Mesoporous Spherical Silica Filler Prepared from Coal Gasification Fine Slag for Styrene Butadiene Rubber Reinforcement and Promoting Vulcanization
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Preparation of the FS-SiO2
2.3. Preparation of the SBR Composites
2.4. Characterization and Performance Tests
2.4.1. Characterization of the Fillers
2.4.2. Testing of the Filled SBR Composites
2.4.3. Crosslink Density of the SBR Composites
3. Results and Discussion
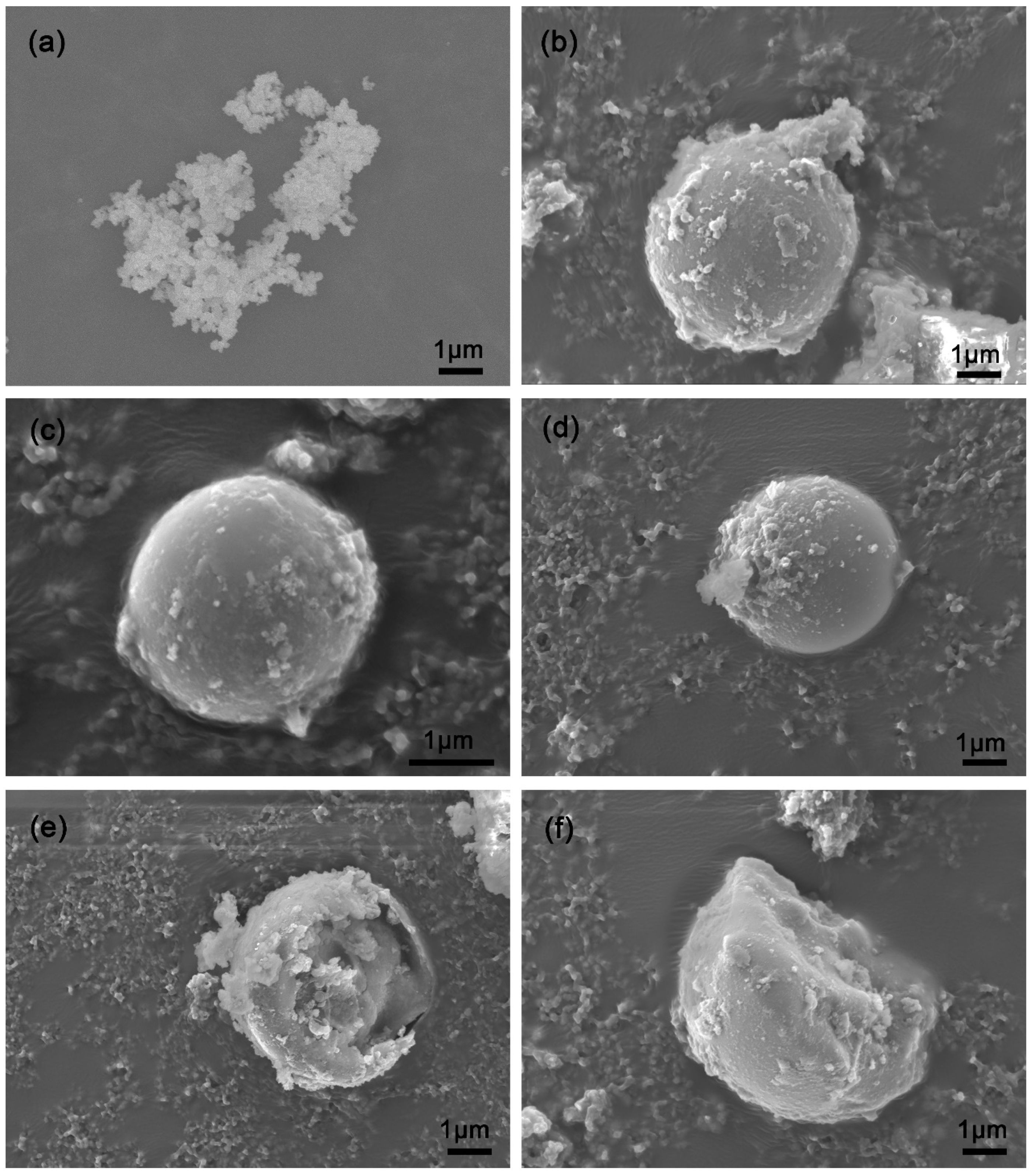
3.1. Characteristics of the Composite Filler Morphology of the Fillers
3.2. Pore Structure of the Fillers
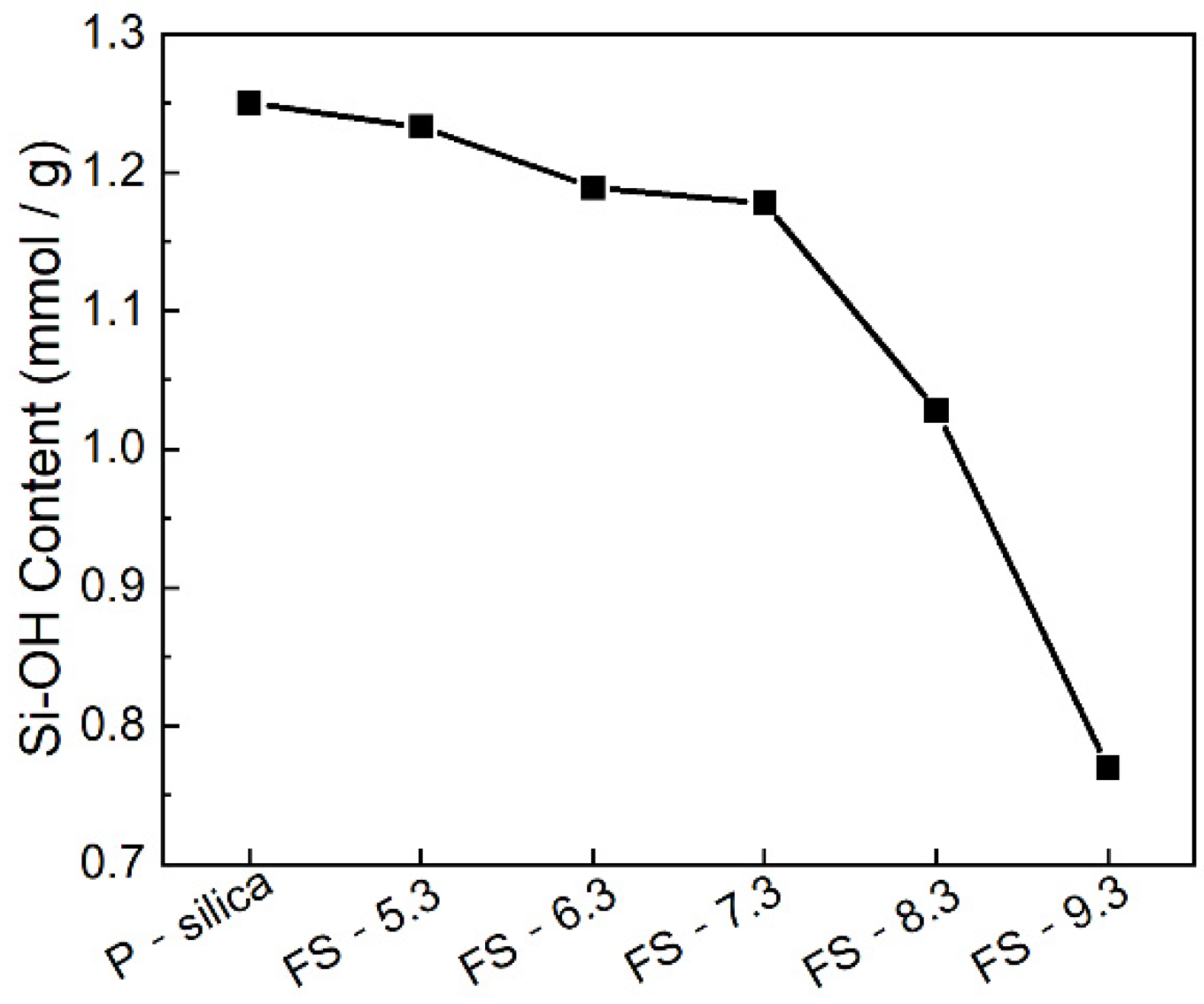
3.3. Surface Silanol Groups
3.4. Particle size of the Fillers
3.5. Cure Characteristics
3.6. Mechanical Properties
3.7. Crosslink Density of SBR Composites
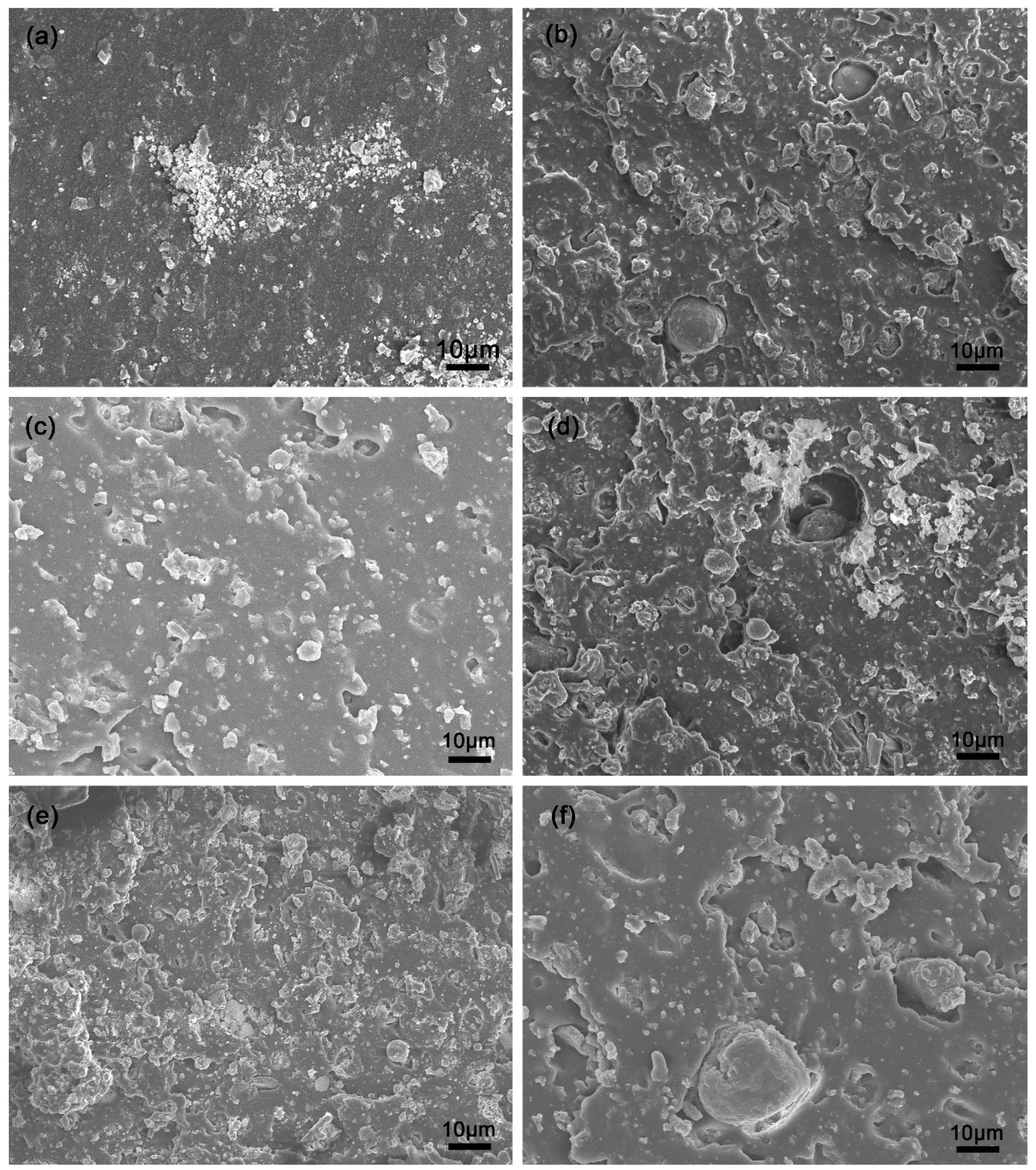
3.8. Microstructure of SBR Composites
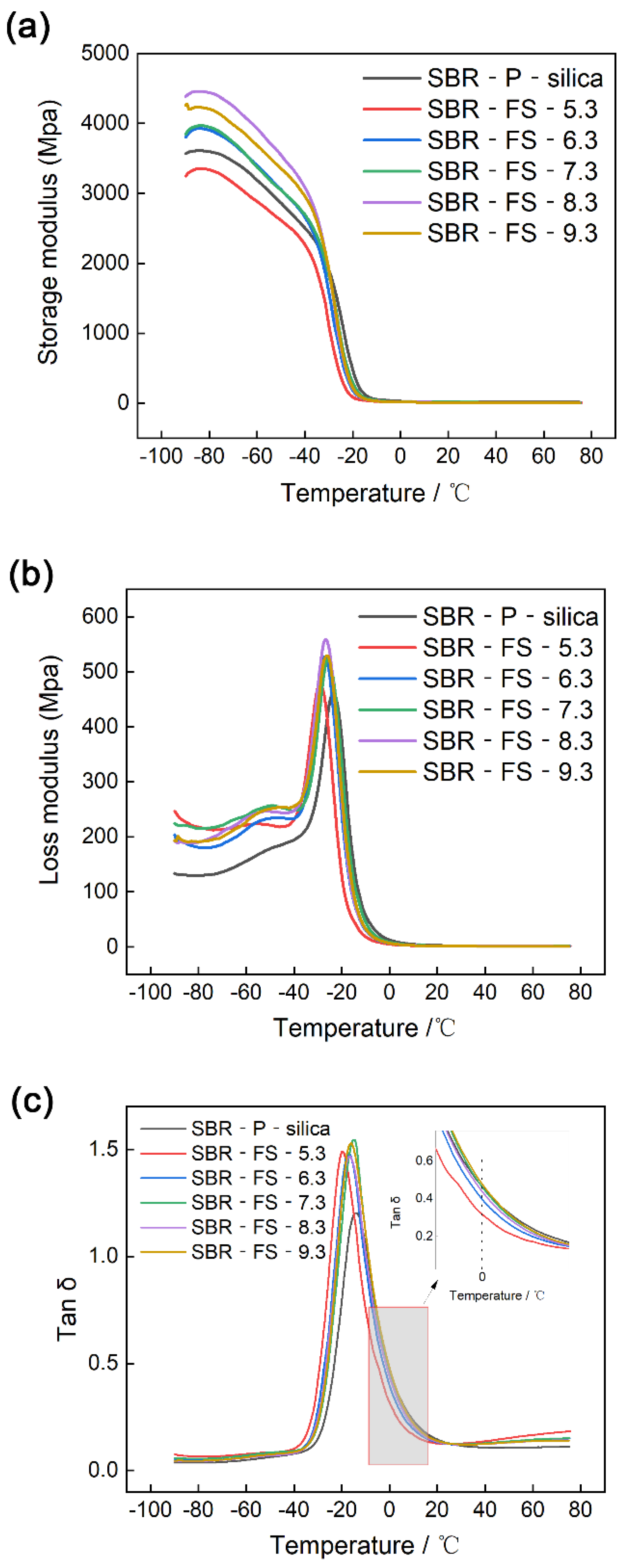
3.9. Dynamic Mechanical Analysis (DMA)
3.10. Rubber Processing Analysis (RPA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, F.; Guo, Y.; Guo, Z.; Miao, Z.; Zhao, X.; Zhang, Y.; Li, J.; Wu, J. Recycling Residual Carbon from Gasification Fine Slag and Its Application for Preparing Slurry Fuels. ACS Sustain. Chem. Eng. 2020, 8, 8830–8839. [Google Scholar] [CrossRef]
- Guo, Q.; Li, H.; Wang, S.; Gong, Y.; Ren, L.; Yu, G. Experimental study on preparation of oxygen reduction catalyst from coal gasification residual carbon. Chem. Eng. J. 2022, 446, 137256. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Zhao, X.; Xu, J.; Qiu, G.; Jia, W.; Wu, J.; Guo, F. Multifaceted evaluation of distribution, occurrence, and leaching features of typical heavy metals in different-sized coal gasification fine slag from Ningdong region, China: A case study. Sci. Total Environ. 2022, 831, 154726. [Google Scholar] [CrossRef]
- Guo, F.; Qiu, G.; Guo, Y.; Jia, W.; Chen, L.; Zhang, Y.; Jiang, L.; Hu, X.; Wu, J.; Zhang, H. Efficient dewatering of waste gasification fine slag based on mechanical pressure-vacuum fields: Dewatering characteristics, energy optimization and potential environmental benefits. J. Environ. Manag. 2022, 320, 115881. [Google Scholar] [CrossRef]
- Wu, T.; Gong, M.; Lester, E.; Wang, F.; Zhou, Z.; Yu, Z. Characterisation of residual carbon from entrained-bed coal water slurry gasifiers. Fuel 2007, 86, 972–982. [Google Scholar] [CrossRef]
- Pan, C.; Liang, Q.; Guo, X.; Dai, Z.; Liu, H.; Gong, X. Characteristics of Different Sized Slag Particles from Entrained-Flow Coal Gasification. Energy Fuels 2016, 30, 1487–1495. [Google Scholar] [CrossRef]
- Wu, S.; Huang, S.; Wu, Y.; Gao, J. Characteristics and catalytic actions of inorganic constituents from entrained-flow coal gasification slag. J. Energy Inst. 2015, 88, 93–103. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, C.; Mao, Y.; Li, W.; Peng, Y.; Wang, T.; Eiteneer, B.; Zamansky, V.; Fletcher, T. The Surface Characteristics and Reactivity of Residual Carbon in Coal Gasification Slag†. Energy Fuels 2010, 24, 91–94. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Ai, W.; Wei, C. A new method to prepare mesoporous silica from coal gasification fine slag and its application in methylene blue adsorption. J. Clean. Prod. 2019, 212, 1062–1071. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Qiu, G.; Jia, W.; Guo, Y.; Guo, F.; Wu, J. Synthesis of Porous Material from Coal Gasification Fine Slag Residual Carbon and Its Application in Removal of Methylene Blue. Molecules 2021, 26, 6116. [Google Scholar] [CrossRef]
- Chai, Z.; Lv, P.; Bai, Y.; Wang, J.; Song, X.; Su, W.; Yu, G. Low-cost Y-type zeolite/carbon porous composite from coal gasification fine slag and its application in the phenol removal from wastewater: Fabrication, characterization, equilibrium, and kinetic studies. RSC Adv. 2022, 12, 6715–6724. [Google Scholar] [CrossRef]
- Niu, Y.; Xu, J.; Miao, Z.; Guo, F.; Zhang, Y.; Wu, J. Distribution modes of residual carbon and ash in coal gasification fine slag and its feasibility analysis as particle electrodes. Chemosphere 2022, 303, 135159. [Google Scholar] [CrossRef]
- Miao, Z.; Wu, J.; Niu, Y.; Guo, Z.; Guo, F.; Zhang, Y. Development of a novel type hierarchical porous composite from coal gasification fine slag for CO2 capture. Chem. Eng. J. 2022, 435, 134909. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, Y.; Li, H.; He, J.; Xu, H.; Wu, C. The microwave absorption properties of residual carbon from coal gasification fine slag. Fuel 2021, 290, 120050. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, J.; Jiang, Y.; Ju, A.; Zhu, D.; Zhang, J.; Wei, C. Synthesis and characterization of composite conductive powders prepared by Sb-SnO2-coated coal gasification fine slag porous microbeads. Powder Technol. 2021, 385, 409–417. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, Y.; Gong, Y.; Zhuang, X.; Richter, A.; Yu, G. Recovered Carbon from Coal Gasification Fine Slag as Electrocatalyst for Oxygen Reduction Reaction and Zinc–Air Battery. Energy Technol. 2021, 9, 2000890. [Google Scholar] [CrossRef]
- Ren, X.J.; Sancaktar, E. Use of fly ash as eco-friendly filler in synthetic rubber for tire applications. J. Clean. Prod. 2019, 206, 374–382. [Google Scholar] [CrossRef]
- Tan, J.; Cheng, H.; Wei, L.; Wei, C.; Xing, Y.; Gui, X. Using low-rank coal slime as an eco-friendly replacement for carbon black filler in styrene butadiene rubber. J. Clean. Prod. 2019, 234, 949–960. [Google Scholar] [CrossRef]
- Chen, J.; Xu, K.; Pan, R.; Peng, Z.; Ma, L. Effect of modification conditions of coal gangue on properties of natural rubber composites. Polym. Compos. 2014, 35, 1911–1917. [Google Scholar] [CrossRef]
- Sombatsompop, N.; Thongsang, S.; Markpin, T.; Wimolmala, E. Fly ash particles and precipitated silica as fillers in rubbers. I. Untreated fillers in natural rubber and styrene-butadiene rubber compounds. J. Appl. Polym. Sci. 2004, 93, 2119–2130. [Google Scholar] [CrossRef]
- Sombatsompop, N.; Wimolmala, E.; Markpin, T. Fly-ash particles and precipitated silica as fillers in rubbers. II. Effects of silica content and Si69-treatment in natural rubber/styrene–butadiene rubber vulcanizates. J. Appl. Polym. Sci. 2007, 104, 3396–3405. [Google Scholar] [CrossRef]
- Alkadasi, N.A.N.; Hundiwale, D.G.; Kapadi, U.R. Effect of coupling agent on the mechanical properties of fly ash-filled polybutadiene rubber. J. Appl. Polym. Sci. 2003, 91, 1322–1328. [Google Scholar] [CrossRef]
- Mahmood, N.; Khan, M.S.; Khan, A.U.; Stöckelhuber, K.W.; Heinrich, G. Purification, surface modification of coal ash silica and its potential application in rubber composites. J. Appl. Polym. Sci. 2010, 117, 1493–1501. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, J.; Ai, W.; Liu, S.; Zhu, D.; Zhang, J.; Wei, C. Preparation of a new high-efficiency resin deodorant from coal gasification fine slag and its application in the removal of volatile organic compounds in polypropylene composites. J. Hazard. Mater. 2020, 384, 121347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zuo, J.; Liu, Y.; Zhang, J.; Fu, W.; Zhang, J.; Miao, S.; Wei, C. Universality of mesoporous coal gasification slag for reinforcement and deodorization in four common polymers. Nanotechnology 2021, 33, 095703. [Google Scholar] [CrossRef]
- Ai, W.; Zhang, J.; Miao, S.; Wei, C. A low-cost and high-value reinforcing filler for styrene butadiene rubber fabricated by a pneumatic separation technique from coal gasification fine slag. Polym. J. 2019, 52, 493–503. [Google Scholar] [CrossRef]
- Li, J.; Isayev, A.I.; Ren, X.; Soucek, M.D. Modified soybean oil-extended SBR compounds and vulcanizates filled with carbon black. Polymer 2015, 60, 144–156. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, J.; Jiang, Y.; Zhu, D.; Zhang, J.; Wei, C. Kinetic analysis on the mesoporous formation of coal gasification slag by acid leaching and its thermal stability. Solid State Sci. 2020, 100, 106084. [Google Scholar] [CrossRef]
- Zhong, B.; Zeng, X.; Chen, W.; Luo, Q.; Hu, D.; Jia, Z.; Jia, D. Nonsolvent-assisted surface modification of silica by silane and antioxidant for rubber reinforcement. Polym. Test. 2019, 78, 105949. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Catalfamo, P.; Primerano, P.; Arrigo, I.; Corigliano, F. Use of a Glass Residue in the Removal of Heavy Metals from Wastewater. Ann. Chim. 2006, 96, 487–492. [Google Scholar] [CrossRef]
- Yong, Y.; Huajun, G.; Qingxiao, Z.; Fang, Z.; Hui, L. Hollow ni-p amorphous alloy nanospheres: An efficient catalyst for sugars hydrogenation to polyols. Catal. Today 2021, 365, 282–290. [Google Scholar] [CrossRef]
- Muhammud, A.M.; Gupta, N.K. Nanostructured SiO2 material: Synthesis advances and applications in rubber reinforcement. RSC Adv. 2022, 12, 18524–18546. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Basu, D.; Kumar, A.; Nawale, M.; Kadam, S.; Bhujbal, A.; Rajkumar, K.; Bhowmick, A.; Chattopadhyay, S. Bio-based oil derived from waste coconut shell: A potential additive for enhancing silanization in silica filled styrene butadiene copolymer. J. Polym. Res. 2022, 29, 311. [Google Scholar] [CrossRef]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Huang, C.-L.; Feng, Y.-H.; Zhang, X.-X.; Li, J.; Wang, G. Thermal conductivity of a kind of mesoporous silica SBA-15. Chin. Phys. B 2013, 22, 064401. [Google Scholar] [CrossRef]
- Şova, D.; Stanciu, M.D.; Belea, E.; Bidu, V.V. Innovative thermal insulation panels with air channels. IOP Conf. Ser. Mater. Sci. Eng. 2018, 444, 062005. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Park, N.; Lim, S.; Ahn, B.; Kim, W.; Moon, H.; Paik, H.-j.; Kim, W. Influence of the silanes on the crosslink density and crosslink structure of silica-filled solution styrene butadiene rubber compounds. Compos. Interfaces 2016, 24, 711–727. [Google Scholar] [CrossRef]
- Ji, X.; Hampsey, J.E.; Hu, Q.; He, J.; Yang, Z.; Lu, Y. Mesoporous Silica-Reinforced Polymer Nanocomposites. Chem. Mater. 2003, 15, 3656–3662. [Google Scholar] [CrossRef]
- Zhong, B.; Jia, Z.; Luo, Y.; Jia, D. A method to improve the mechanical performance of styrene-butadiene rubber via vulcanization accelerator modified silica. Compos. Sci. Technol. 2015, 117, 46–53. [Google Scholar] [CrossRef]
- Ichazo, M.N.; Albano, C.; Hernández, M.; González, J.; Carta, A. Effects of Particle Size and Size Distribution on the Mechanical Properties of EPDM/Silica Vulcanizates. Adv. Mater. Res. 2008, 47–50, 113–116. [Google Scholar] [CrossRef]
- Li, M.; Wang, K.; Xiong, Y. Multiple Intermolecular Interaction to Improve the Abrasion Resistance and Wet Skid Resistance of Eucommia Ulmoides Gum/Styrene Butadiene Rubber Composite. Materials 2021, 14, 5246. [Google Scholar] [CrossRef] [PubMed]
- El Yaagoubi, M.; Juhre, D.; Meier, J.; Alshuth, T.; Giese, U. Tearing energy and path-dependent J-integral evaluation considering stress softening for carbon black reinforced elastomers. Eng. Fract. Mech. 2018, 190, 259–272. [Google Scholar] [CrossRef]
- Zhong, B.; Jia, Z.; Luo, Y.; Jia, D.; Liu, F. Understanding the effect of filler shape induced immobilized rubber on the interfacial and mechanical strength of rubber composites. Polym. Test. 2017, 58, 31–39. [Google Scholar] [CrossRef]
- Xie, T.; Wang, F.; Xie, C.; Lei, S.; Yu, S.; Liu, J.; Huang, D. Mechanical Properties of Natural Rubber Filled with Foundry Waste Derived Fillers. Materials 2019, 12, 1863. [Google Scholar] [CrossRef]
- Abdul Salim, Z.A.S.; Hassan, A.; Ismail, H. A Review on Hybrid Fillers in Rubber Composites. Polym.-Plast. Technol. Eng. 2017, 57, 523–539. [Google Scholar] [CrossRef]
- Yang, S.; Tian, J.; Bian, X.; Wu, Y. High performance NBR/fly ash composites prepared by an environment-friendly method. Compos. Sci. Technol. 2020, 186, 107909. [Google Scholar] [CrossRef]
- Thongsang, S.; Vorakhan, W.; Wimolmala, E.; Sombatsompop, N. Dynamic mechanical analysis and tribological properties of NR vulcanizates with fly ash/precipitated silica hybrid filler. Tribol. Int. 2012, 53, 134–141. [Google Scholar] [CrossRef]
- Chen, B.; Dai, J.; Song, T.; Guan, Q. Research and Development of High-Performance High-Damping Rubber Materials for High-Damping Rubber Isolation Bearings: A Review. Polymers 2022, 14, 2427. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Maslowski, M.; Rybinski, P.; Strzelec, K. Modified Nanoclays/Straw Fillers as Functional Additives of Natural Rubber Biocomposites. Polymers 2021, 13, 799. [Google Scholar] [CrossRef]
- Kim, W.; Kim, D.; Park, N.; Ahn, B. Influence of End-Functionalized Solution Styrene–Butadiene Rubber on Silica-Filled Vulcanizates with Various Silica–Silane Systems. Rubber Chem. Technol. 2018, 92, 364–377. [Google Scholar] [CrossRef]
- Park, N.; Ahn, B.; Lee, J.-Y.; Kim, W.; Moon, H.; Kim, W. Effect of organosilane agents on the vulcanizate structure and physical properties of silica-filled solution styrene butadiene rubber compounds. Compos. Interfaces 2017, 25, 259–273. [Google Scholar] [CrossRef]
- Xu, H.; Song, Y.; Zhang, Q.; Zheng, Q. Contributions of silica network and interfacial fraction in reinforcement and Payne effect of polypropylene glycol nanocomposites. Polymer 2018, 138, 139–145. [Google Scholar] [CrossRef]
- Xu, T.; Jia, Z.; Li, J.; Luo, Y.; Jia, D.; Peng, Z. Study on the dispersion of carbon black/silica in SBR/BR composites and its properties by adding epoxidized natural rubber as a compatilizer. Polym. Compos. 2018, 39, 377–385. [Google Scholar] [CrossRef]
- Zhong, B.; Jia, Z.; Hu, D.; Luo, Y.; Jia, D. Reinforcement and reinforcing mechanism of styrene–butadiene rubber by antioxidant-modified silica. Compos. Part A Appl. Sci. Manuf. 2015, 78, 303–310. [Google Scholar] [CrossRef]

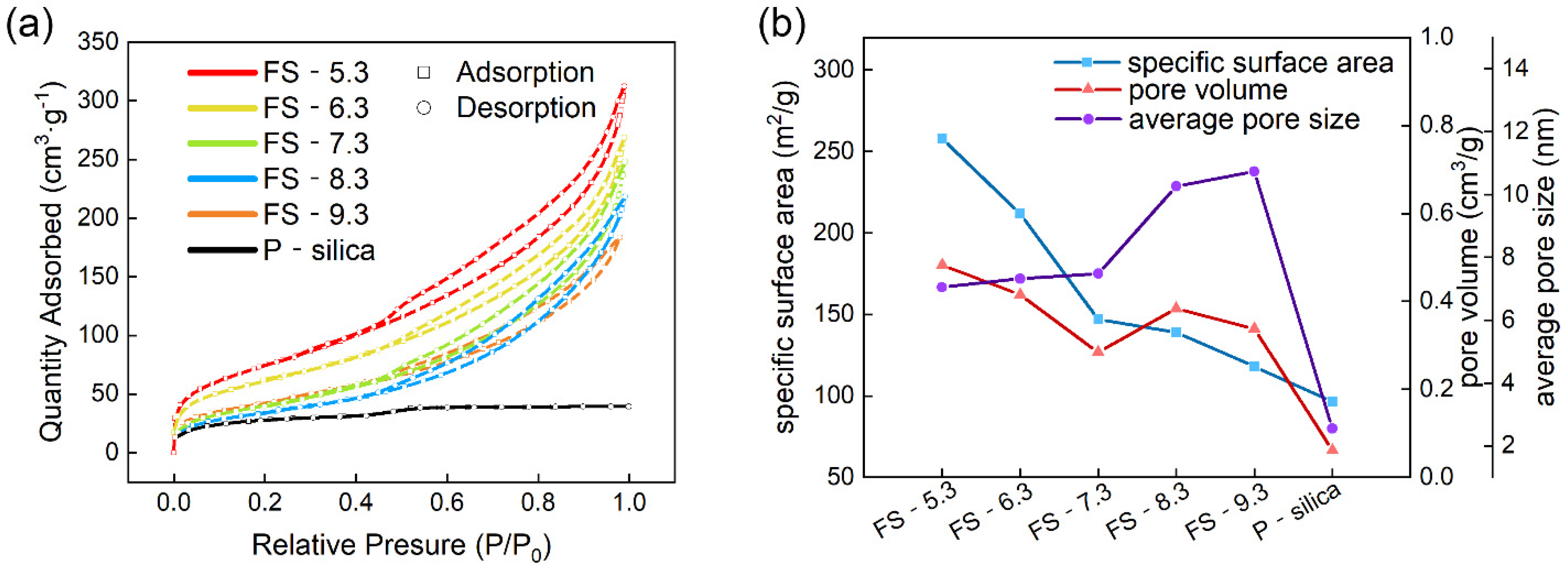




| Samples | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| FS-5.3 | 258 | 0.483 | 7.054 |
| FS-6.3 | 212 | 0.415 | 7.320 |
| FS-7.3 | 147 | 0.284 | 7.484 |
| FS-8.3 | 139 | 0.384 | 10.261 |
| FS-9.3 | 118 | 0.338 | 10.738 |
| P-silica | 96 | 0.061 | 2.557 |
| Sample | D10 (μm) | D50 (μm) | D90 (μm) | Dav (μm) | Standard Deviations | <5 μm | <10 μm |
|---|---|---|---|---|---|---|---|
| FS-5.3 | 1.173 | 4.583 | 9.811 | 5.501 | 5.490 | 56.844% | 90.180% |
| FS-6.3 | 1.265 | 3.258 | 6.475 | 3.606 | 2.332 | 76.633% | 98.840% |
| FS-7.3 | 1.152 | 3.855 | 6.536 | 3.809 | 1.663 | 69.030% | 100.000% |
| FS-8.3 | 1.457 | 4.247 | 6.758 | 4.168 | 2.170 | 64.174% | 99.928% |
| FS-9.3 | 1.268 | 4.048 | 6.745 | 4.021 | 2.293 | 66.585% | 99.686% |
| P-silica | 3.326 | 5.696 | 12.263 | 6.706 | 4.012 | 37.066% | 84.244% |
| Sample | Tc10 (min) | Tc90 (min) | ts1 (min) | ML (dNm) | MH (dNm) | MH-ML (dNm) |
|---|---|---|---|---|---|---|
| SBR-P-silica | 3.83 | 19.41 | 3.84 | 2.32 | 11.14 | 8.82 |
| SBR-FS-5.3 | 4.35 | 24.41 | 5.58 | 2.19 | 8.28 | 6.09 |
| SBR-FS-6.3 | 3.49 | 22.96 | 3.99 | 2.28 | 12.28 | 10.00 |
| SBR-FS-7.3 | 3.58 | 22.24 | 4.06 | 1.94 | 9.92 | 7.98 |
| SBR-FS-8.3 | 3.14 | 21.37 | 3.85 | 2.16 | 9.85 | 7.69 |
| SBR-FS-9.3 | 3.55 | 20.54 | 3.78 | 2.03 | 10.68 | 8.65 |
| Sample | Modulus at 100% | Modulus at 300% | Tensile Strength (Mpa) | Elongation at Break (%) | Tearing Strength (kN/m) |
|---|---|---|---|---|---|
| SBR-P-silica | 1.08 ± 0.04 | 1.54 ± 0.09 | 11.19 ± 0.77 | 2215.2 ± 94.3 | 30.93 ± 1.46 |
| SBR-FS-5.3 | 0.89 ± 0.16 | 1.44 ± 0.07 | 10.50 ± 0.29 | 2494.7 ± 38.1 | 42.74 ± 0.49 |
| SBR-FS-6.3 | 1.05 ± 0.01 | 1.69 ± 0.02 | 13.78 ± 1.91 | 2215.0 ± 105.0 | 37.55 ± 0.77 |
| SBR-FS-7.3 | 0.98 ± 0.09 | 1.64 ± 0.06 | 9.76 ± 1.53 | 2072.8 ± 177.2 | 28.59 ± 1.06 |
| SBR-FS-8.3 | 0.99 ± 0.06 | 1.75 ± 0.08 | 9.83 ± 0.36 | 1869.0 ± 93.9 | 29.74 ± 0.77 |
| SBR-FS-9.3 | 0.94 ± 0.05 | 1.57 ± 0.06 | 9.98 ± 0.64 | 2146.4 ± 81.3 | 27.90 ± 2.47 |
| SBR-P-silica | SBR-FS-5.3 | SBR-FS-6.3 | SBR-FS-7.3 | SBR-FS-8.3 | SBR-FS-9.3 | |
|---|---|---|---|---|---|---|
| ΔG’ | 814.55 | 600.15 | 655.81 | 602.83 | 670.18 | 683.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Ai, W.; Zuo, J.; Yang, W.; Wei, C.; Xu, S. Mesoporous Spherical Silica Filler Prepared from Coal Gasification Fine Slag for Styrene Butadiene Rubber Reinforcement and Promoting Vulcanization. Polymers 2022, 14, 4427. https://doi.org/10.3390/polym14204427
Xu Y, Ai W, Zuo J, Yang W, Wei C, Xu S. Mesoporous Spherical Silica Filler Prepared from Coal Gasification Fine Slag for Styrene Butadiene Rubber Reinforcement and Promoting Vulcanization. Polymers. 2022; 14(20):4427. https://doi.org/10.3390/polym14204427
Chicago/Turabian StyleXu, You, Weidong Ai, Jing Zuo, Wentong Yang, Cundi Wei, and Shaonan Xu. 2022. "Mesoporous Spherical Silica Filler Prepared from Coal Gasification Fine Slag for Styrene Butadiene Rubber Reinforcement and Promoting Vulcanization" Polymers 14, no. 20: 4427. https://doi.org/10.3390/polym14204427
APA StyleXu, Y., Ai, W., Zuo, J., Yang, W., Wei, C., & Xu, S. (2022). Mesoporous Spherical Silica Filler Prepared from Coal Gasification Fine Slag for Styrene Butadiene Rubber Reinforcement and Promoting Vulcanization. Polymers, 14(20), 4427. https://doi.org/10.3390/polym14204427





