Functional Chitosan-Based Composite Film Incorporated with 3-(Methylthio) Propyl Isothiocyanate/α-Cyclodextrin Inclusion Complex for Chicken Meat Preservation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the MTPITC-α-CD Inclusion Complex
2.3. Preparation of the CS-MTPITC-α-CD Film
2.4. Structural Characterization of the MTPITC-α-CD Inclusion Complex and the CS-MTPITC-α-CD Film
2.4.1. FTIR Analysis
2.4.2. XRD Analysis
2.5. Characterization of the CS-MTPITC-α-CD Film
2.5.1. Surface Morphology
2.5.2. Light Transmittance
2.5.3. Water Solubility (WS)
2.5.4. Water Vaper Permeability (WVP)
2.5.5. Antioxidant Property
2.5.6. Antibacterial Property
2.6. Application of the CS-MTPITC-α-CD Film on Fresh Chicken Meat
2.6.1. Fresh Chicken Meat Treatments
2.6.2. Total Viable Count (TVC) Quantification
2.6.3. Total Volatile Base Nitrogen (TVB-N) Measurement
2.6.4. pH Measurement
2.6.5. Thiobarbituric Acid–Reactive Substances (TBARS) Measurement
2.6.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
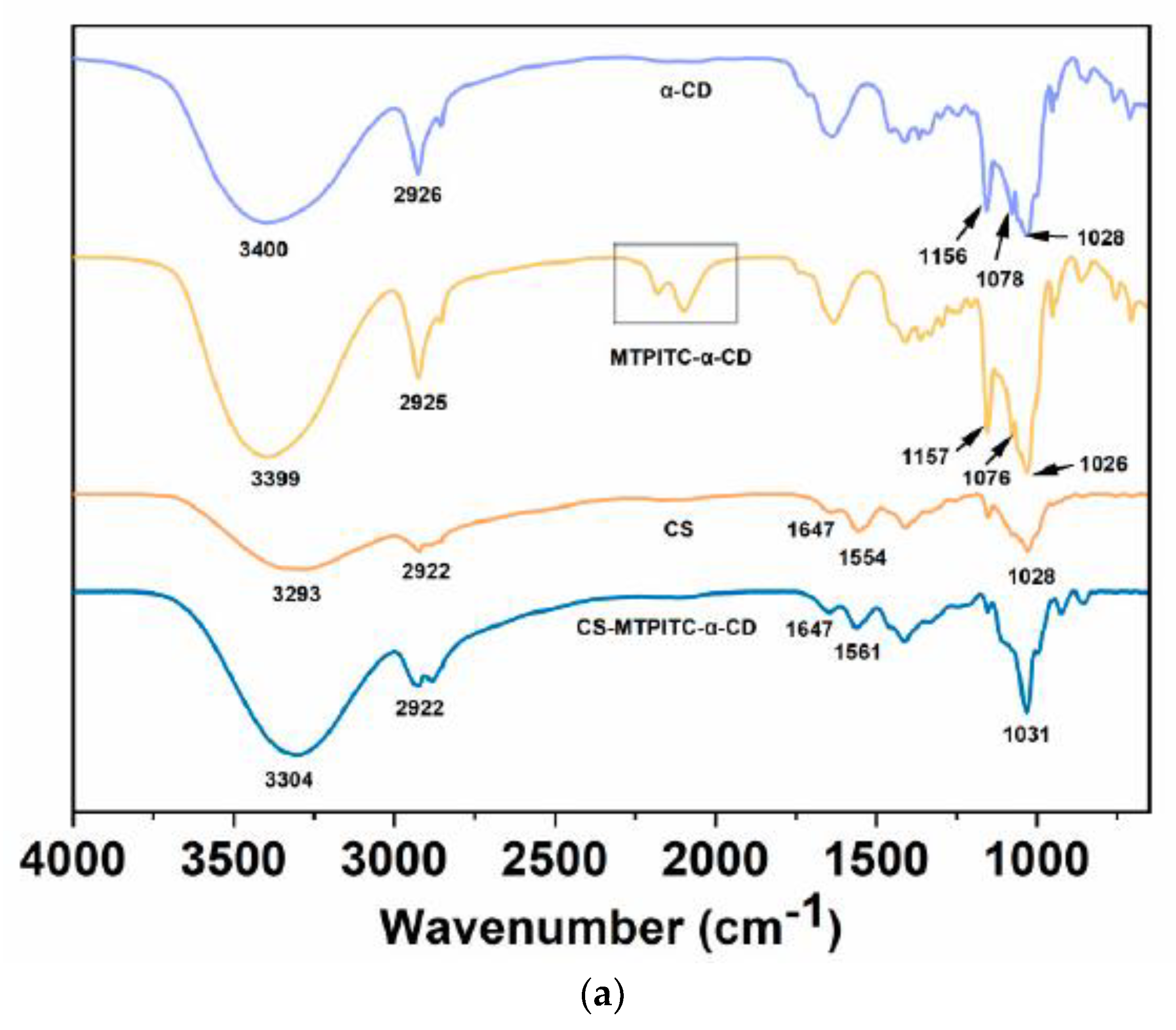
3.1. Structural Characterization of the MTPITC-α-CD Inclusion Complex and CS-MTPITC-α-CD Film
3.2. Physical Appearance, Surface Morphology, and Light Transmittance of CS and CS-MTPITC-α-CD Films
3.3. WS, WVP, and Antioxidant Properties of CS and CS-MTPITC-α-CD Films
3.4. Antibacterial Activities of CS and CS-MTPITC-α-CD Films
3.5. Application of the CS and CS-MTPITC-α-CD Films on Fresh Chicken Meat
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polymers 2021, 273, 118616. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, N.; Członka, S. Natural additives improving polyurethane antimicrobial activity. Polymers 2022, 14, 2533. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Zhang, X.; Bai, R.; Liu, Y.; Liu, J.; Liu, J. Preparation and characterization of antioxidant and antimicrobial packaging films based on chitosan and proanthocyanidins. Int. J. Biol. Macromol. 2019, 134, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, H.; Kang, S.; Xia, L.; Jiang, S.; Chen, M.; Jiang, S. An active packaging film based on yam starch with eugenol and its application for pork preservation. Food Hydrocoll. 2019, 96, 546–554. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ghaderi, J.; Gómez-Guillén, M.C. Tailoring physico-mechanical and antimicrobial/antioxidant properties of biopolymeric films by cinnamaldehyde-loaded chitosan nanoparticles and their application in packaging of fresh rainbow trout fillets. Food Hydrocoll. 2022, 124, 107249. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, Y.; Wu, T.; Wang, L.; Yuan, Y.; Chen, J.; Hu, Y.; Pang, J. Enhanced functional properties of biopolymer film incorporated with curcurmin-loaded mesoporous silica nanoparticles for food packaging. Food Chem. 2019, 288, 139–145. [Google Scholar] [CrossRef]
- Chang, W.; Liu, F.; Sharif, H.R.; Huang, Z.; Goff, H.D.; Zhong, F. Preparation of chitosan films by neutralization for improving their preservation effects on chilled meat. Food Hydrocoll. 2019, 90, 50–61. [Google Scholar] [CrossRef]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Uppal, S.; Kaur, K.; Kumar, R.; Kaur, N.D.; Shukla, G.; Mehta, S.K. Chitosan nanoparticles as a biocompatible and efficient nanowagon for benzyl isothiocyanate. Int. J. Biol. Macromol. 2018, 115, 18–28. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J.; Le, X. Molecular interactions, characterization and antimicrobial activity of curcumin–chitosan blend films. Food Hydrocoll. 2016, 52, 564–572. [Google Scholar] [CrossRef]
- Budinčić, J.M.; Petrović, L.; Đekić, L.; Fraj, J.; Bučko, S.; Katona, J.; Spasojević, L. Study of vitamin E microencapsulation and controlled release from chitosan/sodium lauryl ether sulfate microcapsules. Carbohydr. Polym. 2021, 251, 116988. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chen, X.; Zhang, G.L.; Hao, H.; Hou, H.M.; Bi, J. Preparation of chitosan-cellulose-benzyl isothiocyanate nanocomposite film for food packaging applications. Carbohydr. Polym. 2022, 285, 119234. [Google Scholar] [CrossRef] [PubMed]
- Moller, A.; Leone, C.; Kataria, J.; Sidhu, G.; Rama, E.N.; Kroft, B.; Thippareddi, H.; Singh, M. Effect of a carrageenan/chitosan coating with allyl isothiocyanate on microbial load in chicken breast. LWT 2022, 161, 113397. [Google Scholar] [CrossRef]
- Chen, W.; Karangwa, E.; Yu, J.; Xia, S.; Feng, B.; Zhang, X.; Jia, C. Characterizing red radish pigment off-odor and aroma-active compounds by sensory evaluation, gas chromatography-mass spectrometry/olfactometry and partial least square regression. Food Bioprocess Technol. 2017, 10, 1337–1353. [Google Scholar] [CrossRef]
- Zekie, M.; Radonic, A.; Marijianovic, Z. Glucosinolate profiling of Calepina irregularis. Nat. Prod. Commun. 2016, 11, 1329–1332. [Google Scholar] [CrossRef]
- Wilson, A.E.; Bergaentzlé, M.; Bindler, F.; Marchioni, E.; Lintz, A.; Ennahar, S. In vitro efficacies of various isothiocyanates from cruciferous vegetables as antimicrobial agents against foodborne pathogens and spoilage bacteria. Food Control 2013, 30, 318–324. [Google Scholar] [CrossRef]
- Wu, H.Y.; Xu, Y.H.; Wei, L.N.; Bi, J.R.; Hou, H.M.; Hao, H.S.; Zhang, G.L. Inhibitory effects of 3-(methylthio) propyl isothiocyanate in comparison with benzyl isothiocyanate on Listeria monocytogenes. J. Food Meas. Charact. 2022, 16, 1768–1775. [Google Scholar] [CrossRef]
- Zhu, Y.; Sang, X.; Li, X.; Zhang, Y.; Hao, H.; Bi, J.; Zhang, G.; Hou, H. Effect of quorum sensing and quorum sensing inhibitors on the expression of serine protease gene in Hafnia alvei H4. Appl. Microbiol. Biotechnol. 2020, 104, 7457–7465. [Google Scholar] [CrossRef]
- Inoue, M.; Hisada, H.; Takatori, K.; Koide, T.; Fukami, T.; Roy, A.; Carriere, J. Solid-state analysis of alpha-cyclodextrin inclusion complexes using low-frequency Raman spectroscopy. Anal. Chem. 2020, 93, 704–708. [Google Scholar] [CrossRef]
- Li, X.; Jin, Z.; Wang, J. Complexation of allyl isothiocyanate by α-and β-cyclodextrin and its controlled release characteristics. Food Chem. 2007, 103, 461–466. [Google Scholar] [CrossRef]
- Plackett, D.; Ghanbari-Siahkali, A.; Szente, L. Behavior of α-and β-cyclodextrin-encapsulated allyl isothiocyanate as slow-release additives in polylactide-co-polycaprolactone films. J. Appl. Polym. Sci. 2007, 105, 2850–2857. [Google Scholar] [CrossRef]
- Silvestro, S.; Chiricosta, L.; Gugliandolo, A.; Iori, R.; Rollin, P.; Perenzoni, D.; Mattivi, F.; Bramanti, P.; Mazzon, E. The Moringin/α-CD pretreatment induces neuroprotection in an in vitro model of Alzheimer’s disease: A transcriptomic study. Curr. Issues Mol. Biol. 2021, 43, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ao, X.; Liu, J.; Zhu, J.; Bi, J.; Hou, H.; Hao, H.; Zhang, G. A bioactive chitosan−based film enriched with benzyl isothiocyanate/α−cyclodextrin inclusion complex and its application for beef preservation. Foods 2022, 11, 2687. [Google Scholar] [CrossRef]
- Chen, H.; Shu, J.; Li, P.; Chen, B.; Li, N.; Li, L. Application of coating chitosan film-forming solution combined β-CD-citral inclusion complex on beef fillet. J. Food Nutr. Res. 2014, 2, 692–697. [Google Scholar] [CrossRef]
- Huang, M.; Wang, H.; Xu, X.; Lu, X.; Song, X.; Zhou, G. Effects of nanoemulsion-based edible coatings with composite mixture of rosemary extract and ε-poly-L-lysine on the shelf life of ready-to-eat carbonado chicken. Food Hydrocoll. 2020, 102, 105576. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Yousefi, S.; Ahari, H. Optimization of bioactive preservative coatings of starch nanocrystal and ultrasonic extract of sour lemon peel on chicken fillets. Int. J. Food Microbiol. 2019, 300, 31–42. [Google Scholar] [CrossRef]
- Raeisi, S.; Ojagh, S.M.; Pourashouri, P.; Salaün, F.; Quek, S.Y. Shelf-life and quality of chicken nuggets fortified with encapsulated fish oil and garlic essential oil during refrigerated storage. J. Food Sci. Technol. 2021, 58, 121–128. [Google Scholar] [CrossRef]
- Dikmen, G. Investigation of non-covalent complex formation between 2-(4-hydroxyphenylazo) benzoic acid and α-cyclodextrin in solid and solution forms. J. Mol. Liq. 2021, 335, 116278. [Google Scholar] [CrossRef]
- Dardeer, H.M.; Toghan, A.; Zaki, M.E.; Elamary, R.B. Design, synthesis and evaluation of novel antimicrobial polymers based on the inclusion of polyethylene glycol/TiO2 nanocomposites in cyclodextrin as drug carriers for sulfaguanidine. Polymers 2022, 14, 227. [Google Scholar] [CrossRef]
- Kayaci, F.; Uyar, T. Solid inclusion complexes of vanillin with cyclodextrins: Their formation, characterization, and high-temperature stability. J. Agric. Food Chem. 2011, 59, 11772–11778. [Google Scholar] [CrossRef]
- Liu, J.; Wu, H.; Ao, X.; Hao, H.; Bi, J.; Hou, H.; Zhang, G. Characterization of the inclusion complexes of isothiocyanates with γ-cyclodextrin for improvement of antibacterial activities against Staphylococcus aureus. Foods 2021, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Nazari, F. Thymol essential oil/β-cyclodextrin inclusion complex into chitosan nanoparticles: Improvement of thymol properties in vitro studies. J. Mol. Liq. 2022, 346, 118250. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Chen, Y.; Ma, X.; Xia, M. Chitosan and procyanidin composite films with high antioxidant activity and pH responsivity for cheese packaging. Food Chem. 2021, 338, 128013. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Huang, J.; Li, H.; Pan, Y.; Zhu, B.; Zhao, Y.; Liu, H. Chitosan-riboflavin composite film based on photodynamic inactivation technology for antibacterial food packaging. Int. J. Biol. Macromol. 2021, 172, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Rezaei, M.; Farzi, G. Improvement of active chitosan film properties with rosemary essential oil for food packaging. Int. J. Food Sci. Technol. 2012, 47, 847–853. [Google Scholar] [CrossRef]
- Cazón, P.; Antoniewska, A.; Rutkowska, J.; Vázquez, M. Evaluation of easy-removing antioxidant films of chitosan with Melaleuca alternifolia essential oil. Int. J. Biol. Macromol. 2021, 186, 365–376. [Google Scholar] [CrossRef]
- Bai, M.Y.; Zhou, Q.; Zhang, J.; Li, T.; Cheng, J.; Liu, Q.; Xu, W.R.; Zhang, Y.C. Antioxidant and antibacterial properties of essential oils-loaded β-cyclodextrin-epichlorohydrin oligomer and chitosan composite films. Colloids Surf. 2022, 215, 112504. [Google Scholar] [CrossRef]
- Siva, S.; Li, C.Z.; Cui, H.Y.; Lin, L. Encompassment of isoeugenol in 2-hydroxypropyl-β-cyclodextrin using ultrasonication: Characterization, antioxidant and antibacterial activities. J. Mol. Liq. 2019, 296, 11. [Google Scholar] [CrossRef]
- Sinha, V.R.; Anitha, R.; Ghosh, S.; Nanda, A.; Kumria, R. Complexation of celecoxib with β-cyclodextrin: Characterization of the interaction in solution and in solid state. J. Pharm. Sci. 2005, 94, 676–687. [Google Scholar] [CrossRef]
- Alizadeh, N.; Malakzadeh, S. Antioxidant, antibacterial and anti-cancer activities of β-and γ-CDs/curcumin loaded in chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 147, 778–791. [Google Scholar] [CrossRef]
- Xu, Y.; Hou, K.; Gao, C.; Feng, X.; Cheng, W.; Wu, D.; Meng, L.; Yang, Y.; Shen, X.; Zhang, Y.; et al. Characterization of chitosan film with cinnamon essential oil emulsion co-stabilized by ethyl-Nα-lauroyl-l-arginate hydrochloride and hydroxypropyl-β-cyclodextrin. Int. J. Biol. Macromol. 2021, 188, 24–31. [Google Scholar] [CrossRef]
- Li, M.; Luo, X.; Zhu, R.; Zhong, K.; Ran, W.; Wu, Y.; Gao, H. Development and characterization of active bilayer film incorporated with dihydromyricetin encapsulated in hydroxypropyl-β-cyclodextrin for food packaging application. Food Hydrocoll. 2022, 131, 107834. [Google Scholar] [CrossRef]
- Yang, P.P.; Shi, Y.G.; Li, D.H.; Chen, R.K.; Zheng, M.Z.; Ma, K.Z.; Xu, N.; Dong, L.J.; Li, T. Antimicrobial and mechanical properties of β-cyclodextrin inclusion with octyl gallate in chitosan films and their application in fresh vegetables. Food Biophys. 2022, 17, 598–611. [Google Scholar] [CrossRef]
- Ezati, P.; Riahi, Z.; Rhim, J.W. Carrageenan-based functional films integrated with CuO-doped titanium nanotubes for active food-packaging applications. ACS Sustain. Chem. Eng. 2021, 9, 9300–9307. [Google Scholar] [CrossRef]
- Cui, R.; Yan, J.; Cao, J.; Qin, Y.; Yuan, M.; Li, L. Release properties of cinnamaldehyde loaded by montmorillonite in chitosan-based antibacterial food packaging. Int. J. Food Sci. Technol. 2021, 56, 3670–3681. [Google Scholar] [CrossRef]
- Lu, N.; Liu, Y. Structural, physicochemical, and functional (antioxidant-antimicrobial) properties of 2-O-methyl-β-cyclodextrin inclusion with hexahydro-β-acids in chitosan films. Colloids Surf. B 2020, 191, 111002. [Google Scholar] [CrossRef]
- Liu, J.; Meng, C.G.; Liu, S.; Kan, J.; Jin, C.H. Preparation and characterization of protocatechuic acid grafted chitosan films with antioxidant activity. Food Hydrocoll. 2017, 63, 457–466. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chalyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Characterization of chitosan film incorporated with curcumin extract. Polymers 2021, 13, 963. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Sun, J.; Lu, Y.; Tong, C.; Wang, L.; Yan, Z.; Pang, J. Novel konjac glucomannan films with oxidized chitin nanocrystals immobilized red cabbage anthocyanins for intelligent food packaging. Food Hydrocoll. 2020, 98, 105245. [Google Scholar] [CrossRef]
- Gomaa, M.; Fawzy, M.A.; Hifney, A.F.; Abdel-Gawad, K.M. Use of the brown seaweed Sargassum latifolium in the design of alginate-fucoidan based films with natural antioxidant properties and kinetic modeling of moisture sorption and polyphenolic release. Food Hydrocoll. 2018, 82, 64–72. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- Wang, L.; Guo, H.; Wang, J.; Jiang, G.; Du, F.; Liu, X. Effects of Herba Lophatheri extract on the physicochemical properties and biological activities of the chitosan film. Int. J. Biol. Macromol. 2019, 133, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Butnaru, E.; Stoleru, E.; Brebu, M.A.; Darie-Nita, R.N.; Bargan, A.; Vasile, C. Chitosan-based bionanocomposite films prepared by emulsion technique for food preservation. Materials 2019, 12, 373. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Iori, R. Antioxidant and pro-oxidant capacities of ITCs. Environ. Mol. Mutagen. 2009, 50, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Oscar, T.P. Initial contamination of chicken parts with Salmonella at retail and cross-contamination of cooked chicken with Salmonella from raw chicken during meal preparation. J. Food Prot. 2013, 76, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Dufour, V.; Alazzam, B.; Ermel, G.; Thepaut, M.; Rossero, A.; Tresse, O.; Baysse, C. Antimicrobial activities of isothiocyanates against Campylobacter jejuni isolates. Front. Cell. Infect. Microbiol. 2012, 2, 53. [Google Scholar] [CrossRef]
- Sivarajan, M.; Lalithapriya, U.; Mariajenita, P.; Vajiha, B.A.; Harini, K.; Madhushalini, D.; Sukumar, M. Synergistic effect of spice extracts and modified atmospheric packaging towards non-thermal preservation of chicken meat under refrigerated storage. Poult. Sci. 2017, 96, 2839–2844. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Liu, D.; Fu, M.; Yang, X.; Guo, Y. The preservative effects of chitosan film incorporated with thinned young apple polyphenols on the quality of grass carp (Ctenopharyngodon idellus) fillets during cold storage: Correlation between the preservative effects and the active properties of the film. Food Packag. Shelf Life 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Ayaseh, A.; Alirezalu, K.; Nemati, Z.; Pateiro, M.; Lorenzo, J.M. Effect of chitosan coating incorporated with Artemisia fragrans essential oil on fresh chicken meat during refrigerated storage. Polymers 2021, 13, 716. [Google Scholar] [CrossRef]
- Feng, X.; Bansal, N.; Yang, H. Fish gelatin combined with chitosan coating inhibits myofibril degradation of golden pomfret (Trachinotus blochii) fillet during cold storage. Food Chem. 2016, 200, 283–292. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, T.; Zhang, Y.; Zhang, C.; Lu, Z.; Lu, F.; Zhao, H. Effect of tea polyphenols on curdlan/chitosan blending film properties and its application to chilled meat preservation. Coatings 2019, 9, 262. [Google Scholar] [CrossRef]
- Hu, F.; Sun, T.; Xie, J.; Xue, B.; Li, X.; Gan, J.; Li, L.; Shao, Z. Functional properties and preservative effect on Penaeus vannamei of chitosan films with conjugated or incorporated chlorogenic acid. Int. J. Biol. Macromol. 2020, 159, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, Y.; Shavisi, N. Chitosan coatings containing Mentha spicata essential oil and zinc oxide nanoparticle for shelf life extension of rainbow trout fillets. J. Aquat. Food Prod. Technol. 2018, 27, 986–997. [Google Scholar] [CrossRef]
- Zhao, R.; Guan, W.; Zheng, P.; Tian, F.; Zhang, Z.; Sun, Z.; Cai, L. Development of edible composite film based on chitosan nanoparticles and their application in packaging of fresh red sea bream fillets. Food Control 2022, 132, 108545. [Google Scholar] [CrossRef]
- Liu, F.; Chang, W.; Chen, M.; Xu, F.; Ma, J.; Zhong, F. Tailoring physicochemical properties of chitosan films and their protective effects on meat by varying drying temperature. Carbohydr. Polym. 2019, 212, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Guo, X.; Ji, M.; Wu, J.; Zhu, W.; Wang, J.; Cheng, C.; Chen, L.; Zhang, Q. Preservative effects of fish gelatin coating enriched with CUR/betaCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. Food Chem. 2019, 272, 643–652. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Thavaraj, P.; Yang, X.; Guo, Y. Effects of thinned young apple polyphenols on the quality of grass carp (Ctenopharyngodon idellus) surimi during cold storage. Food Chem. 2017, 224, 372–381. [Google Scholar] [CrossRef]
- Yong, H.; Xu, F.; Yun, D.; Hu, H.; Liu, J. Antioxidant packaging films developed by in-situ cross-linking chitosan with dialdehyde starch-catechin conjugates. Int. J. Biol. Macromol. 2022. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef]
- Feng, Z.; Li, L.; Wang, Q.; Wu, G.; Liu, C.; Jiang, B.; Xu, J. Effect of antioxidant and antimicrobial coating based on whey protein nanofibrils with TiO2 nanotubes on the quality and shelf life of chilled meat. Int. J. Mol. Sci. 2019, 20, 1184. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids Surf. B 2022, 214, 112472. [Google Scholar] [CrossRef] [PubMed]
- Laorenza, Y.; Chonhenchob, V.; Bumbudsanpharoke, N.; Jittanit, W.; Sae-Tan, S.; Rachtanapun, C.; Chanput, W.; Charoensiddhi, S.; Srisa, A.; Promhuad, K.; et al. Polymeric packaging applications for seafood products: Packaging-deterioration relevance, technology and trends. Polymers 2022, 14, 3706. [Google Scholar] [CrossRef] [PubMed]
- Srisa, A.; Promhuad, K.; San, H.; Laorenza, Y.; Wongphan, P.; Wadaugsorn, K.; Sodsai, J.; Kaewpetch, T.; Tansin, K.; Harnkarnsujarit, N. Antibacterial, antifungal and antiviral polymeric food packaging in post-COVID-19 era. Polymers 2022, 14, 4042. [Google Scholar] [CrossRef] [PubMed]
- San, H.; Laorenza, Y.; Behzadfar, E.; Sonchaeng, U.; Wadaugsorn, K.; Sodsai, J.; Kaewpetch, T.; Promhuad, K.; Srisa, A.; Wongphan, P.; et al. Functional polymer and packaging technology for bakery products. Polymers 2022, 14, 3793. [Google Scholar] [CrossRef] [PubMed]




| Samples | Time | |
|---|---|---|
| 12 h | 24 h | |
| CS | 7.3 ± 0.6 mm b | 0.0 ± 0.0 mm b |
| CS-MTPITC−α-CD | 14.7 ± 0.6 mm a | 11.7 ± 0.4 mm a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Ao, X.; Liu, J.; Zhu, J.; Bi, J.; Hou, H.; Hao, H.; Zhang, G. Functional Chitosan-Based Composite Film Incorporated with 3-(Methylthio) Propyl Isothiocyanate/α-Cyclodextrin Inclusion Complex for Chicken Meat Preservation. Polymers 2022, 14, 4655. https://doi.org/10.3390/polym14214655
Wu H, Ao X, Liu J, Zhu J, Bi J, Hou H, Hao H, Zhang G. Functional Chitosan-Based Composite Film Incorporated with 3-(Methylthio) Propyl Isothiocyanate/α-Cyclodextrin Inclusion Complex for Chicken Meat Preservation. Polymers. 2022; 14(21):4655. https://doi.org/10.3390/polym14214655
Chicago/Turabian StyleWu, Hongyan, Xinying Ao, Jianan Liu, Junya Zhu, Jingran Bi, Hongman Hou, Hongshun Hao, and Gongliang Zhang. 2022. "Functional Chitosan-Based Composite Film Incorporated with 3-(Methylthio) Propyl Isothiocyanate/α-Cyclodextrin Inclusion Complex for Chicken Meat Preservation" Polymers 14, no. 21: 4655. https://doi.org/10.3390/polym14214655
APA StyleWu, H., Ao, X., Liu, J., Zhu, J., Bi, J., Hou, H., Hao, H., & Zhang, G. (2022). Functional Chitosan-Based Composite Film Incorporated with 3-(Methylthio) Propyl Isothiocyanate/α-Cyclodextrin Inclusion Complex for Chicken Meat Preservation. Polymers, 14(21), 4655. https://doi.org/10.3390/polym14214655






