Effect of Organosilicon Self-Assembled Polymeric Nanolayers Formed during Surface Modification by Compositions Based on Organosilanes on the Atmospheric Corrosion of Metals
Abstract
1. Introduction
2. Materials and Methods
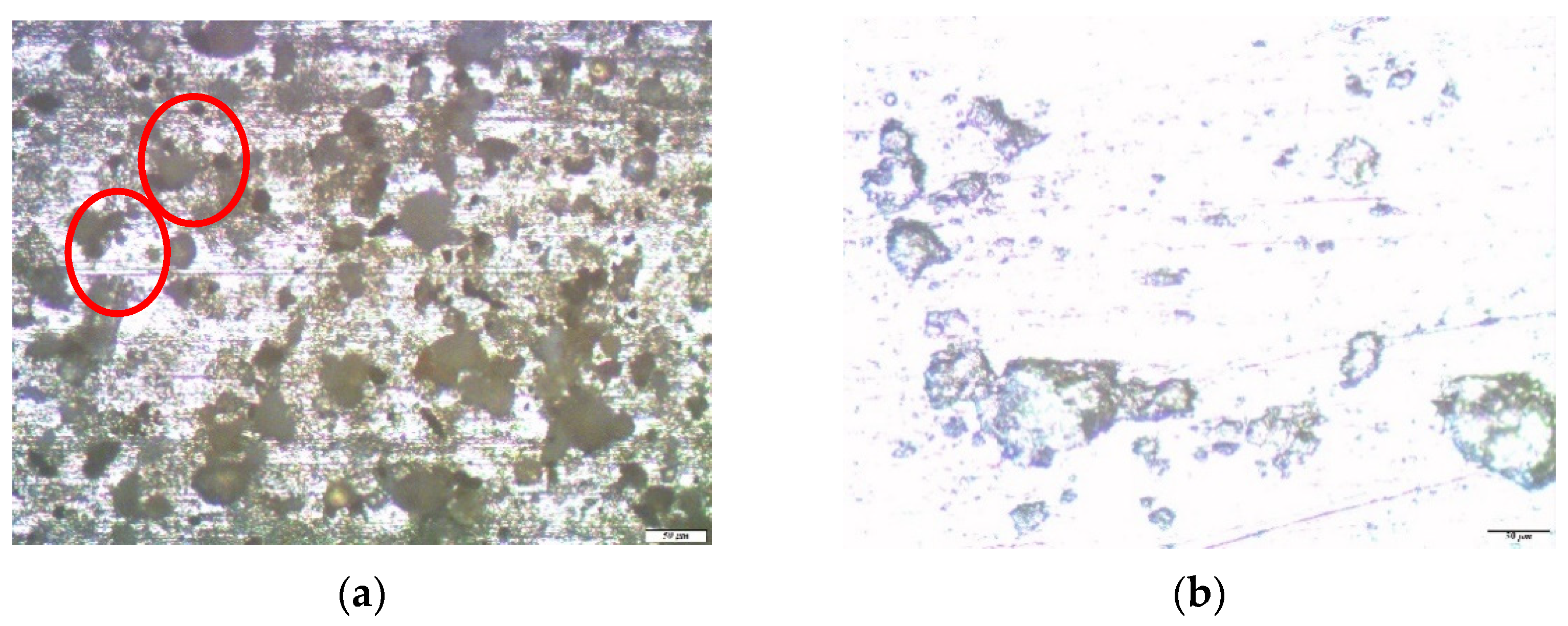
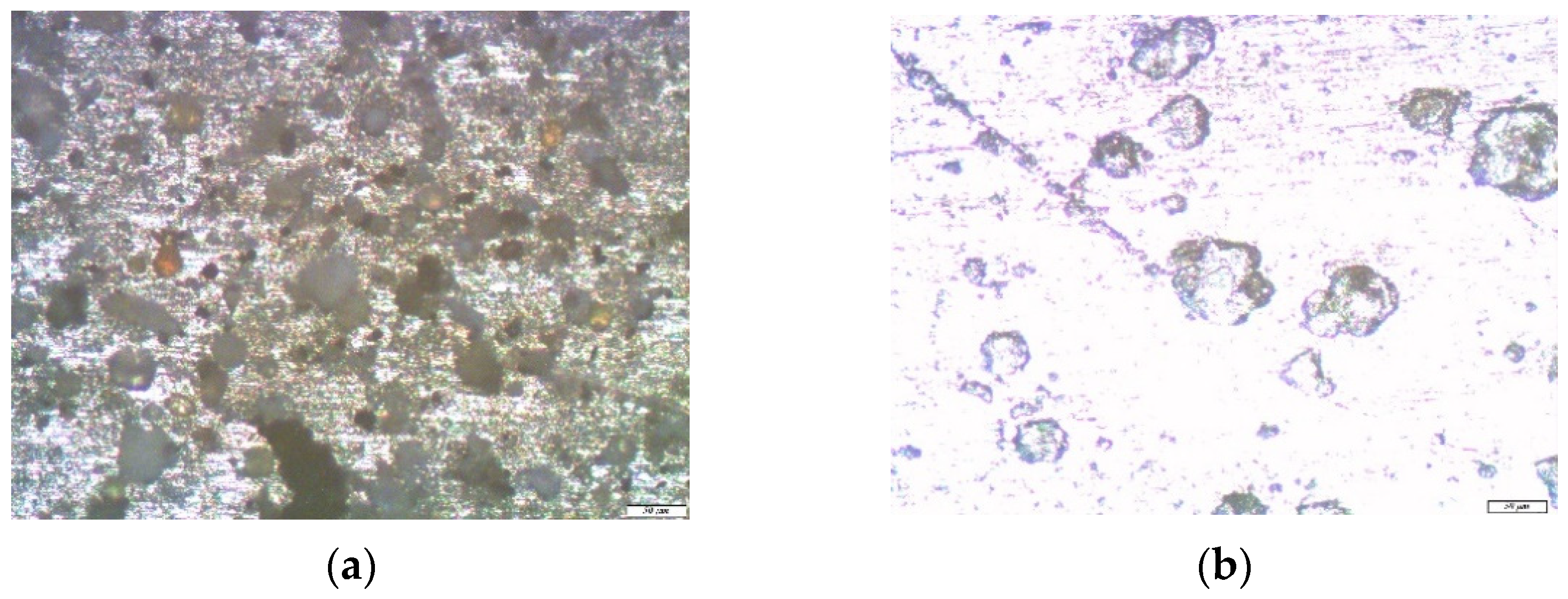
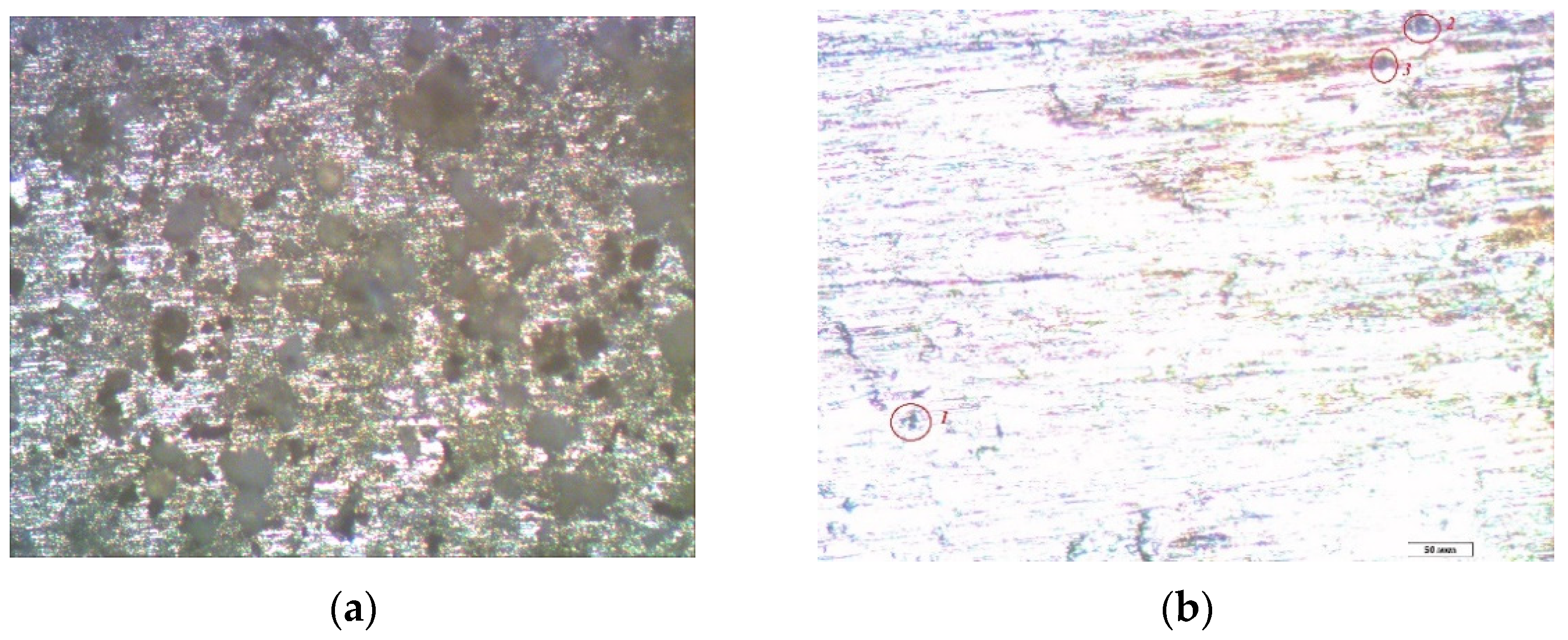
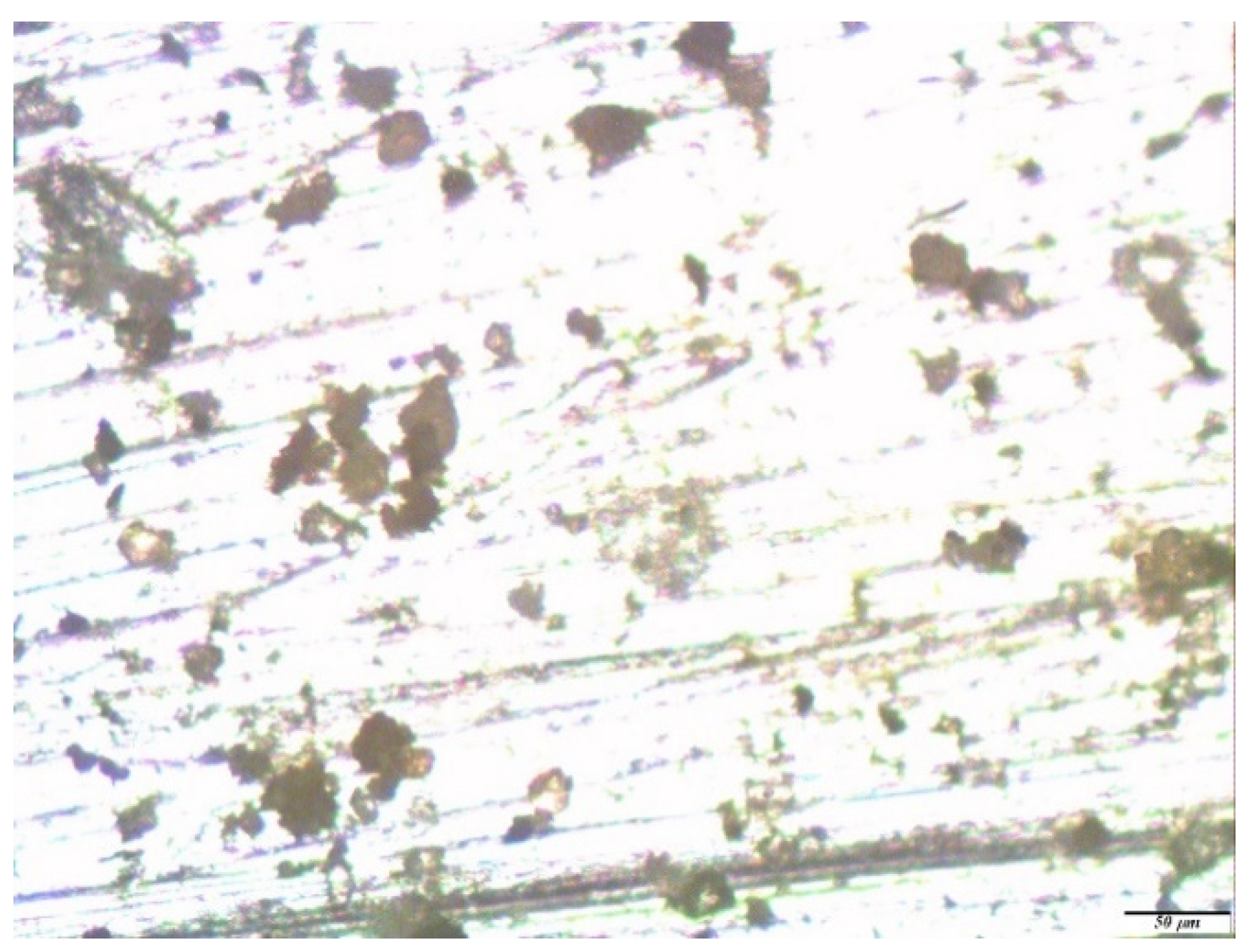
3. Results and Discussion
4. Conclusions
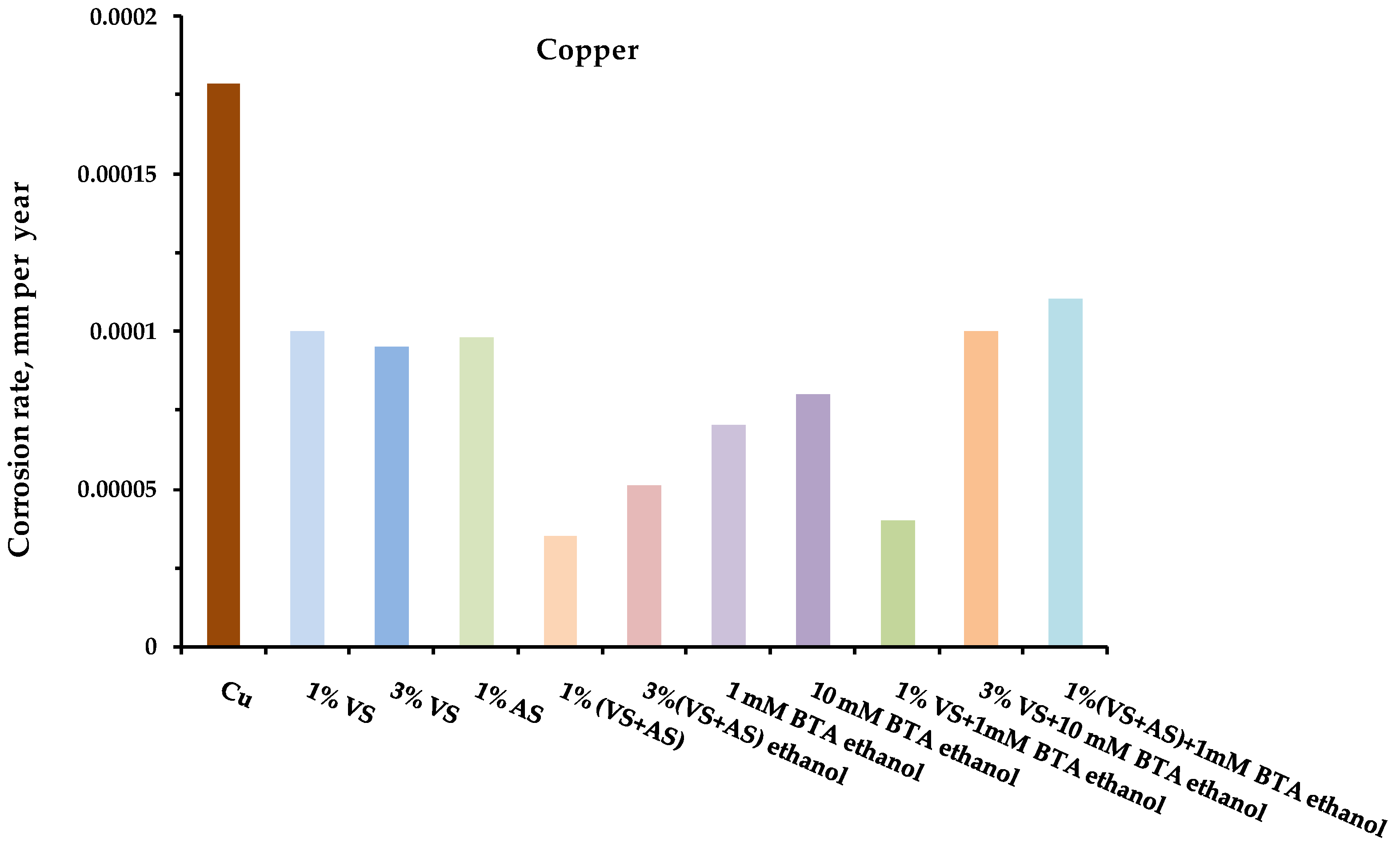
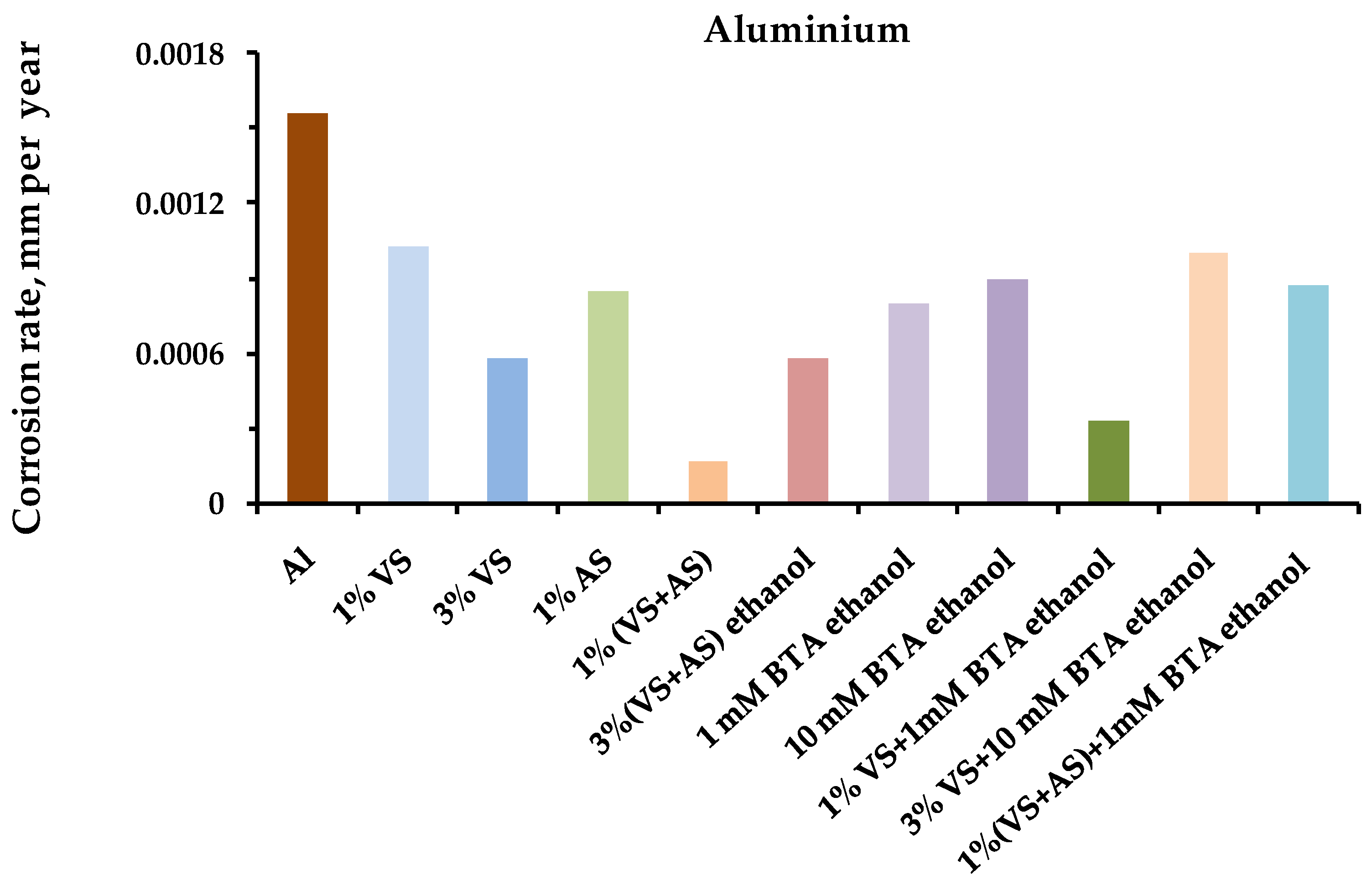
- Full-scale corrosion tests of structural metals were carried out for steel, copper, zinc, and aluminum, modified with organosilane-based compositions, in an urban atmosphere.
- After one year of natural corrosion tests, it was found that preliminary modification of the metal surface with organosilane-based compositions led to the inhibition of both uniform and local corrosion of the metals.
- The greatest inhibitory effect was demonstrated by the mixed two-component modifying compositions: mixtures of vinylsilane with aminosilane and vinylsilane with benzotriazole.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leygraf, C.; Wallinder, I.O.; Tidblad, J.; Graedel, T. Atmospheric Corrosion, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 1–380. [Google Scholar]
- Arkles, B. Silane Coupling Agents: Connecting Across Boundaries, 3rd ed.; Gelest, Inc.: Morrisville, PA, USA, 2014; pp. 1–75. [Google Scholar]
- Petrunin, M.A.; Gladkikh, N.A.; Maleeva, M.A.; Maksaeva, L.B.; Yurasova, T.A. The use of organosilanes to inhibit metal corrosion. A review. Int. J. Corros. Scale Inhib. 2019, 8, 882–907. [Google Scholar]
- Nazarov, A.; Petrunin, M.; Maksaeva, L.; Yurasova, T.; Traverso, P.; Marshakov, A. Vapour phase deposition of thin siloxane coatings on the iron surface. The impact of the layer structure and oxygen adsorption on corrosion stability. Coatings 2021, 11, 1217. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Gladkikh, N.A.; Maleeva, M.A.; Maksaeva, L.B.; Kostina, Y.V.; Shapagin, A.V.; Yurasova, T.A.; Kotenev, V.A.; Tsivadze, A.Y. The formation of self-organizing organosilicone layers on a carbon steel surface and their effect on the electrochemical and corrosion behavior of the metal. Prot. Met. Phys. Chem. Surf. 2019, 55, 895–901. [Google Scholar] [CrossRef]
- Gladkikh, N.; Makarychev, Y.; Maleeva, M.; Petrunin, M.; Maksaeva, L.; Rybkina, A.; Marshakov, A.; Kuznetsov, Y. Synthesis of thin organic layers containing silane coupling agents and azole on the surface of mild steel. Synergism of inhibitors for corrosion protection of underground pipelines. Prog. Org. Coat. 2019, 132, 481–489. [Google Scholar] [CrossRef]
- Gladkikh, N.; Petrunin, M.; Maksaeva, L.; Yurasova, T. Adsorption of organosilanes on the surface of aluminium and the formation of organosilane films to protect it from corrosion. Materials 2021, 14, 5757. [Google Scholar] [CrossRef] [PubMed]
- Peng, N.; Wen, Y.; He, Y. Improved distribution of etched tunnels on aluminum foil with silane treatment. Prog. Org. Coat. 2019, 127, 151–1568. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Maksaeva, L.B.; Gladkikh, N.A.; Narkevich, E.N.; Yurasova, T.A.; Rybkin, A.A.; Terekhova, E.V.; Kotenev, V.A.; Kablov, E.N.; Tsivadze, A.Y. The effect of vinyl-siloxane nanolayers on the corrosion behavior of zinc. Prot. Met. Phys. Chem. Surf. 2018, 54, 795–803. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Maksaeva, L.B.; Yurasova, T.A.; Terekhova, E.V.; Kotenev, V.A.; Kablov, E.N.; Tsivadze, A.Y. The directional formation and protective effect of self-assembling vinyl siloxane nanolayers on copper surface. Prot. Met. Phys. Chem. Surf. 2012, 48, 656–664. [Google Scholar] [CrossRef]
- Gladkikh, N.; Makarychev, Y.; Chirkunov, A.; Shapagin, A.; Petrunin, M.; Maksaeva, L.; Maleeva, M.; Yurasova, T.; Marshakov, A. Formation of polymer-like anticorrosive films based on organosilanes with benzotriazole, carboxylic and phosphonic acids. Protection of copper and steel against atmospheric corrosion. Prog. Org. Coat. 2020, 141, 105544. [Google Scholar] [CrossRef]
- Masi, G.; Josse, C.; Esvan, J.; Chiavari, C.; Bernardi, E.; Martini, C.; Bignozzi, M.C.; Monticelli, C.; Zanotto, F.; Balbo, A.; et al. Evaluation of the protectiveness of an organosilane coating on patinated Cu-Si-Mn bronze for contemporary art. Prog. Org. Coat. 2019, 127, 286–299. [Google Scholar] [CrossRef]
- Yongjuan, G.; Shaochun, L.; Dongshuai, H.; Weifeng, Z.; Zuquan, J.; Qiuyi, L.; Jianlin, L. Fabrication of superhydrophobicity on foamed concrete surface by GO/Silane coating. Mater. Lett. 2020, 265, 27423–127435. [Google Scholar]
- Najibzad, A.S.; Amini, R.; Rostami, M.; Kardar, P.; Fedel, M. Active corrosion performance of magnesium by silane coatings reinforced with polyaniline/praseodymium. Prog. Org. Coat. 2020, 140, 105504–105515. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Haddadi, S.; Motlagh, A.L.; Ghaderi, M.; Ramezanzadeh, B.; Mahdavian, M.; Arjmand, M.; Jalili, M. Epoxy nanocomposite coating based on calcium zinc phosphate with dual active/barrier corrosion mitigation properties. Prog. Org. Coat. 2022, 163, 106677. [Google Scholar] [CrossRef]
- Van Ooij, W.J.; Zhu, D.; Palanivel, V.; Lamar, J.A.; Stacy, M. Overview: The potential of silanes for chromate replacement in metal finishing industries. Silicon Chem. 2006, 3, 11–30. [Google Scholar] [CrossRef]
- ASTM G0050-20; Standard Practice for Conducting Atmospheric Corrosion Tests on Metals. ASTM International: West Conshohocken, PA, USA, 2020; pp. 1–6.
- International Standart ISO 9223; Corrosion of Metals and Alloys–Corrosivity of Atmospheres–Classification, Determination and Estimation. Second Edition 2012-02-01. ISO Switzerland: Bern, Switzerland, 2012; pp. 1–15.
- Reshetnikov, S.M.; Krutkina, T.G.; Burmistr, M.V. The interdependence of adsorption and protective properties of acidic-corrosion inhibitors. Prot. Met. 1980, 16, 173–176. [Google Scholar]
- Tomashev, N.D.; Chernova, G.P. Passivity and Protection of Metals against Corrosion; Plenum Press: New York, NY, USA, 1967; pp. 1–208. [Google Scholar]
- Kuznetsov, Y.I. Physicochemical aspects of metal corrosion inhibition in aqueous solutions. Russ. Chem. Rev. 2004, 73, 75–87. [Google Scholar] [CrossRef]
- Plueddemann, E.P. Bonding through coupling agents. In Molecular Characterization of Composite Interfaces; Ishida, H., Kumar, G., Eds.; Springer Science + Business Media: New York, NY, USA, 1985; pp. 13–24. [Google Scholar]
- Kaas, R.L.; Kardos, J.L. The Interaction of Alkoxy Silane Coupling Agents with Silica Surfaces. Polym. Eng. Sci. 1971, 77, 11–18. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I. Organic Inhibitors of Corrosion of Metals; Springer Science + Business Media: New York, NY, USA, 1996; pp. 1–283. [Google Scholar]
- Kallip, S.; Bastos, A.C.; Yasakau, K.A.; Zheludkevich, M.L.; Ferreira, M.G.S. Synergistic corrosion inhibition on galvanically coupled metallic materials. Electrochem. Commun. 2012, 20, 101–104. [Google Scholar] [CrossRef]
- Gladkikh, N.; Makarychev, Y.; Petrunin, M.; Maleeva, M.; Maksaeva, L.; Marshakov, A. Synergistic effect of silanes and azole for enhanced corrosion protection of carbon steel by polymeric coatings. Prog. Org. Coat. 2020, 138, 105386. [Google Scholar] [CrossRef]
- Balan, P.; Shelton, M.J.; Ching, D.O.L.; Han, G.C.; Palniandy, L.K. Modified silane films for corrosion protection of mild steel. Procedia Mater. Sci. 2014, 6, 244–248. [Google Scholar] [CrossRef]
- Galio, A.F.; Lamaka, S.V.; Zheludkevich, M.L.; Dick, L.F.P.; Müller, I.L.; Ferreira, M.G.S. Inhibitor-doped sol–gel coatings for corrosion protection of magnesium alloy AZ31. Surf. Coat. Technol. 2010, 204, 1479–1486. [Google Scholar] [CrossRef]
- Chirkunov, A.A.; Semiletov, A.M.; Kuznetsov, Y.I.; Andreeva, N.P. Passivation of steel with aqueous solutions of trialkoxysilanes. Prot. Met. Phys. Chem. Surf. 2015, 51, 1154–1159. [Google Scholar] [CrossRef]
- Montemor, M.F.; Pinto, R.; Ferreira, M.G.S. Chemical composition and corrosion protection of silane films modified with CeO2 nanoparticles. Electrochim. Acta 2009, 54, 5179–5189. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I.; Semiletov, A.M.; Chirkunov, A.A.; Arkhipushkin, I.A.; Kazanskii, L.P.; Andreeva, N.P. Protecting aluminum from atmospheric corrosion via surface hydrophobization with stearic acid and trialkoxysilanes. Russ. J. Phys. Chem. A 2018, 92, 621–629. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I.; Andreev, N.N.; Marshakov, A.I. Physicochemical aspects of metal corrosion inhibition. Russ. J. Phys. Chem. A 2020, 94, 505–515. [Google Scholar] [CrossRef]
- Bober, Y.G.; Kuznetsov, Y.I.; Andreeva, N.P. Adsorption at iron and passivation effect of anions of substituted phenylanthranilic acids. Prot. Met. 2008, 44, 84–90. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Maksaeva, L.B.; Yurasova, T.A.; Terekhova, E.V.; Maleeva, M.A.; Shcherbina, A.A.; Kotenev, V.A.; Kablov, E.N.; Tsivadze, A.Y. The effect of self-organizing vinyl siloxane nanolayers on the corrosion behavior of aluminum in neutral chloride-containing solutions. Prot. Met. Phys. Chem. Surf. 2014, 50, 784–791. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Maksaeva, L.B.; Yurasova, T.A.; Terekhova, E.V.; Maleeva, M.A.; Kotenev, V.A.; Kablov, E.N.; Tsivadze, A.Y. Formation of organosilicon self-organizing nanolavers on an iron surface from vapor phase and their effect on corrosion behavior of metal. Prot. Met. Phys. Chem. Surf. 2015, 51, 1010–1017. [Google Scholar] [CrossRef]
- Petrunin, M.; Maksaeva, L.; Gladkikh, N.; Makarychev, Y.; Maleeva, M.; Yurasova, T.; Nazarov, A. Thin benzotriazole films for inhibition of carbon steel corrosion in neutral electrolytes. Coatings 2020, 10, 362. [Google Scholar] [CrossRef]

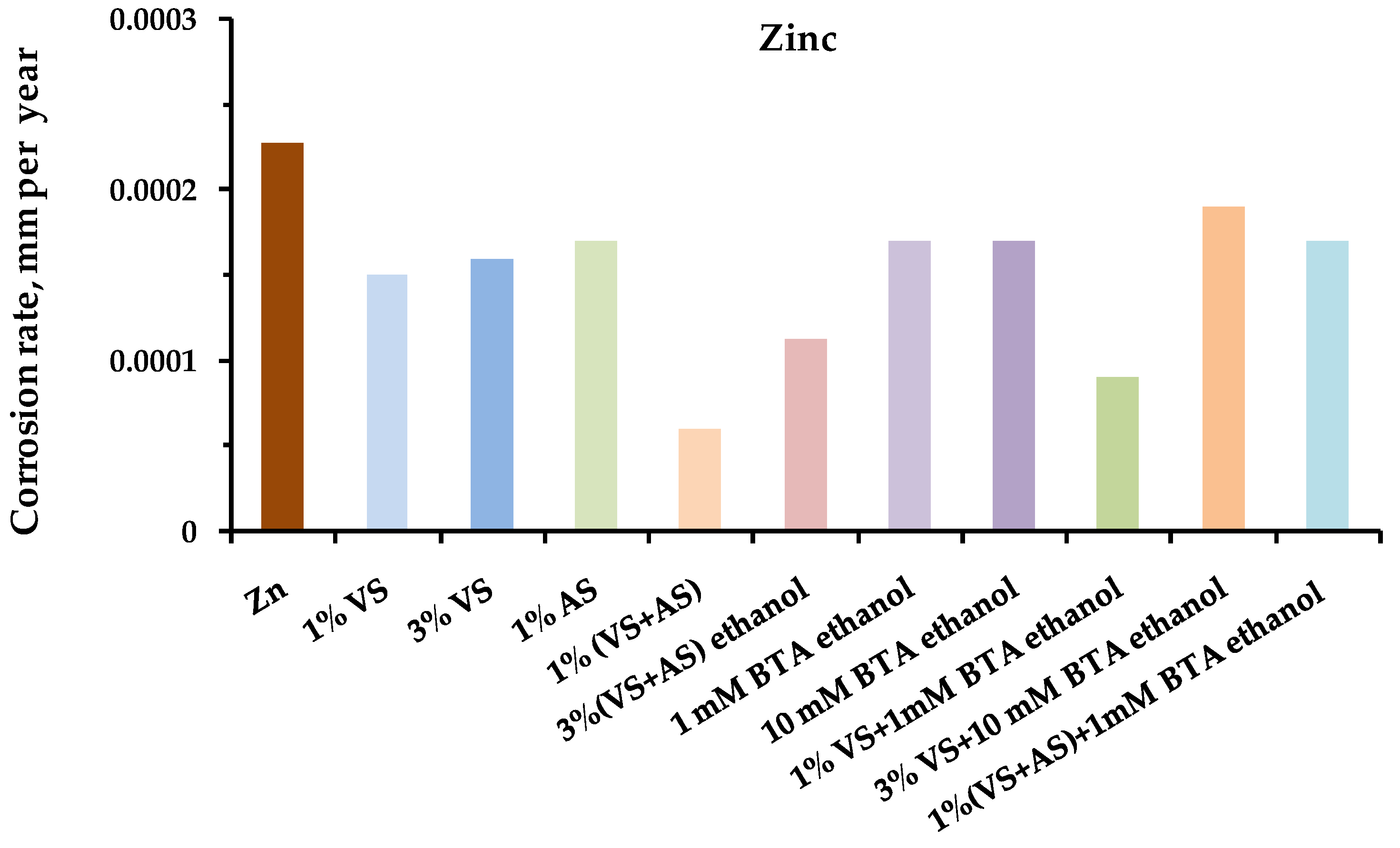


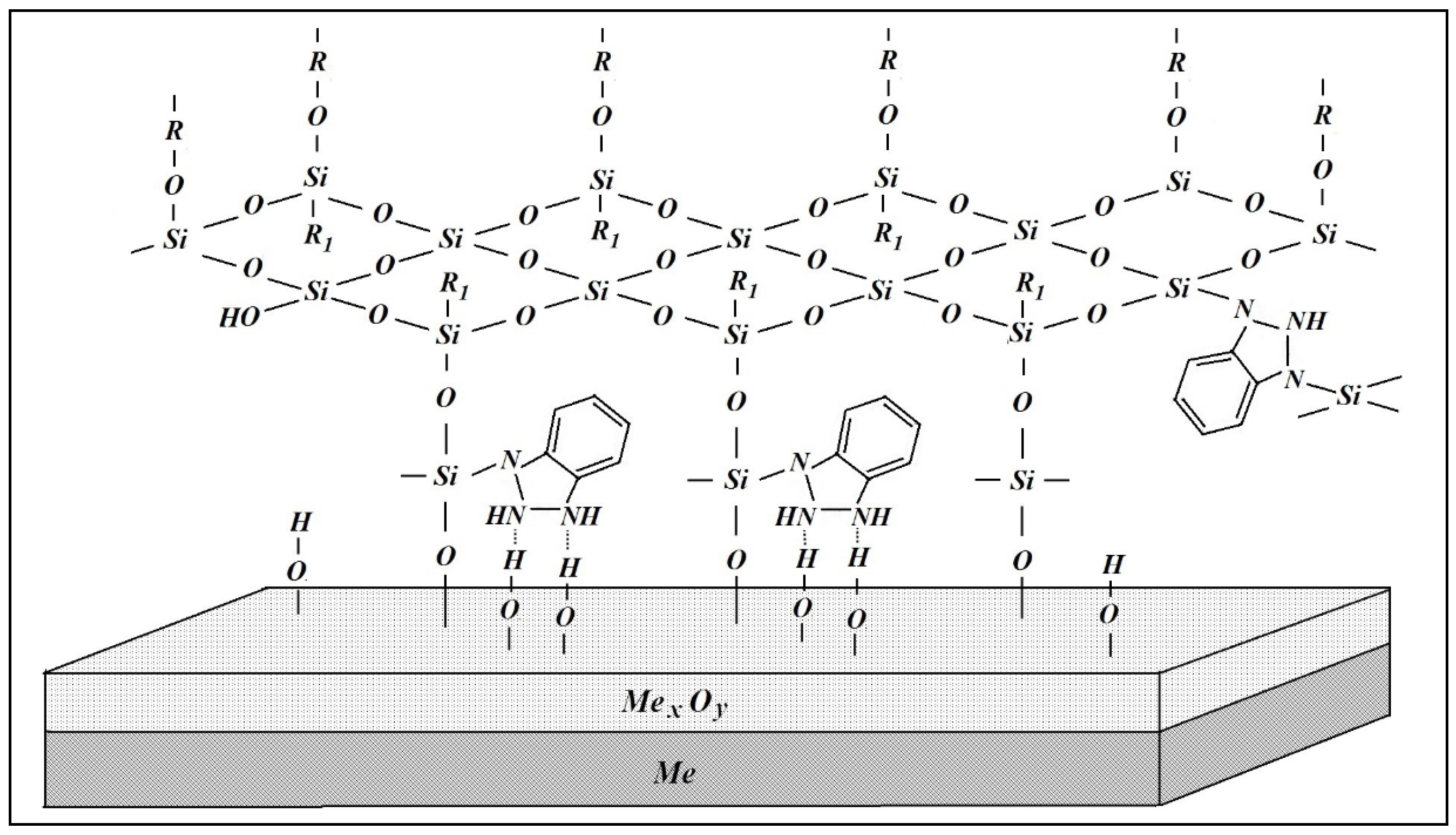

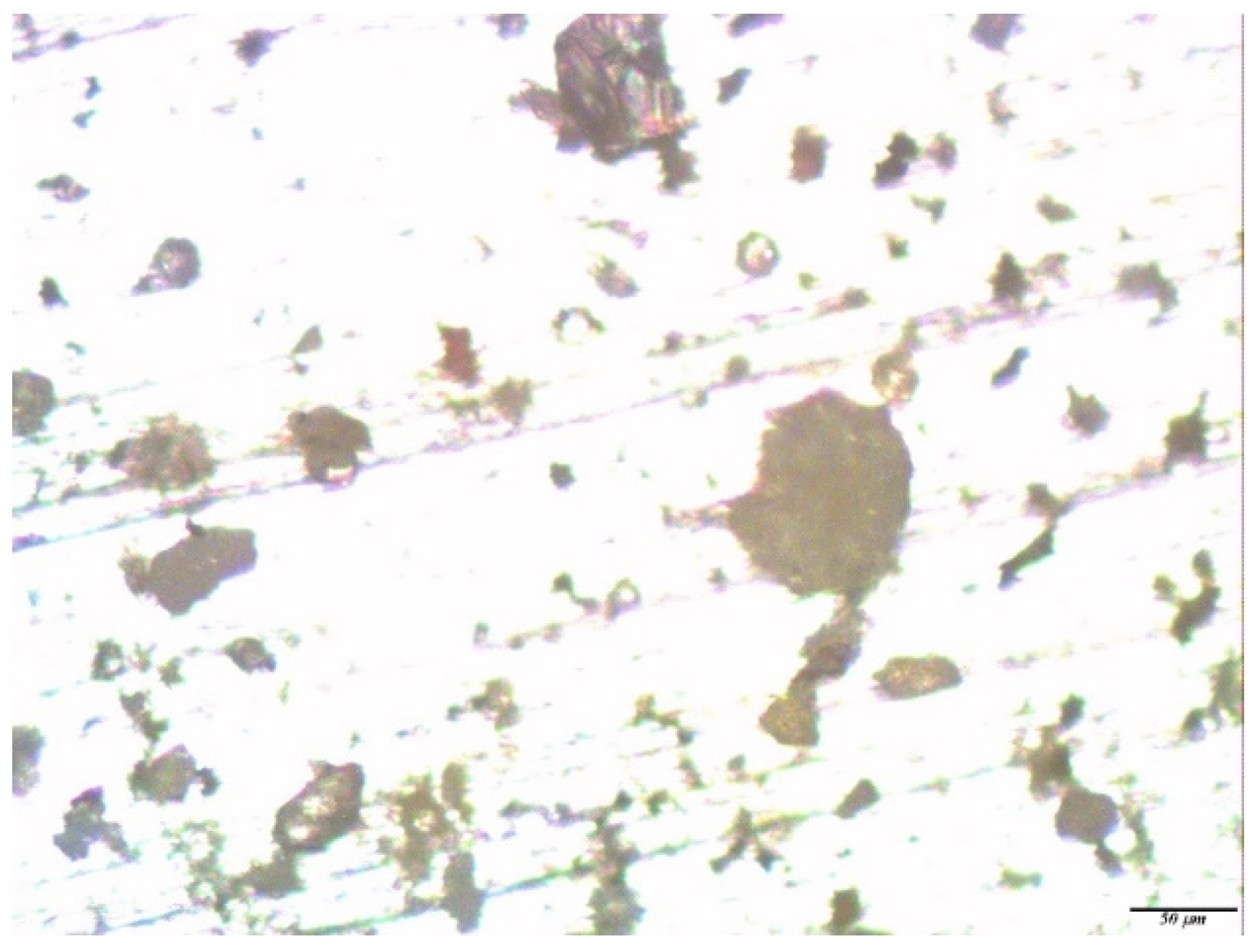

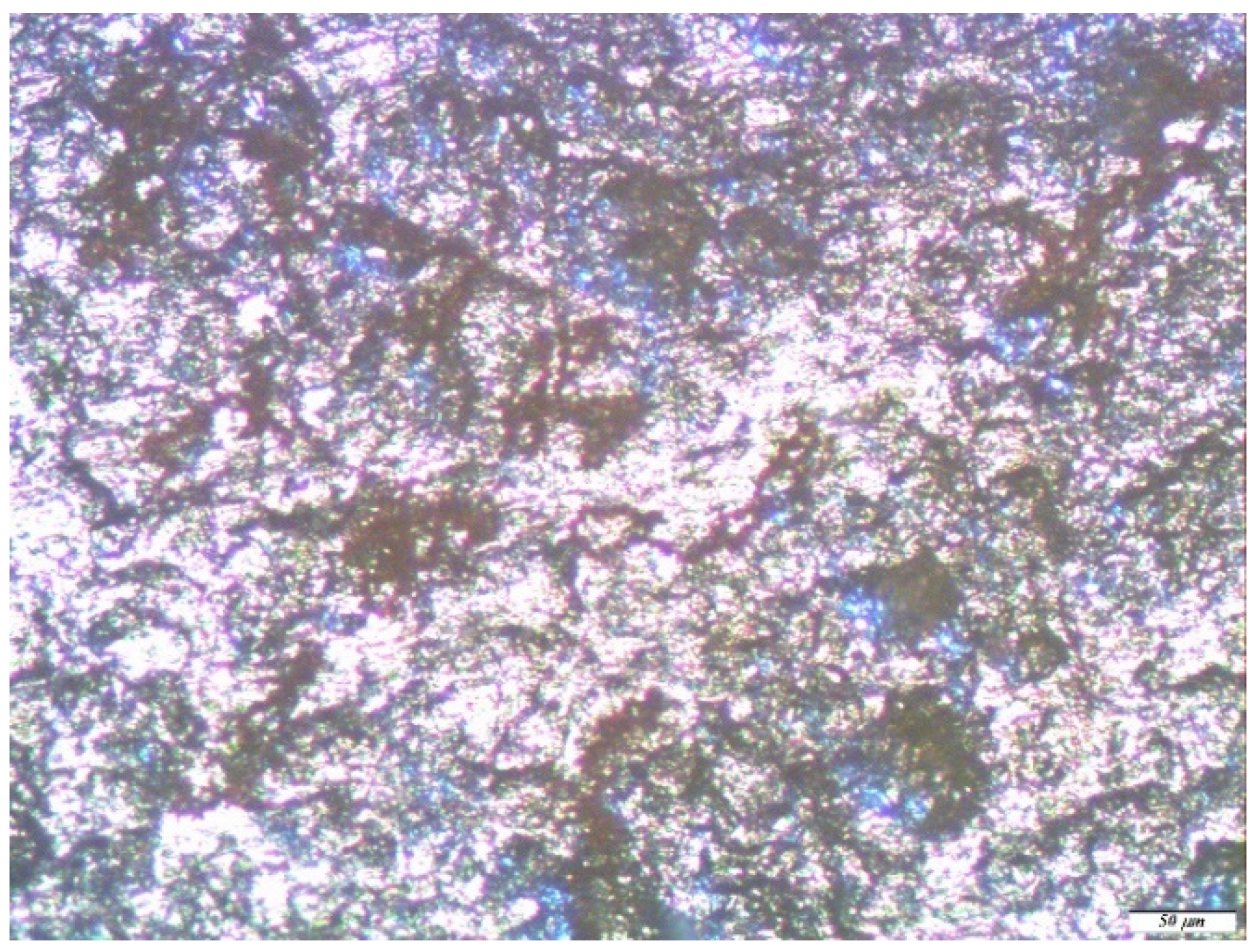
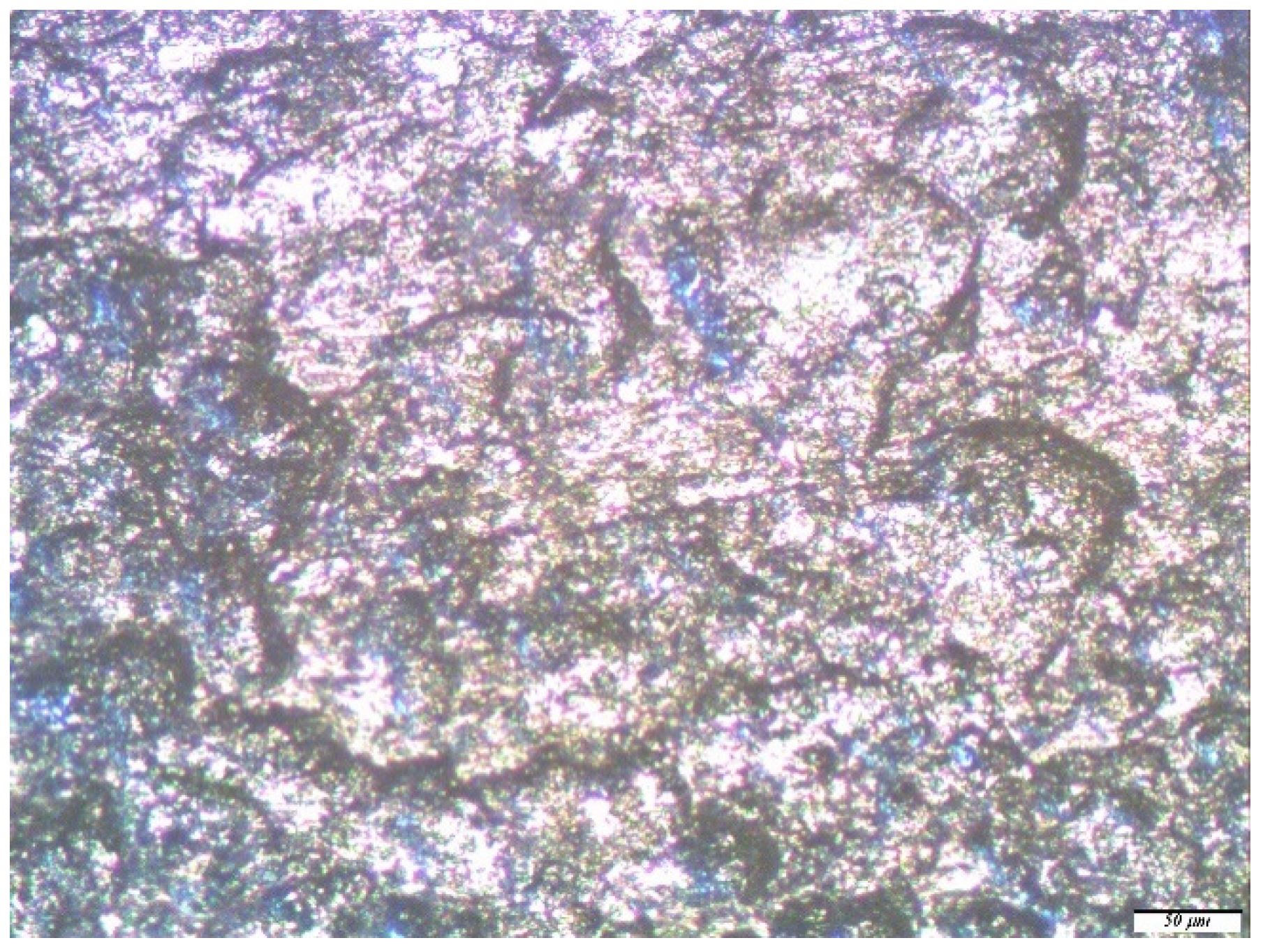
| No. | Conditional Designation | Title | Chemical Formula |
|---|---|---|---|
| 1 | VS | Vinyltrimethoxysilane | CH2=CH–Si(OC2H5)3 |
| 2 | AS | γ-Aminopropyltriethoxysilane | NH2(CH2)3–Si(OC2H5)3 |
| 3 | DAS | Aminoethylaminopropyltrimethoxy silane- Diaminsilane | NH2–CH2–CH2–NH–CH2–CH2–CH2–Si(OCH3)3 |
| 4 | MS | γ-Methacryloxypropyltrime- thoxysilane |  |
| 5 | BTA | 1.2.3-benzotriazole | 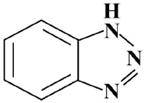 |
| No. | System for Modifying the Sample Surface | Ecor, V | |||
|---|---|---|---|---|---|
| Copper | Zinc | Aluminum | Carbon Steel | ||
| 1 | Blank | −0.020 | −0.835 | −0.653 | −0.405 |
| 2 | 1% VS | −0.027 | −0.834 | −0.640 | −0.137 |
| 3 | 3% VS | −0.83 | −0.770 | −0.652 | −0.098 |
| 4 | 1% VS + 1% AS | 0.048 | −0.796 | −0.532 | −0.078 |
| 5 | 1% VS + 1 mM BTA | 0.001 | −0.830 | −0.453 | −0.116 |
| Modifying Solution | Inhibition Coefficient of Uniform Corrosion γ (Gravimetric Data) | |||
|---|---|---|---|---|
| Copper | Zinc | Aluminum | Carbon Steel | |
| Blank | 1.00 | 1.00 | 1.00 | 1.00 |
| 1% water solution of VS | 1.79 | 1.52 | 1.52 | 1.44 |
| 3% water solution of VS | 1.88 | 1.43 | 2.70 | 1.76 |
| 1% water solution of AS | 1.82 | 1.34 | 1.84 | 1.57 |
| Alcohol solution of the mixture: [1% VS + 1 mM BTA] | 4.46 | 2.53 | 4.74 | 3.09 |
| Water solution of the mixture: [1% VS + 1% AS] | 5.10 | 3.80 | 9.20 | 3.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrunin, M.; Rybkina, A.; Yurasova, T.; Maksaeva, L. Effect of Organosilicon Self-Assembled Polymeric Nanolayers Formed during Surface Modification by Compositions Based on Organosilanes on the Atmospheric Corrosion of Metals. Polymers 2022, 14, 4428. https://doi.org/10.3390/polym14204428
Petrunin M, Rybkina A, Yurasova T, Maksaeva L. Effect of Organosilicon Self-Assembled Polymeric Nanolayers Formed during Surface Modification by Compositions Based on Organosilanes on the Atmospheric Corrosion of Metals. Polymers. 2022; 14(20):4428. https://doi.org/10.3390/polym14204428
Chicago/Turabian StylePetrunin, Maxim, Alevtina Rybkina, Tatyana Yurasova, and Liudmila Maksaeva. 2022. "Effect of Organosilicon Self-Assembled Polymeric Nanolayers Formed during Surface Modification by Compositions Based on Organosilanes on the Atmospheric Corrosion of Metals" Polymers 14, no. 20: 4428. https://doi.org/10.3390/polym14204428
APA StylePetrunin, M., Rybkina, A., Yurasova, T., & Maksaeva, L. (2022). Effect of Organosilicon Self-Assembled Polymeric Nanolayers Formed during Surface Modification by Compositions Based on Organosilanes on the Atmospheric Corrosion of Metals. Polymers, 14(20), 4428. https://doi.org/10.3390/polym14204428








