1. Introduction
Car tires are the most relevant rubber items in terms of volume and importance, accounting for about 70% of the synthetic and natural rubber produced in the world. In general, they are composed of 40–50% elastomers such as raw rubber (NR, SBR, etc.), 20–30% fillers (carbon black, silica, etc.), 5–10% fibers and reinforcement (rayon, polyester, steel, etc.) and finally a tiny percentage of vulcanizing agents [
1,
2]. Unfortunately, the search for products with high performance, durability, and long life results in a polymer that is difficult to discard and complex to recycle [
1].
At the end of the tire’s life cycle, its destination can be recovery, incineration, pyrolysis, or landfill. In incineration, waste tires are completely burned for energy generation in cement kilns and for producing steam, electricity, pulp, paper, lime, and steel [
1,
2]. In pyrolysis, the rubber component decomposes in the presence of heat and in the absence of oxygen, which prevents oxidation, and makes it possible to obtain pyrolysis oil, carbon black, and others [
1,
2]. Other approaches include the use of GTR in polymer blends. These mixtures are prepared with thermoplastics such as polyethylene (PE), polypropylene (PP), ethylene-vinyl acetate copolymer (EVA) [
3], and polylactic acid (PLA) [
4]. This approach allows for better-quality blends when devulcanization occurs [
5]. In the devulcanization process, the focus is on selective scission, or in a way that allows the elastomer to be reprocessed. When scission is selective, the sulfide bonds undergo scission using specific conditions, which reduces the crosslinked fraction and often allows revulcanization [
6]. Overall, these processes require rubber separation, and the wire and steel mesh are recovered as qualified iron scrap, and the nylon fabric is recovered and can be used as reinforcement in cardboard packaging [
7].
Devulcanization or recovery methods have particularities, and their acceptability must be associated with the final destination of the treated product. Regarding devulcanization, the most efficient methods are the chemical method [
8], the microwave-assisted method, the ultrasonic method, the biological method, the thermal method, and the mechanical method [
7,
8]. Mechanical processes are those in which the rubber is subjected to high shear stress forces [
1,
2,
7,
8]. Thermo-mechanical processes can be carried out continuously or in batches. In the continuous process, a twin-screw extruder is commonly used, in which the thread profile must be precisely adjusted to promote a good balance of bond breaks that allows the reuse of the elastomer. The batch process is usually performed in roller mixers or some other equipment specifically designed to generate the high levels of shear that benefit this process [
1,
2,
6,
8]. Furthermore, these methods can be aided by chemical agents to aid in processing conditions or chain breaking.
Ujianto and coworkers compared elastomer devulcanization using single- and twin-screw extruders. They reported that the twin-screw extruder operating at 300 °C and 100 rpm reduced the gel content by 50% for an elastomer with an initial gel content of 85%, while for the single-screw, the reduction was 40% [
9]. Diaz et al. performed thermomechanical devulcanization of waste tires and compared two mixers, one with high shear and the other with pressure. In the study, the authors varied the temperature of the rubber and the mechanical energy consumed in operation, feeding the instrument with large pieces of rubber [
10]. The machine reduced the particle size by rotating the rotor, thus increasing the content of soluble material in the rubber by 5% and decreasing its crosslink density by five times for the smallest particle size (100 μm) [
10,
11]. Studies show that using shear forces can reduce the particle size and the fraction of gelled material.
WO 2011/113148 deals with a method and equipment used to regenerate ground vulcanized rubber. The equipment consists of a thermokinetic mixer with a hermetic stationary chamber with a non-uniform internal surface, a rotor shaft that extends coaxially into the chamber, and a speed controller that varies up to at least about 2000 rpm. The method comprises pre-mixing the shredded rubber with oil at room temperature and then transferring the mixture to the thermokinetic mixer. The speed is increased until a devulcanization temperature is reached (300 to 330 °C for 0.25 to 3 s). After this condition, the speed is reduced until a new mixture temperature condition is reached (150 to 250 °C for 2 to 30 s). The best devulcanization condition leads to a 61% reduction in crosslink density. One of the advantages of this method is that there is no need to use auxiliary devulcanization chemicals, making this method ecologically correct or “green” [
12].
Cavalieri and coworkers evaluated the effect of mechanochemical activation on the surface of solid-state devulcanized mid-tire rubber [
13]. The authors show a reduction of 3% in the gel content and more than 50% in the crosslink density in 15 h of treatment in a ball mill. Interestingly, surface activation often allows the GTR to be reincorporated into fabrics with other elastomers and thermoplastics polymers more easily. Furthermore, during the GTR recovery process, shear forces increase friction between particles and exothermic reactions. Consequently, it can generate self-heating in the elastomer during its recovery [
14].
Thermokinetic mixers can be used to generate heat and often melt the material. A crosslinked PU recovery approach was used by Gonela and coworkers, in which PU particles were depolymerized in a thermokinetic mixer. For the processing time of 90% to 30% reduction in gel content occurred [
15]. Subsequently, the authors use PU after thermomechanical treatment to obtain blends with TPU and PA12. Other studies for the use of thermokinetic mixers deal with obtaining blends with crosslinked PE and EVA with high amounts (>60 wt.%) of crosslinked material [
16,
17]. Tire waste generally has a high gel content (>60%) and a more significant limitation for production if blended with thermoplastics or reused in industrial processes. In addition, high vulcanization limits adhesion between phases when used in mixtures and results in products with poor surface finish. Thus, thermokinetic mixers represent good opportunities to be used to treat crosslinked elastomers to improve and expand their use in the polymer and elastomers industry.
Thus, in this study, an alternative was proposed for the treatment of ground rubber from tires (GTR) using mechanochemical treatments using a high-speed mixer. Processing parameters that affect gel content, crosslink density, and particle morphology were investigated. In addition, the selectivity of chain scission was evaluated using the Horikx approach. Finally, an artificial neural network (ANN) was used to correlate the processing characteristics with the thermal degradation of the samples after mechanochemical treatment. For the system studied, it is noted that random splitting dominates the degradation process from processing times of 10 min at speeds of 5145 rpm (n2). Furthermore, the proposed treatment can be described through a percolation relationship that establishes that the chain’s scission results in the formation of the soluble fraction.
3. Results and Discussion
Table 1 shows the temperature, acetone, and hexane soluble fractions for GTR samples before and after mechanochemical treatment. As the processing time was increased, the temperature increased proportionally from 32 °C for 1 min in n
1 to 68 °C in 60 min. This result is expected since the thermokinetic mixer generates heat by friction. However, as the speed has practically doubled, a much more significant increase in temperature is noted, reaching 185 °C in 10 min for n
2. Although, the soluble fractions presented constant values in n
1 and 1 min processing in n
2, an increase in these fractions was later noticed. This increase in the soluble fraction may be related to chain scission that produces smaller fragments that can be extracted.
The gel fraction (
ξ) and crosslink density (
ν) are important parameters to characterize the efficiency of the mechanochemical process of GTR recovery. However, the gel fraction is less sensitive to structural changes. Therefore, it is often possible to activate the surface of the GTR particle with faster treatments and reincorporate it into a production process. Although some approaches, such as using ball mills, produce exciting results [
13], the thermokinetic mixer is capable of generating high shear rates [
24] (>10
4 s
−1), and it plays an essential role in reducing the crosslink density. On the other hand, at higher shear rates, the oxidation of the elastomeric compound may increase [
14]. Thus, depending on which application the recovered elastomer will be used, the best processing conditions for recovery will be defined.
Table 2 shows the results of the gel fraction, crosslink density, and mechanochemical treatment efficiency for samples treated under different conditions in a thermokinetic mixer. Here, the gel fraction was estimated after extractions in acetone and hexene. The gel content is less sensitive to the mechanochemical treatment since some crosslinks on the surface are initially broken, and only when the medium is more aggressive is there a chain thermomechanical degradation by random scission, which results in changes in the gel content. In the crosslink density, it can be verified that they already produce an efficiency of approximately 50% in milder processing conditions. These results show that depending on where the elastomer will be used, as in mixtures with other elastomers or thermoplastics, there may not be a need for further degradation of the polymer chain, as reported by Cavalieri and coworkers [
8].
Ujianto et al. [
9], using twin-screw extruders, reached gel values of approximately 50%, from samples with 85% gel content at 300 °C and 100 rpm. Considering that the speed used in extrusion is 100 rpm and that in the thermokinetic mixer, the degree of devulcanization is similar. The shear rate is believed to be essential in reducing the gel content, as the rotor speeds are about 50 times higher in the thermokinetic mixer.
On the other hand, when the temperature increases due to friction, it can result in greater chain scissions that will reflect in the reduction in gel content and increase in density. For example, Simon, and coworkers [
22,
23] compared the devulcanization of the GTR in a laboratory microwave oven and an internal mixer. For a sample treated at 120 rpm in the mixer, which reached a temperature of 200 °C, the sol content was 30.2% by weight, and the crosslink density was 3.7 × 10
−4 mol·cm
−3, while a sample treated at 40 rpm, which reached a temperature of 160 °C, the sol content was 14.4% and the crosslink density was 7.3 × 10
−4 mol·cm
−3. Therefore, a more significant reduction in the gelled fraction and crosslink density is noticed when the system reaches higher temperatures. However, thermomechanical degradation favors the reduction in the polymer fraction as light fractions of degradation products are evaporated.
The DSC results not shown here show only a glass transition (T
g) for the GTR at −58.9 °C, which agrees with the literature [
25,
26]. Note that there is no variation in the processing times of 1 and 3 min at speed n
1. On the other hand, when GTR was processed for 10 min in n
2 there was a reduction to −69.8 °C. Thus, when there is a reduction in T
g, there is an increase in the segmental mobility of the chains. This may be related to the possible scission of chains under more severe shear conditions for a longer time.
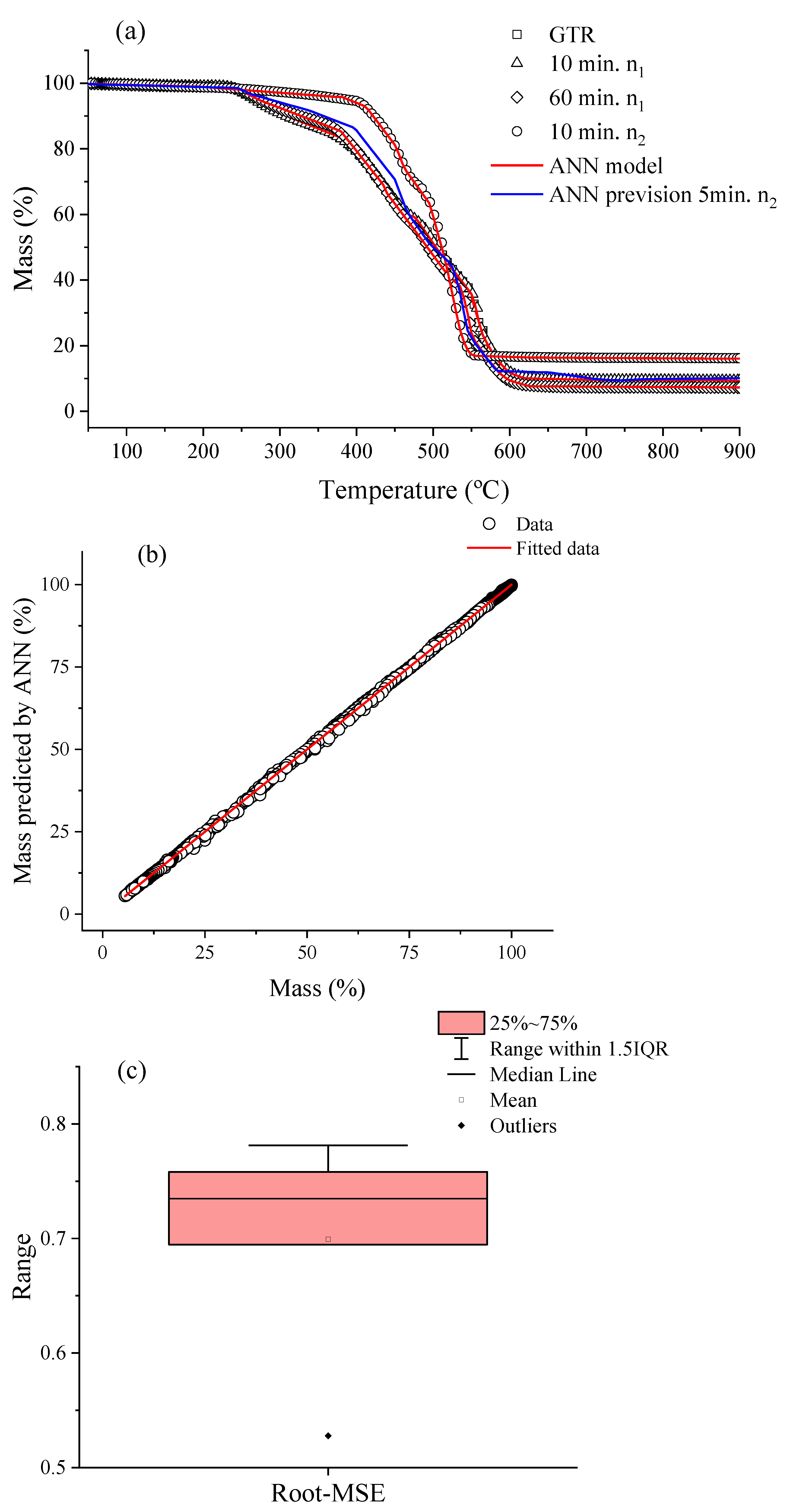
Figure 2a illustrates the TGA curves for the GTR sample after processing in a thermokinetic mixer. Here, it was chosen to present the conditions in 10 min in n
1, 60 min in n
1, and 10 min in n
2. Along with the experimental data, the neural network’s prediction for the behavior of the thermal degradation profile of the samples and an experimental prediction is being shown. All samples presented the temperature of the beginning of thermal degradation ~250 °C, with maximum rates around 500 °C. Due to the GTR being formed by different elastomers, the thermal loss mass profile occurs in multiple steps, as can be seen for a maximum around 350 °C which can be related to natural rubber, and at 470 °C it is related to styrene–butadiene rubber, which is the main elastomers used in the manufacture of automobile tires [
1,
7]. In addition, chain scission forms molecules of a lower molecular mass composed of hydrocarbon species (-CH
2-) and CO
2 [
1]. For the samples processed in n
1, a carbon black content of around 31 wt.% was found. However, when they were processed in n
2 a change was noticed, and this amount increased to 36 wt.% as well as the other relative amounts of fillers (silica and others). This change in the mass loss profile is associated with the fact that during the mechanochemical process, chain scission and volatilization of low molecular mass compounds occur. As a result, there is a change in the relative fraction of carbon black and inorganic compounds.
The ANN was used to predict the observed behavior of the complex mass loss profile. First, experimental data were compared with predicted theoretical data, as shown in
Figure 2b, and a good agreement R
2 > 0.999 was noted. Subsequently, it was possible to predict the experimental data for other conditions (blue curve,
Figure 2a). It is also noted that the cross-validation (
Figure 2c) results in low dispersion.
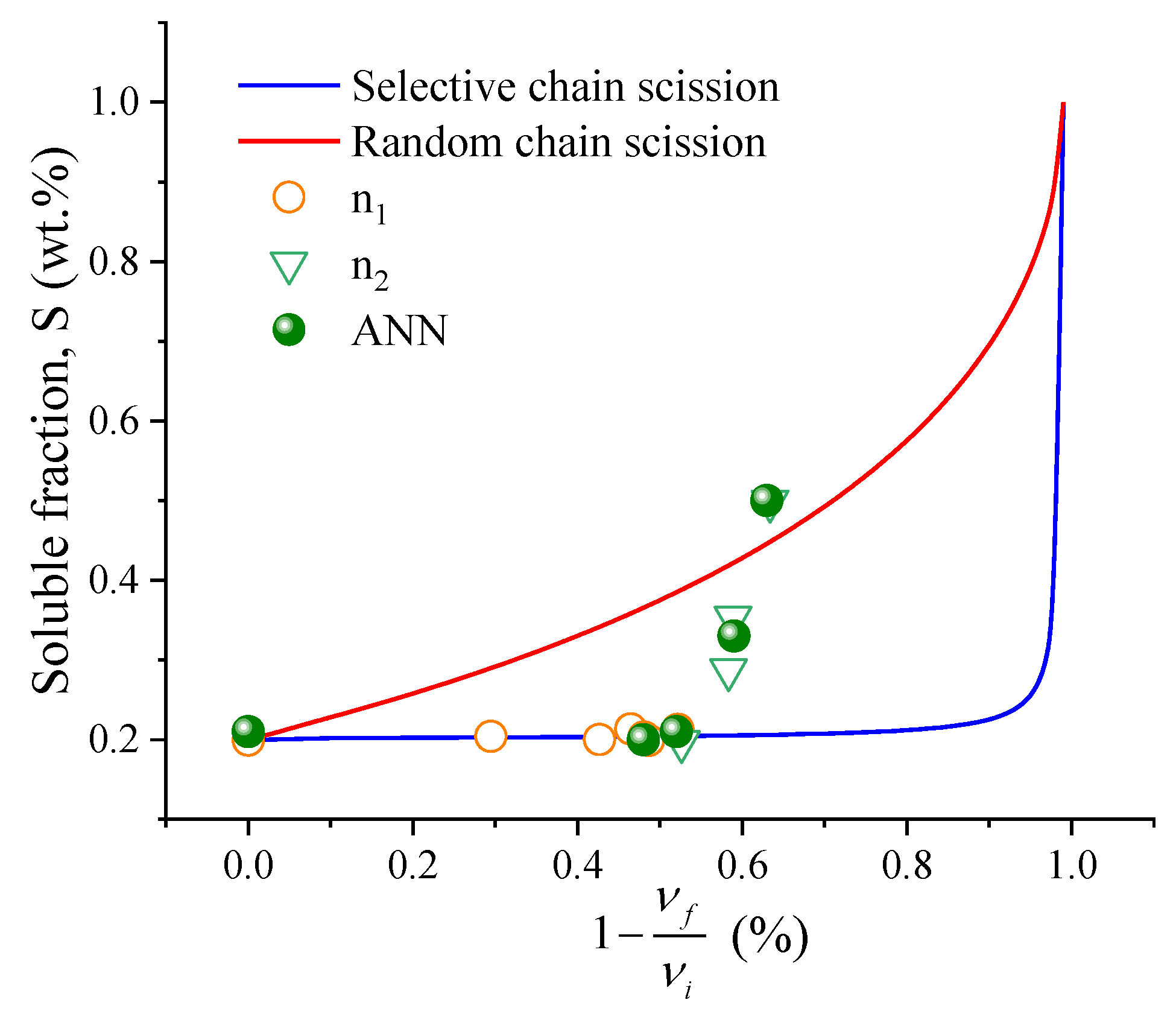
The selectivity of the scission of chains generated by the mechanochemical process in a thermokinetic mixer, the Horikx method, was used, which allows distinguishing whether the scission of chains tends to be more selective or random. For this analysis, it is necessary to carry out a mass balance in the material since the filler and polymer fractions are altered during the treatment.
The theoretical lines in
Figure 3 were constructed using Equations (6) and (7), respectively, varying the fraction of solubles from 0.10 to 0.99 concerning the decrease in crosslink density. The crosslinking index was obtained through Equation (8), considering the numerical average molecular weight of the NR polymer of 200,000 g·mol
−1, according to the literature [
21,
22]. Based on the proximity of the curve (random or selective), it is possible to infer whether the dominant phenomenon during the mechanochemical process is more selective or random [
21,
27]. Selectivity can be achieved using a coefficient (
K). Two possible approaches are to set the selectivity parameter towards the soluble fraction (vertical) or the crosslink density (horizontal). The selectivity parameter of the soluble fraction (
Ks) can be calculated by Equation (9) [
21].
where
Sc is the theoretical sol fraction for random scission,
Sx is the theoretical sol fraction for selective crosslinking scission, and
S is the measured soluble fraction. The selectivity parameter in the crosslink density direction (
Kx) can be calculated by Equation (10) [
18].
where
Xc is the theoretical values related to the relative decrease in crosslink density for random scission, and
Xx is the theoretical values of the relative decrease in crosslink density for selective scission. The general selectivity parameter (
K) is then defined according to Equation (11) [
21].
Thus, when random chain scission is dominant, K = 0, and when selective K = 1.
The soluble fraction showed a practically linear variation for the experiments at n
1 (
and n
2 (
) in relation to the processing time. Thus, the gel fraction changes much more intensely when shear is increased. Regarding the decrease in crosslinks (
) can be fitted by logistic equations in relation to the processing time. For n
1,
, R > 0.98, and for n
2,
, R > 0.99. This observation may be related to the reduction in crosslinks following a sigmoidal process [
28]. That is, there is an initial period plus reading, a period of acceleration, and a limit is reached for the processing technique employed in the mechanochemical treatment.
Figure 3 shows the results of the Horikx analysis for the two speeds (n
1 and n
2) and times of mechanochemical processing. The blue line illustrates the region in which the process is selective, while the red line illustrates the random scission. A comparison was also made with the data generated by ANN. All samples processed at n
1 show selective chain scission with
K = 0.98–1) and at 1 min in s
2. Subsequently, there is an increase in thermomechanical degradation by the random scission in the mechanochemical treatment with K, going from 0.80 to 0.39 in the 10 min in n
2 condition, which indicates that the high shear induces the scission of the C–C bonds of the backbone chain.
Edwards and coworkers [
21] used Horikx analysis to indicate the selectivity of crosslink scission during the devulcanization of GTR by mechanical shear at elevated temperatures in a twin-screw extruder. The extruder used operated at 30–80 rpm and 175–275 °C. The best selectivity condition found by the authors was obtained in the sample submitted to 275 °C and 55 rpm. This best shear condition is about forty-six times lower than the best condition in this study. Furthermore, the working temperature was five times higher than the final temperature of the best condition evaluated by Horikx in the study in the thermokinetic mixer. Simon et al. [
27,
29], compared the thermomechanical treatment in a mixing chamber (Brabender Plasti-Corder) with microwave processes. They noticed that chain scissions are more selective in the internal mixer. However, when there is an increase in temperature, there is a tendency for the polymer chain to degrade, as reported by the same authors. The literature [
11] reports that the devulcanization process will occur extremely slowly if the operating temperature is below 50 °C. This observation is in line with the data presented in this study, in which similar conditions and greater selectivity are achieved in n
1.
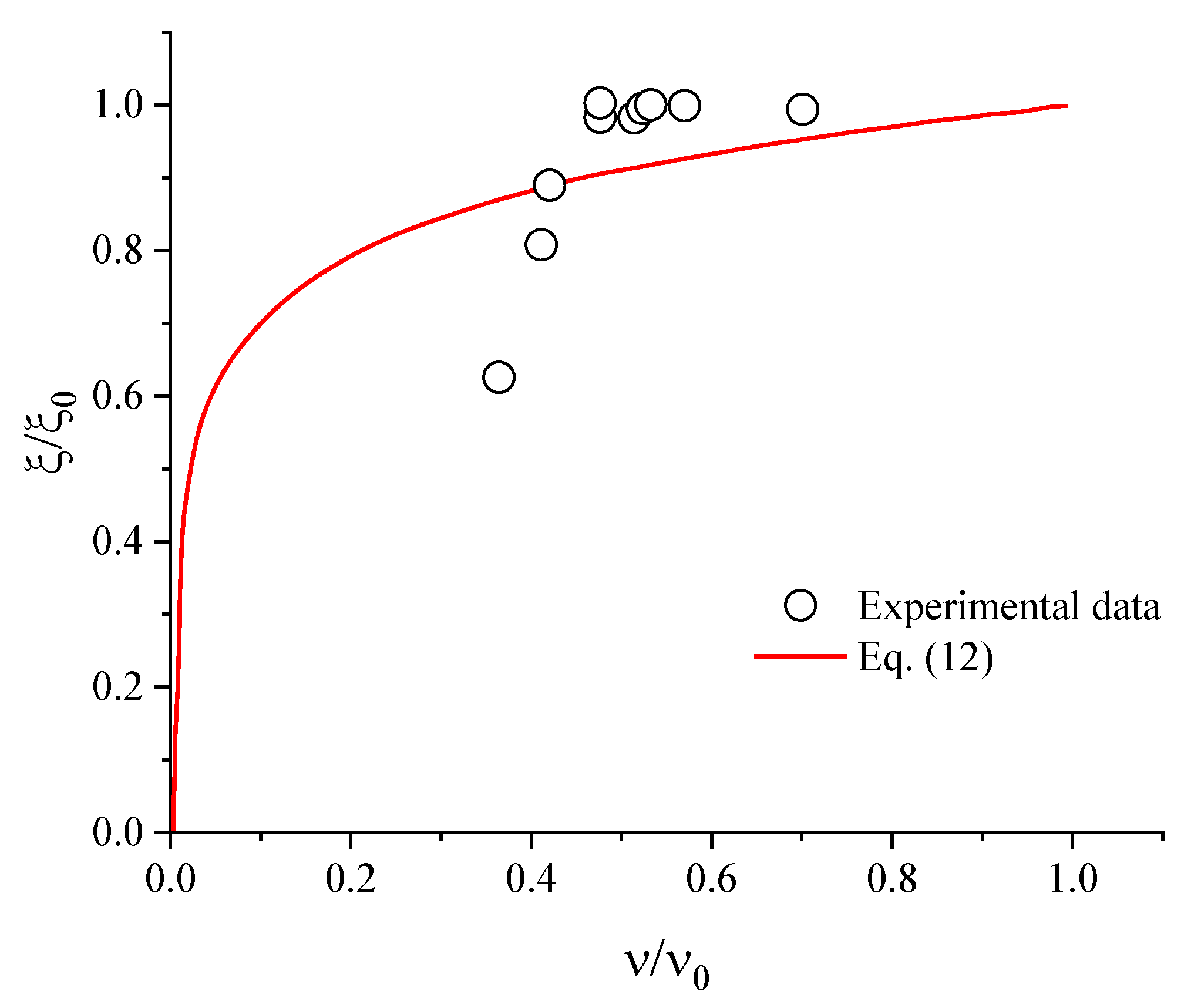
Figure 4 shows that the normalized gel fraction (
ξ/
ξ0) as a function of the normalized crosslink density (
ν/
ν0). In this Figure the experimental data was fitted with the empirical model proposed by Isayev, according to Equation (12) [
30]:
where
H is the Heavi-side step function because the gel fraction is always positive and negative values and has no physical meaning, such that
. The parameter
ψ represents the scales of the relative change in gel fraction concerning the change in the initial crosslink density,
ν0 should be controlled by the relative to chain scission probability.
The empirical model (Equation (12)) was able to describe all data in a universal curve. Thus, using the same assumptions as Isayev [
30], it is possible to infer that the mechanochemical treatment can be described by a network percolation model, in which the ratio between the amounts of intermolecular bond breakage and main chain bond breakage resulting in the formation of the fraction soluble [
30,
31]. This model implies that devulcanization and degradation coincide at random throughout the rubber network, a condition believed to be sufficiently satisfied during processing in a thermokinetic mixer. The value of the parameter
found was 0.13 ± 0.05, which is in agreement with the values found by Isayev [
30] for ultrasonic devulcanization of GTR (
).
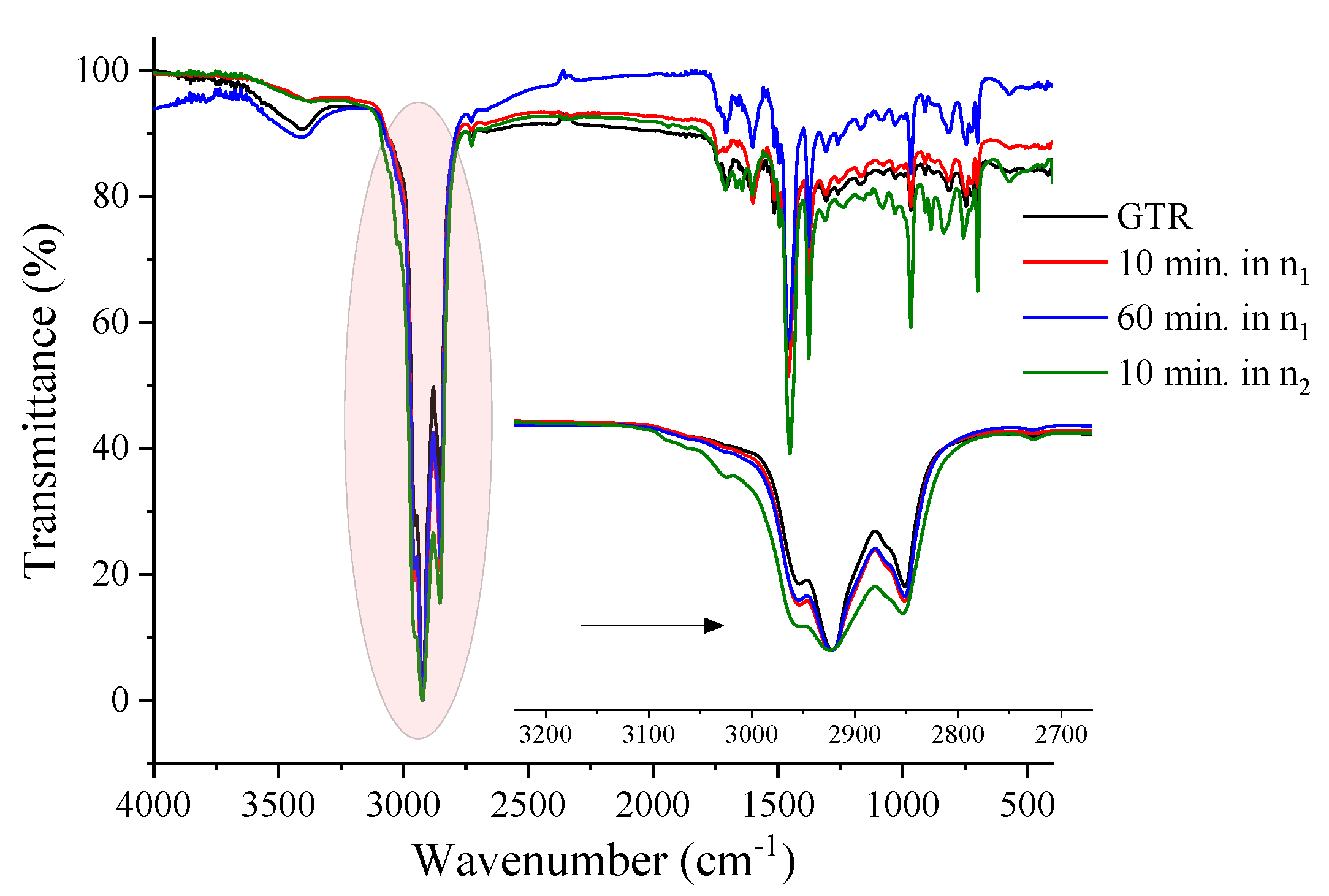
Figure 5 shows the FTIR spectra for the hexane-extracted GTR samples. In the 2950 and 2853 cm
−1, the asymmetric and symmetrical stretching bands of the C-H bonds present in NR methyl groups are observed. At 1450 and 1375 cm
−1 it is possible to observe the symmetrical deformation bands in the CH
2 and CH
3 planes and the cis-isoprene bands, around 885 cm
−1, 1370 cm
−1, and 1630 cm
−1. At 700 cm
−1 and 775 cm
−1 characteristic bands of SBR were found, and at 965 cm
−1 to butadiene [
32,
33]. The band that characterizes the vibration absorption related to the S-S bond in the GTR is found close to 462 cm
−1 and can reach 495 cm
−1 [
33]. Regarding the effect of processing time and speed, it is noted that the region between 2950 and 2853 cm
−1 increases proportionally with the intensity of the mechanochemical treatment.
The intensive mechanochemical treatment results in degradation and almost always oxidation. The oxidative process can result in the formation of new crosslinks, but it is counterbalanced by the high shear that results in chain scission, as noted by the samples processed in n
2. The relative increase in bands at 1800 to 1500 cm
−1 can relate to this effect (C=C and C=O), as well as the appearance of bands at 1500 to 750 cm
−1 (C-O, S-O-C, C-O-C, S=O, C-C) [
32,
33,
34].
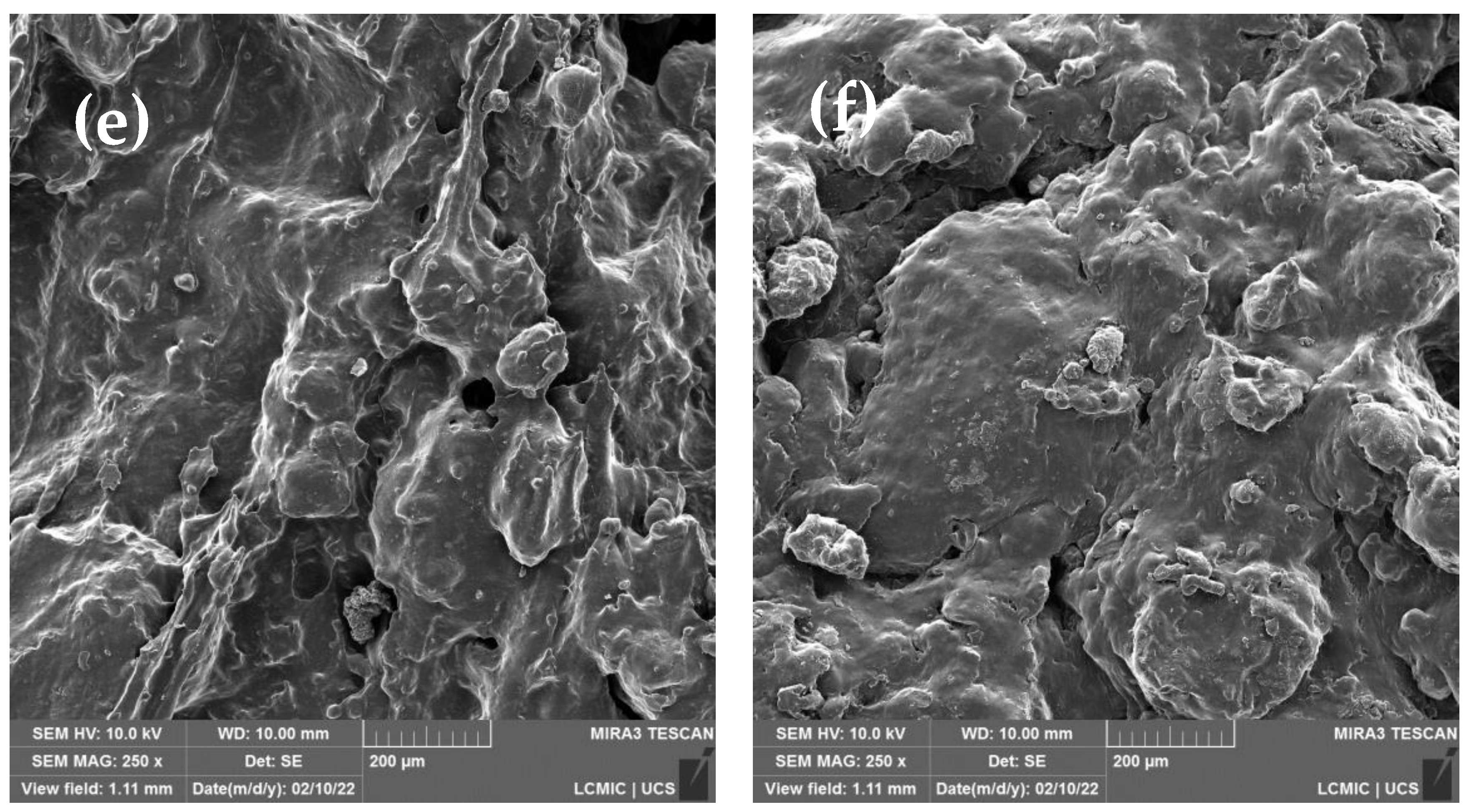
Figure 6a–f illustrates SEM micrographs of the GTR samples before (
Figure 6a,b) and after treatment in the thermokinetic mixer. The particles have elongated sizes, typical of a non-cryogenic milling process. Sizes range from 111 μm to 1345 μm, with a higher incidence of particles larger than 400 μm. The EDX analyses in the
supplementary files show the presence of carbon, sulfur, silicon, aluminum, calcium, oxygen, zinc, and sodium. These elements are part of the compounds commonly used to manufacture tires [
7]. After 3 min and 60 min of processing at n
1, it is noted that the beginning of the fragmentation of the sample occurs (
Figure 6c,d). The increase in the processing speed (n
2) resulted in the formation of a sample with a smoother and more continuous surface. This characteristic is related to the increase in the amount of soluble phase. These results are similar to those found in the literature [
33] using mechanochemical treatment in extrusion. As the conditions are intensified, the rubber particles present smooth contours.