Effect of Functionalization of the Polycaprolactone Film Surface on the Mechanical and Biological Properties of the Film Itself
Abstract
1. Introduction
2. Materials and Methods
2.1. PCL Film Formation and Aminolysis
2.2. Water Contact Angle
2.3. Evaluation of the Mechanical Properties of the Films
2.4. Cultivation of MSCs
2.5. Fluorescence Staining of MSCs
2.6. Cell Counts
2.7. The Determination of Synthesized Extracellular Matrix Protein
2.8. Statistical Analysis
3. Results and Discussion
3.1. Water Contact Angles
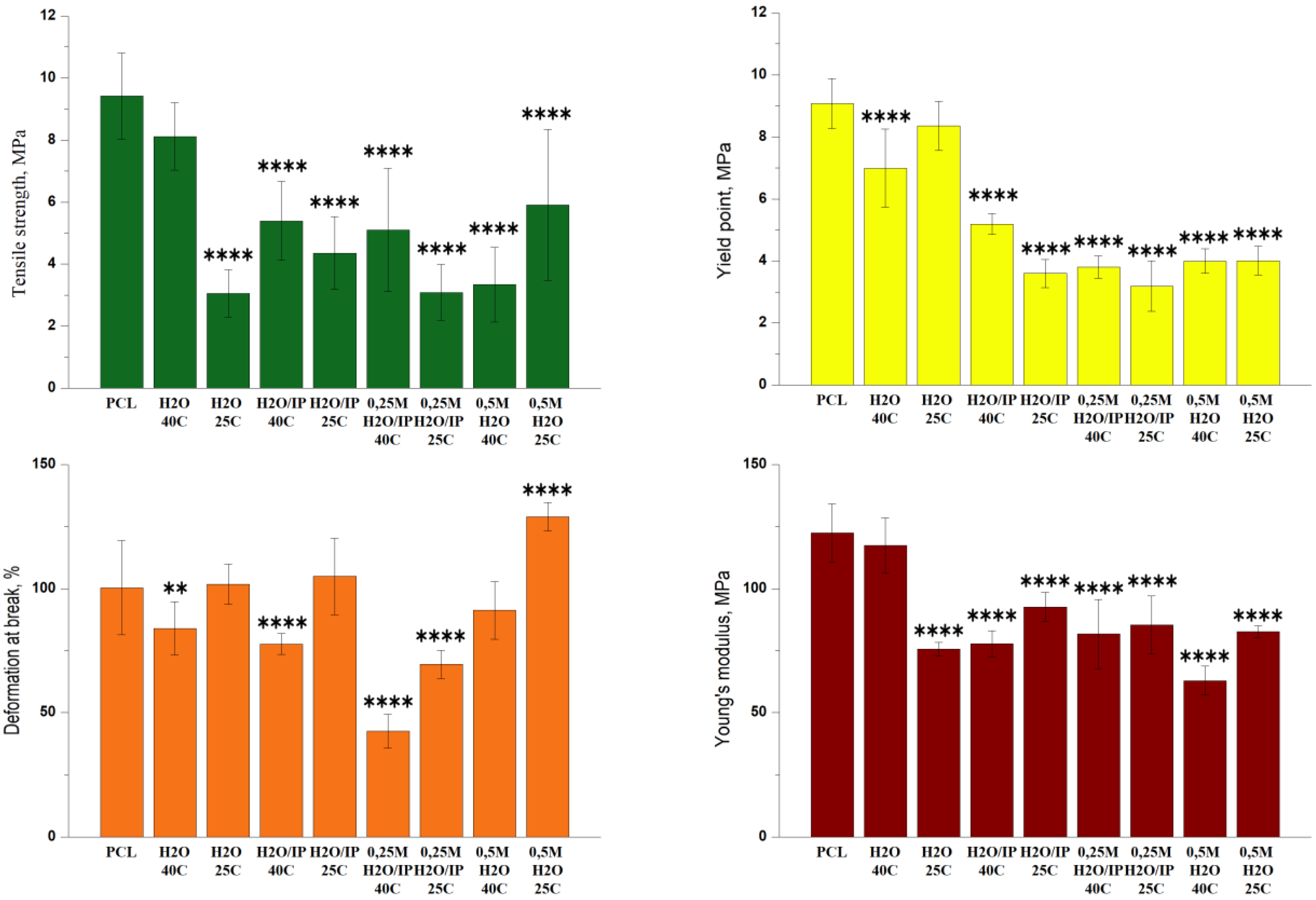
3.2. Mechanical Tests
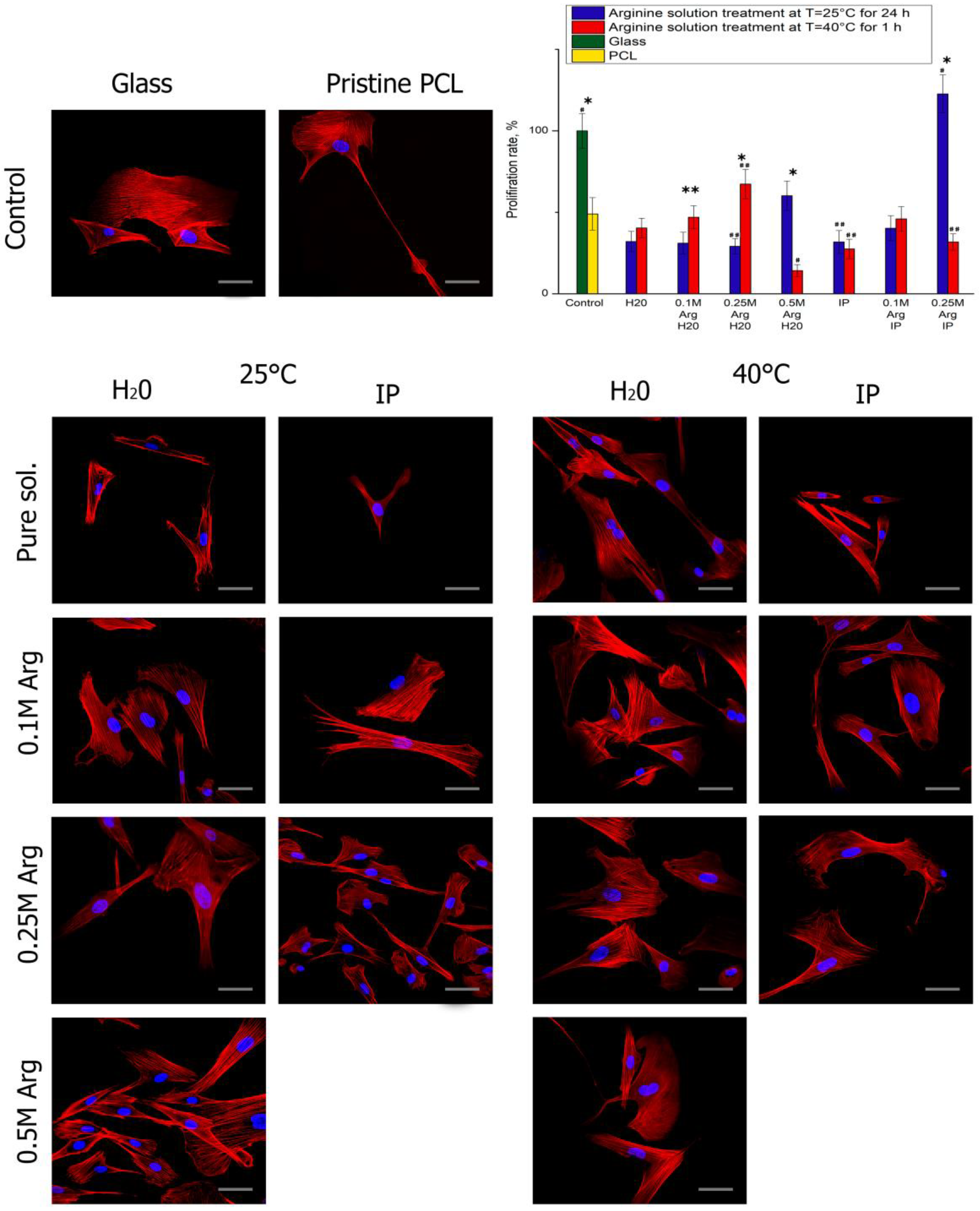
4. Adhesion and Proliferation of MSC on PCL Films
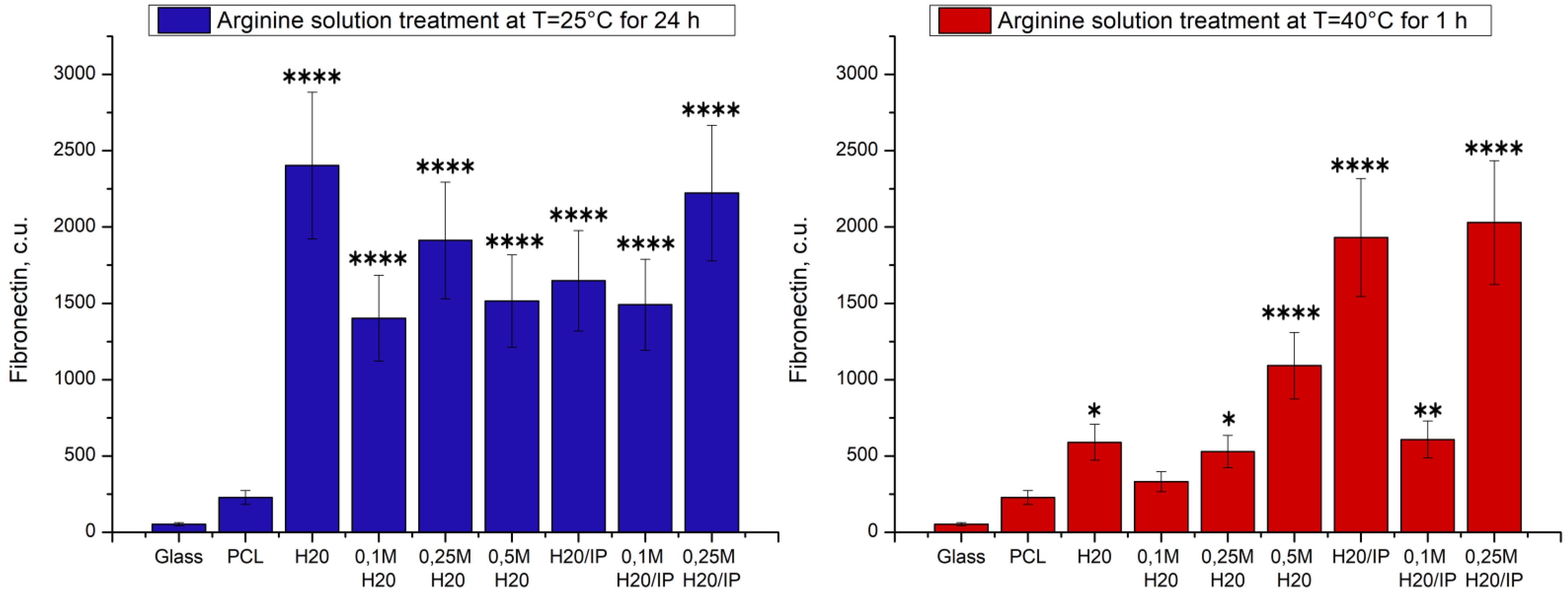
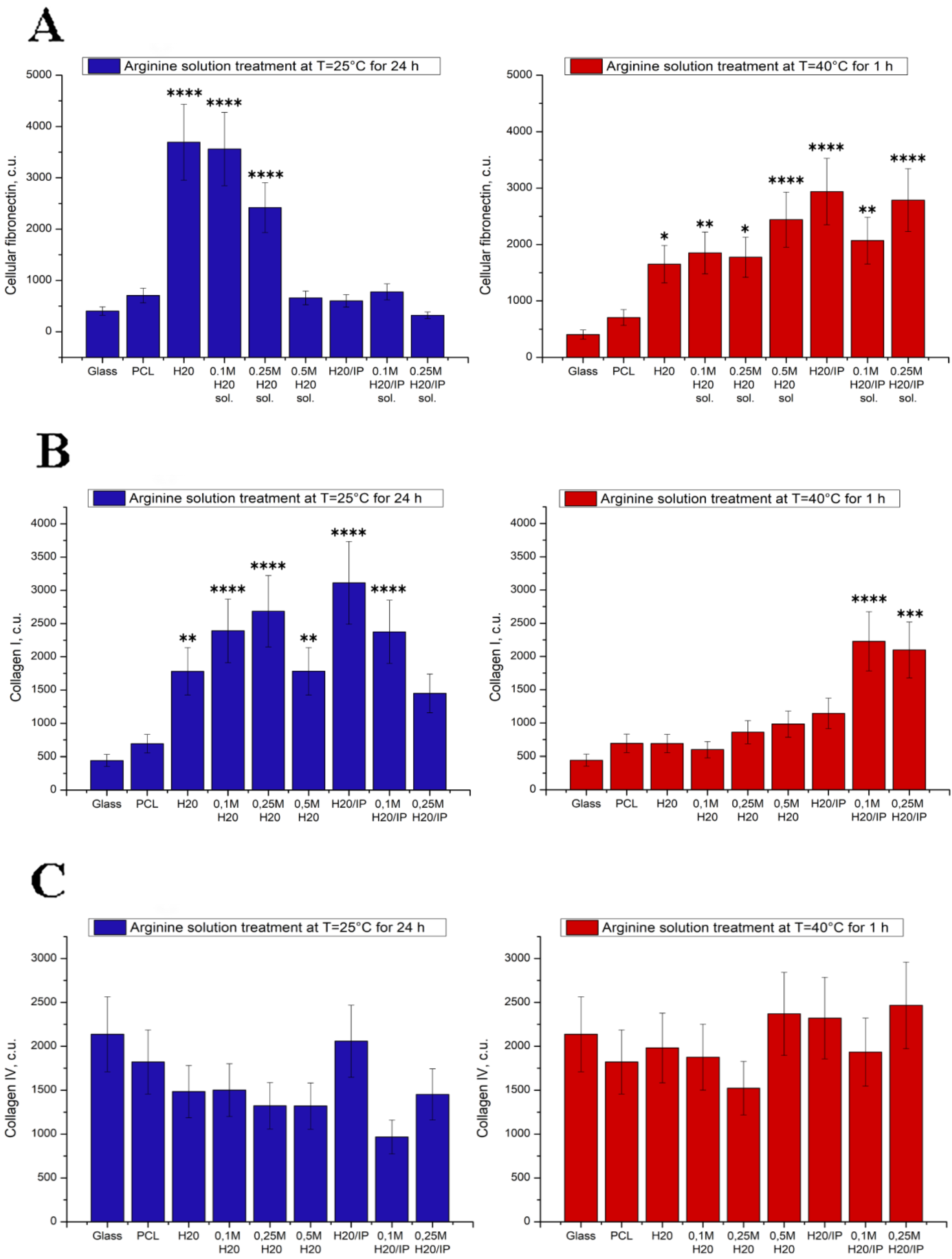
5. Study of Protein Adsorption
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Jiao, Y.P.; Cui, F.Z. Surface modification of polyester biomaterials for tissue engineering. Biomed. Mater. 2007, 2, 24–37. [Google Scholar] [CrossRef]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Geometric control of cell life and death. Science 1997, 276, 1425–1428. [Google Scholar] [CrossRef]
- Ayala, R.; Zhang, C.; Yang, D.; Hwang, Y.; Aung, A.; Shroff, S.S.; Arce, F.T.; Lal, R.; Arya, G.; Varghese, S. Engineering the cell-material interface for controlling stem cell adhesion, migration, and differentiation. Biomaterials 2011, 32, 3700–3711. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Won, Y.; Ma, P.X. Surface modification of interconnected porous scaffolds. J. Biomed. Mater. Res. Part A 2005, 74, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Quirk, R.A.; Chan, W.C.; Davies, M.C.; Tendler, S.J.B.; Shakesheff, K.M. Poly(L-lysine)-GRGDS as a biomimetic surface modifier for poly(lactic acid). Biomaterials 2001, 22, 865–872. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, X.; Shen, J. Surface modification of polycaprolactone membrane via aminolysis and biomacromolecule immobilization for promoting cytocompatibility of human endothelial cells. Biomacromolecules 2002, 3, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhengwei, M.; Huayu, S.; Changyou, G. In-depth study on aminolysis of poly(ε-caprolactone): Back to the fundamentals. Sci. China Chem. 2012, 55, 2419–2427. [Google Scholar] [CrossRef]
- Nashchekina, Y.; Chabina, A.; Nashchekin, A.; Mikhailova, N. Polycaprolactone films modified by L-Arginine for mesenchymal stem cell cultivation. Polymers 2020, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Gaona, L.A.; Gómez Ribelles, J.L.; Perilla, J.E.; Lebourg, M. Hydrolytic degradation of PLLA/PCL microporous membranes prepared by freeze extraction. Polym. Degrad. Stab. 2012, 97, 1621–1632. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. Arginine physiology and its implication for wound healing. Wound Repair Regen. 2003, 11, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Nashekina, Y.; Chabina, A.; Nashekin, A.; Mikhailona, N. Different Conditions for the Modification of Polycaprolactone Films with L-Arginine. Int. J. Mol. Sci. 2020, 21, 6989. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mao, Z.; Gao, C. Aminolysis-based surface modification of polyesters for biomedical applications. RSC Adv. 2013, 3, 2509–2519. [Google Scholar] [CrossRef]
- Leroux, A.; Ngoc Nguyen, T.; Rangel, A.; Cacciapuoti, I.; Duprez, D.; Castner, D.G.; Migonney, V. Long-term hydrolytic degradation study of polycaprolactone films and fibers grafted with poly (sodium styrene sulfonate): Mechanism study and cell response. Biointerphases 2020, 15, 061006. [Google Scholar] [CrossRef]
- Sánchez-González, S.; Diban, N.; Urtiaga, A. Hydrolytic degradation and mechanical stability of poly(ε-Caprolactone)/reduced graphene oxide membranes as scaffolds for in vitro neural tissue regeneration. Membranes 2018, 8, 12. [Google Scholar] [CrossRef]
- França, D.C.; Bezerra, E.B.; Morais, D.D.S.; Araújo, E.M.; Wellen, R.M.R. Effect of hydrolytic degradation on mechanical properties of PCL. Mater. Sci. Forum 2016, 869, 342–345. [Google Scholar] [CrossRef]
- Sevim, K.; Pan, J. A model for hydrolytic degradation and erosion of biodegradable polymers. Acta Biomater. 2018, 66, 192–199. [Google Scholar] [CrossRef]
- Marrese, M.; Cirillo, V.; Guarino, V.; Ambrosio, L. Short-Term Degradation of Bi-Component Electrospun Fibers: Qualitative and Quantitative Evaluations via AFM Analysis. J. Funct. Biomater. 2018, 9, 27. [Google Scholar] [CrossRef]
- Horzum, U.; Ozdil, B.; Pesen-Okvur, D. Step-by-step quantitative analysis of focal adhesions. MethodsX 2014, 1, 56–59. [Google Scholar] [CrossRef]
- Kaewkong, P.; Uppanan, P.; Thavornyutikarn, B.; Kosorn, W.; Janvikul, W. Chondrocyte Infiltration and ECM Production on Surface- Treated PCL Scaffolds: Alkaline Hydrolysis versus Plasma Treatment. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 429–432. [Google Scholar] [CrossRef]
- Zhang, H.; Hollister, S. Comparison of bone marrow stromal cell behaviors on poly(caprolactone) with or without surface modification: Studies on cell adhesion, survival and proliferation. J. Biomater. Sci. Polym. Ed. 2009, 20, 1975–1993. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Xiong, G.; Wang, X.; Zhang, S.; Choong, C. Surface modification of polycaprolactone substrates using collagen-conjugated poly(methacrylic acid) brushes for the regulation of cell proliferation and endothelialisation. J. Mater. Chem. 2012, 22, 13039–13049. [Google Scholar] [CrossRef]
- Jeznach, O.; Kolbuk, D.; Sajkiewicz, P. Aminolysis of various aliphatic polyesters in a form of nanofibers and films. Polymers 2019, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, L.A.; Downes, S. Physicochemical characterisation of degrading polycaprolactone scaffolds. Polym. Degrad. Stab. 2010, 95, 2269–2276. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Savalani, M.M.; Teoh, S.H.; Hutmacher, D.W. Dynamics of in vitro polymer degradation of polycaprolactone-based scaffolds: Accelerated versus simulated physiological conditions. Biomed. Mater. 2008, 3, 034108. [Google Scholar] [CrossRef]
- Keselowsky, B.G.; Collard, D.M.; García, A.J. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 2004, 25, 5947–5954. [Google Scholar] [CrossRef]
- Wilson, C.J.; Clegg, R.E.; Ph, D.; Leavesley, D.I.; Ph, D.; Pearcy, M.J.; Ph, D. Mediation of Biomaterial–Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Gandavarapu, N.R.; Mariner, P.D.; Schwartz, M.P.; Anseth, K.S. Extracellular matrix protein adsorption to phosphate-functionalized gels from serum promotes osteogenic differentiation of human mesenchymal stem cells. Acta Biomater. 2013, 9, 4525–4534. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Preferential adsorption of cell adhesive proteins from complex media on self-assembled monolayers and its effect on subsequent cell adhesion. Acta Biomater. 2015, 26, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Hayman, E.G.; Ruoslahti, E. Distribution of fetal bovine serum fibronectin and endogenous rat cell fibronectin in extracellular matrix. Cell Biol. 1979, 83, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Peng, Y.; Liu, X.; Ding, J. Effects of Functional Groups of Materials on Nonspecific Adhesion and Chondrogenic Induction of Mesenchymal Stem Cells on Free and Micropatterned Surfaces. ACS Appl. Mater. Interfaces 2017, 9, 23574–23585. [Google Scholar] [CrossRef] [PubMed]
- Ogura, N.; Kawada, M.; Chang, W.J.; Zhang, Q.; Lee, S.Y.; Kondoh, T.; Abiko, Y. Differentiation of the human mesenchymal stem cells derived from bone marrow and enhancement of cell attachment by fibronectin. J. Oral Sci. 2004, 46, 207–213. [Google Scholar] [CrossRef]
- Doran, M.R.; Mills, R.J.; Parker, A.J.; Landman, K.A.; Cooper-White, J.J. A cell migration device that maintains a defined surface with no cellular damage during wound edge generation. Lab Chip 2009, 9, 2364–2369. [Google Scholar] [CrossRef]
- Tang, L.; Thevenot, P.; Hu, W. Surface Chemistry Influences Implant Biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. [Google Scholar] [CrossRef]
- Hopp, I.; Michelmore, A.; Smith, L.E.; Robinson, D.E.; Bachhuka, A.; Mierczynska, A.; Vasilev, K. The influence of substrate stiffness gradients on primary human dermal fibroblasts. Biomaterials 2013, 34, 5070–5077. [Google Scholar] [CrossRef]
- Kirchhof, K.; Groth, T. Surface modification of biomaterials to control adhesion of cells. Clin. Hemorheol. Microcirc. 2008, 39, 247–251. [Google Scholar] [CrossRef]
- Bullett, N.A.; Whittle, J.D.; Short, R.D.; Douglas, C.W.I. Adsorption of immunoglobulin G to plasma-co-polymer surfaces of acrylic acid and 1,7-octadiene. J. Mater. Chem. 2003, 13, 1546–1553. [Google Scholar] [CrossRef]
- Siow, K.S.; Britcher, L.; Kumar, S.; Griesser, H.J. Plasma methods for the generation of chemically reactive surfaces for biomolecule immobilization and cell colonization—A review. Plasma Process. Polym. 2006, 3, 392–418. [Google Scholar] [CrossRef]
- Chen, C.Z.C.; Raghunath, M. Focus on collagen: In vitro systems to study fibrogenesis and antifibrosis_state of the art. Fibrogenes. Tissue Repair 2009, 2, 7. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nashchekina, Y.; Chabina, A.; Moskalyuk, O.; Voronkina, I.; Evstigneeva, P.; Vaganov, G.; Nashchekin, A.; Yudin, V.; Mikhailova, N. Effect of Functionalization of the Polycaprolactone Film Surface on the Mechanical and Biological Properties of the Film Itself. Polymers 2022, 14, 4654. https://doi.org/10.3390/polym14214654
Nashchekina Y, Chabina A, Moskalyuk O, Voronkina I, Evstigneeva P, Vaganov G, Nashchekin A, Yudin V, Mikhailova N. Effect of Functionalization of the Polycaprolactone Film Surface on the Mechanical and Biological Properties of the Film Itself. Polymers. 2022; 14(21):4654. https://doi.org/10.3390/polym14214654
Chicago/Turabian StyleNashchekina, Yuliya, Alina Chabina, Olga Moskalyuk, Irina Voronkina, Polina Evstigneeva, Gleb Vaganov, Alexey Nashchekin, Vladimir Yudin, and Nataliya Mikhailova. 2022. "Effect of Functionalization of the Polycaprolactone Film Surface on the Mechanical and Biological Properties of the Film Itself" Polymers 14, no. 21: 4654. https://doi.org/10.3390/polym14214654
APA StyleNashchekina, Y., Chabina, A., Moskalyuk, O., Voronkina, I., Evstigneeva, P., Vaganov, G., Nashchekin, A., Yudin, V., & Mikhailova, N. (2022). Effect of Functionalization of the Polycaprolactone Film Surface on the Mechanical and Biological Properties of the Film Itself. Polymers, 14(21), 4654. https://doi.org/10.3390/polym14214654







