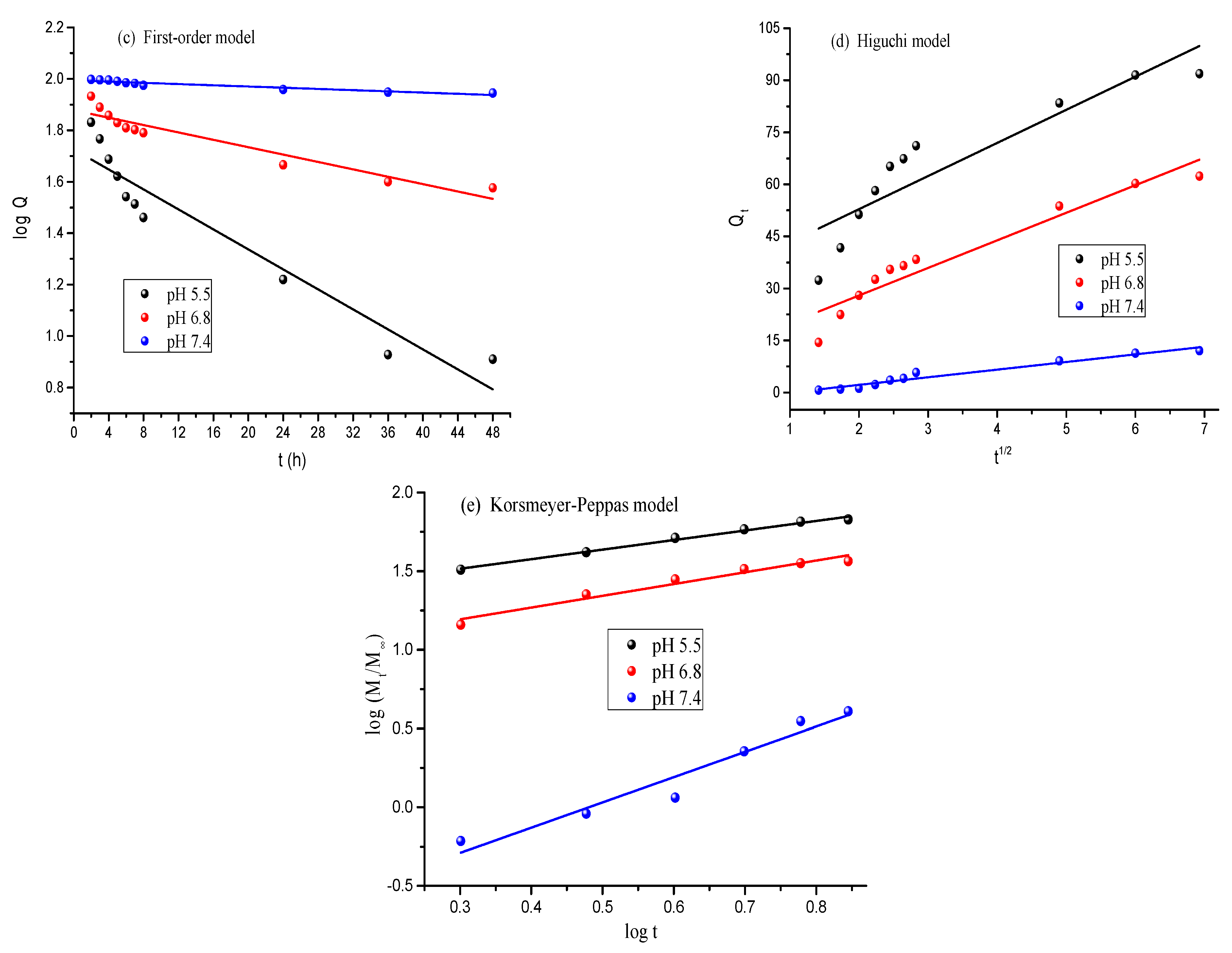
3.4. DOX Loading and Release Studies
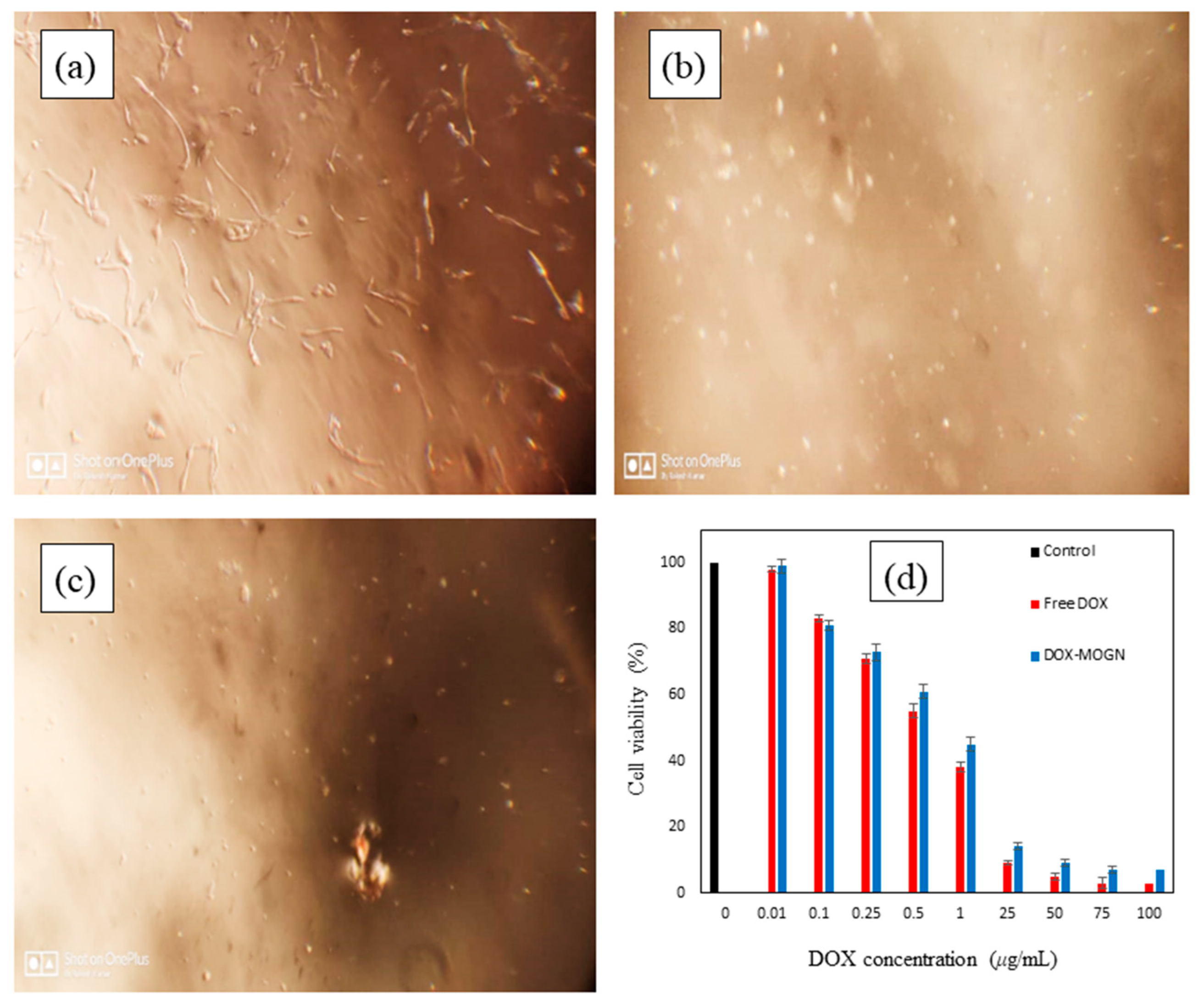
3.5. In Vitro Cytotoxicity Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. Available online: https://publications.iarc.fr/586 (accessed on 4 February 2020).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2018, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Alle, M.; Kim, T.H.; Park, S.H.; Lee, S.-H.; Kim, J.-C. Doxorubicin-Carboxymethyl Xanthan Gum Capped Gold Nanoparticles: Microwave Synthesis, Characterization, and Anti-Cancer Activity. Carbohydr. Polym. 2020, 229, 115511. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Liu, P. pH-Responsive Surface Charge Reversal Carboxymethyl Chitosan-Based Drug Delivery System for pH and Reduction Dual-Responsive Triggered DOX Release. Carbohydr. Polym. 2020, 236, 116093. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yang, D.; Long, T.; Yuan, L.; Qiu, S.; Li, D.; Mu, C.; Ge, L. pH-Sensitive Nanoparticles Based on Amphiphilic Imidazole/Cholesterol Modified Hydroxyethyl Starch for Tumor Chemotherapy. Carbohydr. Polym. 2022, 277, 118827. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Liu, P. Core-Shell-Corona Chitosan-Based Micelles for Tumor Intracellular pH-Triggered Drug Delivery: Improving Performance by Grafting Polycation. Int. J. Biol. Macromol. 2019, 141, 161–170. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.-G.; Kim, I. Recent Advances in Poly(α-L-glutamic acid)-Based Nanomaterial for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]
- Liao, J.; Zheng, H.; Fei, Z.; Lu, B.; Zheng, H.; Li, D.; Xiong, X.; Yi, Y. Tumor-Targeting and pH-Responsive Nanoparticles from Hyaluronic Acid for the Enhanced Delivery of Doxorubicin. Int. J. Biol. Macromol. 2018, 113, 737–747. [Google Scholar] [CrossRef]
- Liu, P.; Chen, N.; Yan, L.; Gao, F.; Ji, D.; Zhang, S.; Zhang, L.; Li, Y.; Xiao, Y. Preparation, Characterisation and In Vitro and In Vivo Evaluation of CD44-Targeted Chondroitin Sulphate-Conjugated Doxorubicin PLGA Nanoparticles. Carbohydr. Polym. 2019, 213, 17–26. [Google Scholar] [CrossRef]
- Tolle, C.; Riedel, J.; Mikolai, C.; Winkel, A.; Stiesch, D.W.; Menzel, H. Biocompatible Coatings from Smart Biopolymer Nanoparticles for Enzymatically Induced Drug Release. Biomolecules 2018, s8, 103. [Google Scholar] [CrossRef]
- Prabaharan, M. Chitosan-Based Nanoparticles for Tumor-Targeted Drug Delivery. Int. J. Biol. Macromol. 2015, 72, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Elbialy, N.S.; Fathy, M.M.; Khalil, W.M. Doxorubicin Loaded Magnetic Gold Nanoparticles for In Vivo Targeted Drug Delivery. Int. J. Pharm. 2015, 490, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.-C.; Dou, S.; Xiong, M.-H.; Sun, T.-M.; Wang, J. Doxorubicin-Tethered Responsive Gold Nanoparticles Facilitate Intracellular Drug Delivery for Overcoming Multidrug Resistance in Cancer Cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef] [PubMed]
- van Elk, M.; Deckers, R.; Oerlemans, C.; Shi, Y.; Storm, G.; Vermonden, T.; Hennink, W.E. Triggered Release of Doxorubicin from Temperature-Sensitive Poly (N-(2-Hydroxypropyl)-Methacrylamide Mono/Dilactate) Grafted Liposomes. Biomacromolecules 2014, 15, 1002–1009. [Google Scholar] [CrossRef]
- Rao, K.M.; Parambadath, S.; Kumar, A.; Ha, C.-S.; Han, S.S. Tunable Intracellular Degradable Periodic Mesoporous Organosilica Hybrid Nanoparticles for Doxorubicin Drug Delivery in Cancer Cells. ACS Biomater. Sci. Eng. 2018, 4, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, F.; Nguyen, K.T.; Ma, X.; Wang, X.; Xing, B.; Zhao, Y. Multifunctional Mesoporous Silica Nanoparticles for Cancer-Targeted and Controlled Drug Delivery. Adv. Funct. Mater. 2012, 22, 5144–5156. [Google Scholar] [CrossRef]
- Du, Y.; Chen, W.; Zheng, M.; Meng, F.; Zhong, Z. pH-Sensitive Degradable Chimaeric Polymersomes for the Intracellular Release of Doxorubicin Hydrochloride. Biomaterials 2012, 33, 7291–7299. [Google Scholar] [CrossRef]
- Shafiee, S.; Ahangar, H.A.; Saffar, A. Taguchi Method Optimization for Synthesis of Fe3O4@ Chitosan/Tragacanth Gum Nanocomposite as a Drug Delivery System. Carbohydr. Polym. 2019, 222, 114982. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-K.; Wang, Q.; Jones, C.H.; Yu, Y.; Zhang, H.; Law, W.-C.; Lai, C.K.; Zeng, Q.; Prasad, P.N.; Pfeifer, B.A. Synthesis of pH-Responsive Chitosan Nanocapsules for the Controlled Delivery of Doxorubicin. Langmuir 2014, 30, 4111–4119. [Google Scholar] [CrossRef]
- Hoelzer, D.; Leiske, M.N.; Hartlieb, M.; Bus, T.; Pretzel, D.; Hoeppener, S.; Kempe, K.; Thierbach, R.; Schubert, U.S. Tumor Targeting with pH-Responsive Poly (2-Oxazoline)-Based Nanogels for Metronomic Doxorubicin Treatment. Oncotarget 2018, 9, 22316–22331. [Google Scholar] [CrossRef]
- Arunraj, T.R.; Rejinold, N.S.; Kumar, N.A.; Jayakumar, R. Doxorubicin-Chitin-Poly (Caprolactone) Composite Nanogel for Drug Delivery. Int. J. Biol. Macromol. 2013, 62, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Rameshbabu, A.P.; Ghosh, P.; Patra, P.; Dhara, S.; Pal, S. Biocompatible Nanogel Derived from Functionalized Dextrin for Targeted Delivery of Doxorubicin Hydrochloride to MG 63 Cancer Cells. Carbohydr. Polym. 2017, 171, 27–38. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liang, H.; Lin, L.; Shah, B.R.; Li, Y.; Chen, Y.; Li, B. Green-Step Assembly of Low Density Lipoprotein/Sodium Carboxymethyl Cellulose Nanogels for Facile Loading and pH-Dependent Release of Doxorubicin. Colloids Surf. B Biointerfaces 2015, 126, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, L.; Li, D.; Wang, X.; Zhang, P.; Wang, J.; Yan, G.; Tang, R. Carboxymethyl Chitosan-Based Nanogels via Acid-Labile Ortho Ester Linkages Mediated Enhanced Drug Delivery. Int. J. Biol. Macromol. 2019, 129, 477–487. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nair, A.; Rejinold, N.S.; Maya, S.; Nair, S.V. Doxorubicin-Loaded pH-Responsive Chitin Nanogels for Drug Delivery to Cancer Cells. Carbohydr. Polym. 2012, 87, 2352–2356. [Google Scholar] [CrossRef]
- Manchun, S.; Cheewatanakornkool, K.; Dass, C.R.; Sriamornsak, P. Novel pH-Responsive Dextrin Nanogels for Doxorubicin Delivery to Cancer Cells with Reduced Cytotoxicity to Cardiomyocytes and Stem Cells. Carbohydr. Polym. 2014, 114, 78–86. [Google Scholar] [CrossRef]
- Pikabea, A.; Villar-Alvarez, E.; Forcada, J.; Taboada, P. pH-Controlled Doxorubicin Delivery from PDEAEMA-Based Nanogels. J. Mol. Liq. 2018, 66, 321–329. [Google Scholar] [CrossRef]
- Sun, Z.; Yi, Z.; Zhang, H.; Ma, X.; Su, W.; Sun, X.; Li, X. Bio-responsive Alginate-Keratin Composite Nanogels with Enhanced Drug Loading Efficiency for Cancer Therapy. Carbohydr. Polym. 2017, 175, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, F.; Li, M.; Yu, Z.; Qi, R.; Ding, J.; Zhang, Z.; Chen, X. Self-Stabilized Hyaluronate Nanogel for Intracellular Codelivery of Doxorubicin and Cisplatin to Osteosarcoma. Adv. Sci. 2018, 5, 1700821. [Google Scholar] [CrossRef]
- Zheng, Y.; Lv, X.; Xu, Y.; Cheng, X.; Wang, X.; Tang, R. pH-Sensitive and Pluronic-Modified Pullulan Nanogels for Greatly Improved Antitumor In Vivo. Int. J. Biol. Macromol. 2019, 139, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Kong, M.; Mu, Y.; Feng, C.; Chen, X. Chitosan Based Nanogels Stepwise Response to Intracellular Delivery Kinetics for Enhanced Delivery of Doxorubicin. Int. J. Biol. Macromol. 2017, 104, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Dispenza, C.; Giacomazza, D.; Jonsson, M. Micro- to Nanoscale Bio-Hybrid Hydrogels Engineered by Ionizing Radiation. Biomolecules 2021, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Arjama, M.; Mehnath, S.; Rajan, M.; Jeyaraj, M. Sericin/RBA Embedded Gellan Gum Based Smart Nanosystem for pH Responsive Drug Delivery. Int. J. Biol. Macromol. 2018, 120, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of Polysaccharide Particle-Based Functional Drug Delivery. Carbohydr. Polym. 2019, 22, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Polysaccharide Based Nanogels in the Drug Delivery System: Application as the Carrier of Pharmaceutical Agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.S. Evaluation of Nanogels as Supports for Enzyme Immobilization. Polym. Int. 2014, 63, 1889–1894. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Sun, W.; Wang, C.; Ranson, D.; Ye, Y.; Weng, Y.; Gu, Z. Internalized Compartments Encapsulated Nanogels for Targeted Drug Delivery. Nanoscale 2016, 8, 9178–9184. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, J.; Wen, Q.; Ni, C. Preparation of Xanthan Gum Nanogels and Their pH/Redox Responsiveness in Controlled Release. J. Appl. Polym. Sci. 2019, 136, 47921. [Google Scholar] [CrossRef]
- Wasti, A.T.; Mandeville, H.; Gatz, S.; Chisholm, J.C. Rhabdomyosarcoma. Paediatr. Child Health 2018, 28, 157–163. [Google Scholar] [CrossRef]
- Werner, M.; Atil, B.; Sieczkowski, E.; Chiba, P.; Hohenegger, M. Simvastatin-induced compartmentalisation of doxorubicin sharpens up nuclear topoisomerase II inhibition in human rhabdomyosarcoma cells. Naunyn-Schmiedebergs Arch. Pharmacol. 2013, 386, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Ranote, S.; Ram, B.; Kumar, D.; Chauhan, G.S.; Joshi, V. Functionalization of Moringa oleifera Gum for use as Hg2+ Ions Adsorbent. J. Environ. Chem. Eng. 2018, 6, 1805–1813. [Google Scholar] [CrossRef]
- Francis, S.; Mitra, D.; Dhanawade, B.R.; Varshney, L.; Sabharwal, S. Gamma Radiation Synthesis of Rapid Swelling Superporous Polyacrylamide Hydrogels. Radiat. Phys. Chem. 2009, 78, 951–953. [Google Scholar] [CrossRef]
- Toti, U.S.; Aminabhavi, T.M. Modified Guar Gum Matrix Tablet for Controlled Release of Diltiazem Hydrochloride. J. Control. Release 2004, 95, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.S.; Chauhan, S. Synthesis, Characterization, and Swelling Studies of pH- and Thermosensitive Hydrogels for Specialty Applications. J. Appl. Polym. Sci. 2008, 109, 47–55. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical Models of Drug Release. In Book Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Shaston, UK, 2015; Volume 2, pp. 63–86. ISBN 978-0-08-100092. [Google Scholar]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic Modeling on Drug Release from Controlled Drug Delivery Systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar]
- Wagner, J.G. Interpretation of Percent Dissolved-Time Plots Derived from In Vitro Testing of Conventional Tablets and Capsules. J. Pharm. Sci. 1969, 58, 1253–1257. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of Sustained-Action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Peppas, N.A. Macromolecular and Modeling Aspects of Swelling–Controlled Systems in Controlled Release Delivery Systems. In Controlled Release Delivery Systems; Roseman, T.J., Mansdorf, S.Z., Eds.; Marcel Dekker: New York, NY, USA, 1983; pp. 77–90. [Google Scholar]
- Hu, H.; Li, Y.; Zhou, Q.; Ao, Y.; Yu, C.; Wan, Y.; Xu, H.; Li, Z.; Yang, X. Redox-Sensitive Hydroxyethyl Starch–Doxorubicin Conjugate for Tumor Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 16, 30833–30844. [Google Scholar] [CrossRef]
- Liu, J.; Ma, X.; Jin, S.; Xue, X.; Zhang, C.; Wei, T.; Guo, W.; Liang, X.-J. Zinc Oxide Nanoparticles as Adjuvant to Facilitate Doxorubicin Intracellular Accumulation and Visualize pH-Responsive Release for Overcoming Drug Resistance. Mol. Pharmaceutics 2016, 13, 1723–1730. [Google Scholar] [CrossRef]
- Ranote, S.; Chauhan, G.S.; Joshi, V. Etherified Moringa oleifera Gum as Rapid and Effective Dye Adsorbents. Chem. Eng. J. 2020, 387, 124056. [Google Scholar] [CrossRef]
- Ranote, S.; Kumar, D.; Kumari, S.; Kumar, R.; Chauhan, G.S.; Joshi, V. Green Synthesis of Moringa oleifera Gum-Based Bifunctional Polyurethane Foam Braced with Ash for Rapid and Efficient Dye Removal. Chem. Eng. J. 2019, 361, 1586–1596. [Google Scholar] [CrossRef]
- Hemvichian, K.; Chanthawong, A.; Suwanmala, P. Synthesis and Characterization of Superabsorbent Polymer Prepared by Radiation-Induced Graft Copolymerization of Acrylamide onto Carboxymethyl Cellulose for Controlled Release of Agrochemicals. Radiat. Phys. Chem. 2014, 103, 167–171. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Li, L.; Wu, R.; Zhang, S.; Wu, J.; Wu, W. A Novel Polyacrylamide-Based Superabsorbent with Temperature Switch for Steam Breakthrough Blockage. J. Appl. Polym. Sci. 2015, 132, 42067. [Google Scholar] [CrossRef]
- Li, Y.-M.; Jiang, T.; Lv, Y.; Wu, Y.; He, F.; Zhuo, R.-X. Amphiphilic Copolymers with Pendent Carboxyl Groups for High-Efficiency Loading and Controlled Release of Doxorubicin. Colloids Surf. B Biointerfaces 2015, 132, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Fangueiro, J.F.; Smitha, J.; Cinu, T.A.; Chacko, A.J.; Premaletha, K.; Souto, E.B. Predictive Modeling of Insulin Release Profile from Cross-Linked Chitosan Microspheres. Eur. J. Med. Chem. 2013, 60, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Chakraborty, S.; Bhattacharya, S.; Mishra, R.; Kundu, P.P. pH-Sensitive Chitosan/Alginate Core-Shell Nanoparticles for Efficient and Safe Oral Insulin Delivery. Int. J. Biol. Macromol. 2015, 72, 640–648. [Google Scholar] [CrossRef]
- Kumari, S.; Ram, B.; Kumar, D.; Ranote, S.; Chauhan, G.S. Nanoparticles of Oxidized-Cellulose Synthesized by Green Method. Mater. Sci. Energy Technol. 2018, 1, 22–28. [Google Scholar] [CrossRef]
- Oishi, M.; Hayashi, H.; Iijima, M.; Nagasaki, Y. Endosomal Release and Intracellular Delivery of Anticancer Drugs Using pH-Sensitive Pegylated Nanogels. J. Mater. Chem. 2007, 17, 3720–3725. [Google Scholar] [CrossRef]
- Soares, P.I.; Sousa, A.I.; Silva, J.C.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Chitosan-Based Nanoparticles as Drug Delivery Systems for Doxorubicin: Optimization and Modelling. Carbohydr. Polym. 2016, 147, 34–312. [Google Scholar] [CrossRef]
- Borandeh, S.; Abdolmaleki, A.; Abolmaali, S.S.; Tamaddon, A.M. Synthesis, Structural and In-Vitro Characterization of Β-Cyclodextrin Grafted L-Phenylalanine Functionalized Graphene Oxide Nanocomposite: A Versatile Nanocarrier for pH-Sensitive Doxorubicin Delivery. Carbohydr. Polym. 2018, 201, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Pooresmaeil, M.; Namazi, H.; Salehi, R. Synthesis of Photoluminescent Glycodendrimer with Terminal Β-Cyclodextrin Molecules as a Biocompatible pH-Sensitive Carrier for Doxorubicin Delivery. Carbohydr. Polym. 2020, 246, 116658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Asghar, S.; Tian, C.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, F.; Xiao, Y. Lactoferrin/Phenylboronic Acid-Functionalized Hyaluronic Acid Nanogels Loading Doxorubicin Hydrochloride for Targeting Glioma. Carbohydr. Polym. 2021, 253, 117194. [Google Scholar] [CrossRef] [PubMed]
- Abasalta, M.; Asefnejad, A.; Khorasani, M.T.; Saadatabadi, A.R. Fabrication of Carboxymethyl Chitosan/Poly(ε-Caprolactone)/Doxorubicin/Nickel Ferrite Core-Shell Fibers for Controlled Release of Doxorubicin Against Breast Cancer. Carbohydr. Polym. 2021, 257, 117631. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-J.; Gulfam, M.; Jo, S.-H.; Gal, Y.-S.; Oh, C.-W.; Park, S.-H.; Lim, K.T. Multi-Stimuli Responsive Hydrogels Derived from Hyaluronic Acid for Cancer Therapy Application. Carbohydr. Polym. 2022, 286, 119303. [Google Scholar] [CrossRef]
- Ye, W.-L.; Du, J.-B.; Zhang, B.-L.; Na, R.; Song, Y.-F.; Mei, Q.-B.; Zhao, M.-G.; Zhou, S.-Y. Cellular Uptake and Antitumor Activity of DOX-hyd-PEGFA Nanoparticles. PLoS ONE 2014, 9, e97358. [Google Scholar]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive ZnO Quantum Dots–Doxorubicin Nanoparticles for Lung Cancer Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 16793–16802. [Google Scholar]




| Release Models | Equations | Parameters |
|---|---|---|
| Zero-order | Qt = amount of drug released in time ‘t’ Q0 = initial amount of drug ko = zero-order rate constant | |
| First-order | Q = amount of drug remaining in time ‘t’ k1 = first-order rate constant | |
| Higuchi | kH = Higuchi dissolution constant | |
| Korsmeyer–Peppas | or | Mt/M∞ = fraction of drug released in time ‘t’ kKP = Korsmeyer–Peppas release rate constant n = drug release exponent |
| Kinetic Model | Parameters | pHs | ||
|---|---|---|---|---|
| 5.5 | 6.8 | 7.4 | ||
| Zero-order | R2 | 0.682 | 0.816 | 0.886 |
| ko | 1.06 | 0.902 | 0.254 | |
| First-order | R2 | 0.907 | 0.897 | 0.895 |
| k1 | 0.019 | 0.007 | 0.001 | |
| Higuchi | R2 | 0.808 | 0.916 | 0.956 |
| kH | 9.55 | 7.95 | 2.2 | |
| Korsmeyer–Peppas | R2 | 0.99 | 0.952 | 0.933 |
| kKP | 3.79 | 2.63 | 0.46 | |
| n | 0.61 | 0.75 | 1.61 |
| Drug Delivery Devices | %L | pH | Pr | Time (h) | References |
|---|---|---|---|---|---|
| CMXG@AuNPs | 96.4 ± 0.6 | 5.3 6.6 7.4 | 98 ± 4.2 89 ± 1.8 6.67 ± 2.5 | 12 | [4] |
| PEG-CMCS-SS-PDPA | ----- | 5.0 | >81.32 | 57 | [5] |
| IHC nanoparticles | 79.0 ± 2.1 | 5.0 6.5 7.4 | 67.8 50.2 35 | 100 | [6] |
| (Cys-PMO) | 76 | 5.5 7.4 | 56 10 | 48 | [16] |
| Chitin-PCLCNGs | 80.0 | 6.0 7.4 | 45.3 36.3 | 72 | [22] |
| n-Dxt-p(MBA)-pAA | 86.0 | 5.5 7.4 | 57.0 34.0 | 72 | [23] |
| Carboxymethyl-chitosan | 94.7 | 5.0 6.5 7.4 | 95.1 67.2 11.7 | 240 | [25] |
| Dextrin | 65–70 | 5.0 6.8 7.4 | 100 94.0 40.0 | 72 | [27] |
| Hyaluronate | 63.2 | 5.5 6.8 7.4 | 86.0 60.1 38.5 | 72 | [30] |
| Pullulan | 70.0 | 5.0 6.5 7.4 | 46.0 34.2 20.5 | 190 | [31] |
| CTNGs | 9.9 | 5.5 7.4 | 70.0 35.0 | 12 | [32] |
| Xanthan gum nanogel | --------- | 5.0 6.5 7.4 | 72.1 55.2 38.3 | 72 | [40] |
| PEGylated nanogel | 80.0 | 5.3 7.4 | 39.0 13.0 | 50 | [62] |
| Chitosan-based nanoparticles | ~75 | 4.5 6.5 7.4 | >80 ~70 ~25 | 48 | [63] |
| GO-Phe-CD nanocomposite | 78.7 | 5.3 7.4 | 40 12 | 72 | [64] |
| GQDs-PAMAM-β-CD | 61.2 | 5.0 7.4 | 73.87 24.5 | 96 | [65] |
| Hyaluronic acid nanogels | 83.33 ± 3.21 | 5.5 7.4 | 61.4 27.2 | 48 | [66] |
| CMC/PCL nanofibers | >90 | 5.5 7.4 | ~80 ~35 | ~160 | [67] |
| HAHG-B hydrogel | 94 | 5.0 7.4 | ~68 ~32 | 96 | [68] |
| DOX-hyd-PEG-FA | --- | 5.0 | 94 | 58 | [69] |
| 7.4 | 12 | ||||
| MOGN | 98.35 | 5.5 6.8 7.4 | 91.92 62.62 12.18 | 72 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranote, S.; Musioł, M.; Kowalczuk, M.; Joshi, V.; Chauhan, G.S.; Kumar, R.; Chauhan, S.; Kumar, K. Functionalized Moringa oleifera Gum as pH-Responsive Nanogel for Doxorubicin Delivery: Synthesis, Kinetic Modelling and In Vitro Cytotoxicity Study. Polymers 2022, 14, 4697. https://doi.org/10.3390/polym14214697
Ranote S, Musioł M, Kowalczuk M, Joshi V, Chauhan GS, Kumar R, Chauhan S, Kumar K. Functionalized Moringa oleifera Gum as pH-Responsive Nanogel for Doxorubicin Delivery: Synthesis, Kinetic Modelling and In Vitro Cytotoxicity Study. Polymers. 2022; 14(21):4697. https://doi.org/10.3390/polym14214697
Chicago/Turabian StyleRanote, Sunita, Marta Musioł, Marek Kowalczuk, Veena Joshi, Ghanshyam S. Chauhan, Rakesh Kumar, Sandeep Chauhan, and Kiran Kumar. 2022. "Functionalized Moringa oleifera Gum as pH-Responsive Nanogel for Doxorubicin Delivery: Synthesis, Kinetic Modelling and In Vitro Cytotoxicity Study" Polymers 14, no. 21: 4697. https://doi.org/10.3390/polym14214697
APA StyleRanote, S., Musioł, M., Kowalczuk, M., Joshi, V., Chauhan, G. S., Kumar, R., Chauhan, S., & Kumar, K. (2022). Functionalized Moringa oleifera Gum as pH-Responsive Nanogel for Doxorubicin Delivery: Synthesis, Kinetic Modelling and In Vitro Cytotoxicity Study. Polymers, 14(21), 4697. https://doi.org/10.3390/polym14214697










