Influence of Incorporating 5% Weight Titanium Oxide Nanoparticles on Flexural Strength, Micro-Hardness, Surface Roughness and Water Sorption of Dental Self-Cured Acrylic Resin
Abstract
1. Introduction
2. Materials and Methods
2.1. Flexural Strength Test
2.2. Micro-Hardness Test
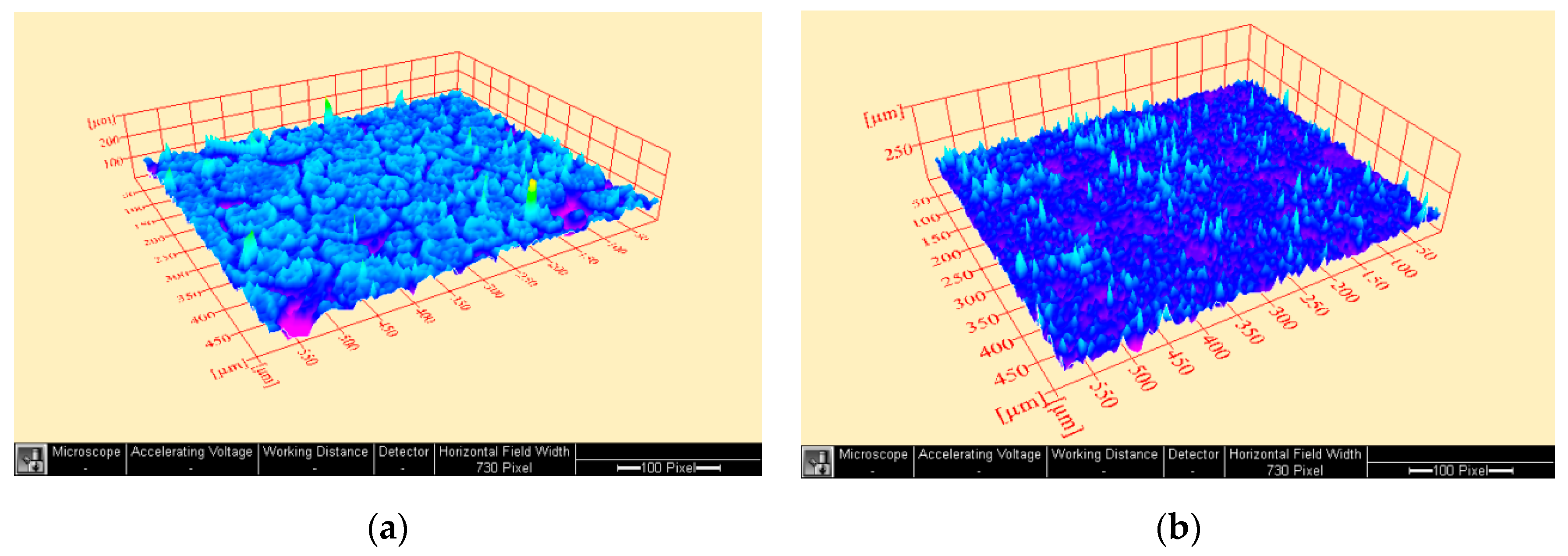
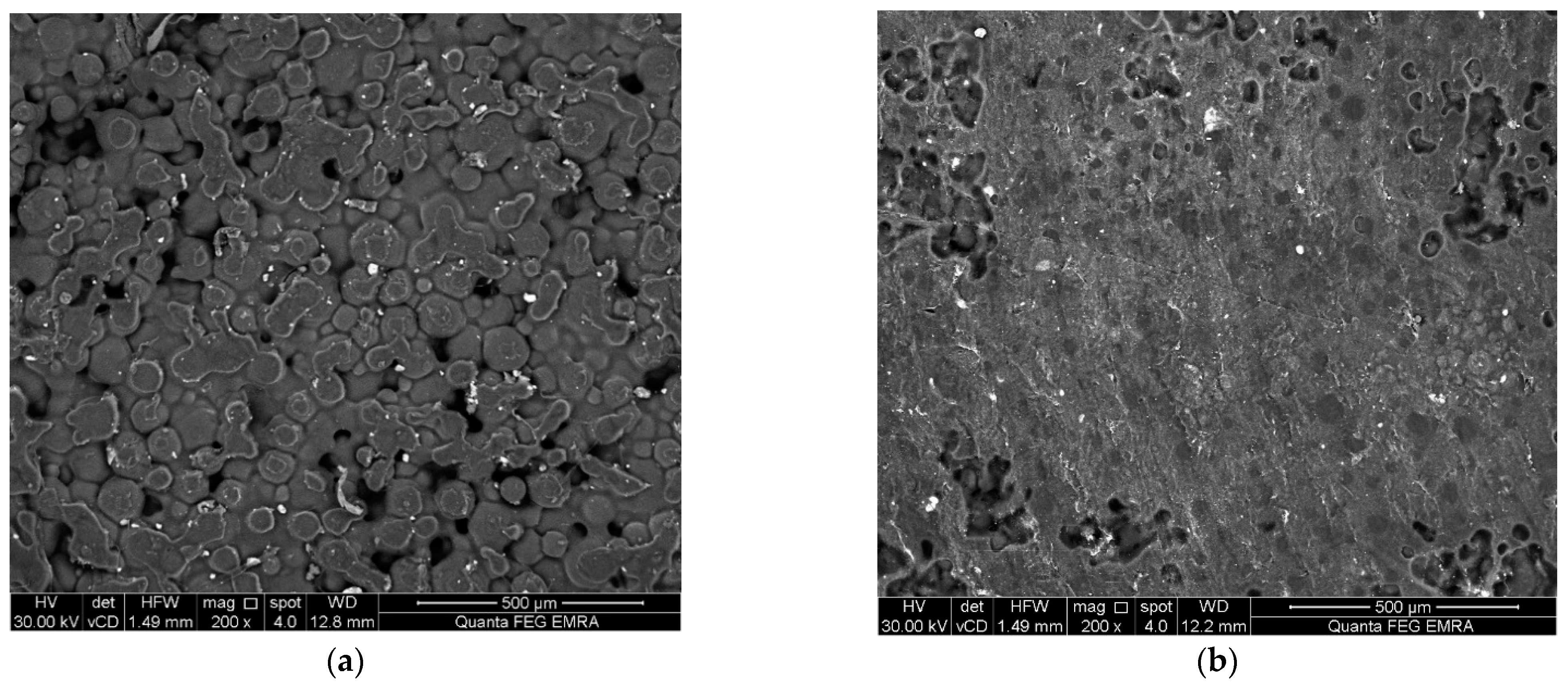
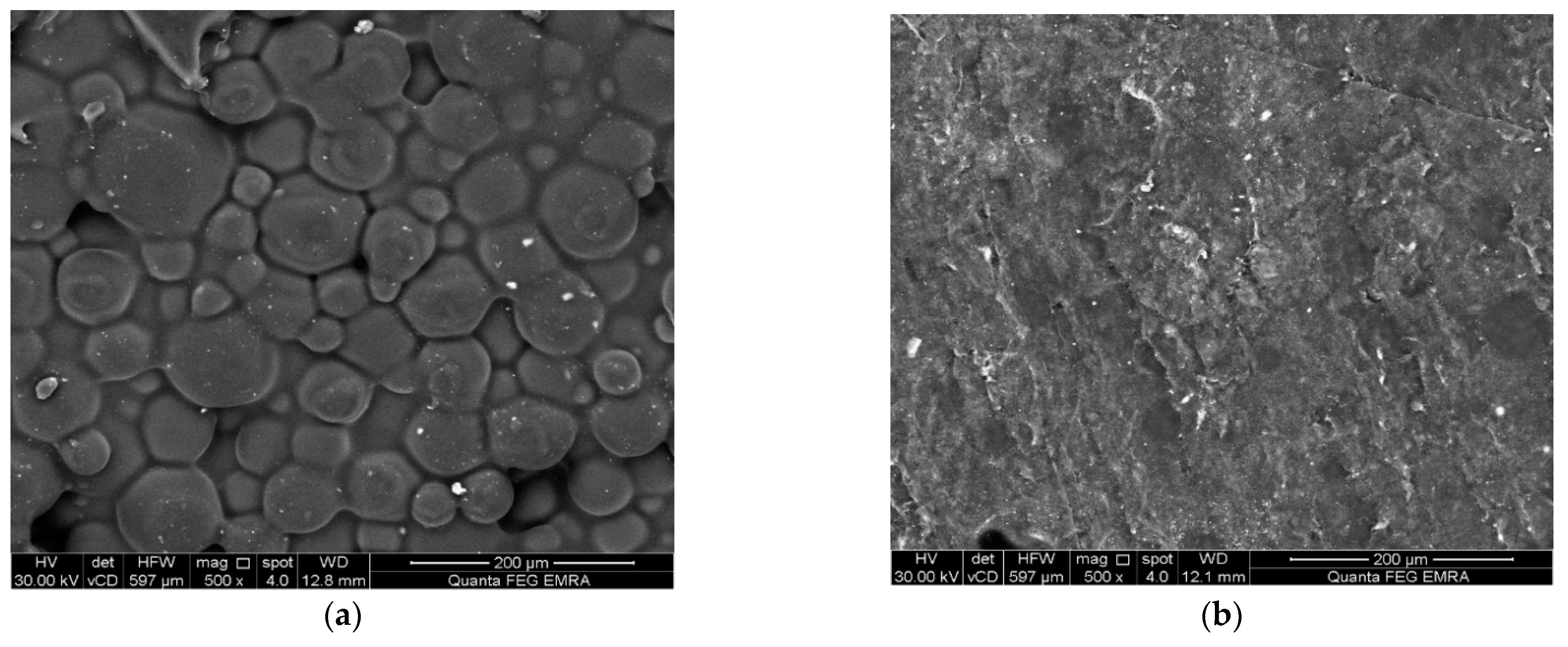
2.3. Surface Examination and Surface Roughness Test
2.4. Water Sorption Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelraouf, R.M. Chemical analysis and microstructure examination of extended-pour alginate impression versus conventional one (characterization of dental extended-pour alginate). Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 612–618. [Google Scholar] [CrossRef]
- Abdelraouf, R.M.; Bayoumi, R.E.; Hamdy, T.M. Effect of powder/water ratio variation on viscosity, tear strength and detail reproduction of dental alginate impression material (In vitro and clinical study). Polymers 2021, 13, 2923. [Google Scholar] [CrossRef] [PubMed]
- Abdelraouf, R.M.; Mohammed, M.; Abdelgawad, F. Evaluation of shear-bond-strength of dental self-adhering flowable resin-composite versus total-etch one to enamel and dentin surfaces: An in-vitro study. Open Access Maced. J. Med. Sci. 2019, 7, 2162–2166. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.F.; Jassim, M.M.; HA, M. Evaluating some Mechanical and Physical Properties of Vertex Thermosens Denture base Material in Comparison with Heat Cure Acrylicdenture Base Material. Int. J. Sci. Res. 2017, 6, 394–397. [Google Scholar] [CrossRef]
- Hamouda, I.M. Retention of Probase Hot Versus the Conventional Heat-Cured Acrylic Resin Denture Bases. Biomed. J. Sci. Tech. Res. 2017, 1, 906–911. [Google Scholar] [CrossRef]
- Minami, H.; Suzuki, S.; Kurashige, H.; Minesaki, Y.; Tanaka, T. Flexural strengths of denture base resin repaired with autopolymerizing resin and reinforcements after thermocycle stressing. J. Prosthodont. 2005, 14, 12–18. [Google Scholar] [CrossRef]
- Alhareb, A.O.; Akil, H.M.; Ahmad, Z.A. Impact strength, fracture toughness and hardness improvement of PMMA denture base through addition of nitrile rubber/ceramic fillers. Saudi J. Dent. Res. 2017, 8, 26–34. [Google Scholar] [CrossRef]
- DAR-ODEH, N.S.; HARRISON, A.; ABU-HAMMAD, O. An evaluation of self-cured and visible light-cured denture base materials when used as a denture base repair material. J. Oral Rehabil. 2008, 24, 755–760. [Google Scholar] [CrossRef]
- Choksi, R.H.; Mody, P.V. Flexural properties and impact strength of denture base resins reinforced with micronized glass flakes. J. Indian Prosthodont. Soc. 2016, 16, 264–270. [Google Scholar] [CrossRef]
- Naji, S.A.; Behroozibakhsh, M.; Kashi, T.S.J.; Eslami, H.; Masaeli, R.; Mahgoli, H.; Tahriri, M.; Lahiji, M.G.; Rakhshan, V. Effects of incorporation of 2.5 and 5 wt% TiO2 nanotubes on fracture toughness, flexural strength, and microhardness of denture base poly methyl methacrylate (PMMA). J. Adv. Prosthodont. 2018, 10, 113–121. [Google Scholar] [CrossRef]
- ISO 20795-1: 2013; Dentistry—Base Polymers—Part 1: Denture Base Polymers. 2nd ed. International Organization for Standardization: Geneva, Switzerland, 2013; 35p. Available online: https://www.iso.org/obp/ui/#iso:std:iso:20795:-1:ed-2:v1:en (accessed on 7 September 2022).
- Meng, T.R.; Latta, M.A. Physical properties of four acrylic denture base resisns. J. Contemp. Dent. Pract. 2005, 6, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Alaa, M. Effect of aluminum oxide powder reinforcement on degree of conversion, monomer release and flexural strength of heat-cured acrylic resin. Biomed. Res. Clin. Rev. 2020, 1, 1–5. [Google Scholar] [CrossRef]
- Zaki, D.Y.; Safwat, E.M.; Nagi, S.M.; Salem, H.N.; Hamdy, T.M.; Moharam, L.M.; Hassan, M.L.; Hamzawy, E.M.A. A novel dental re-mineralizing blend of hydroxyethyl-cellulose and cellulose nanofibers oral film loaded with nepheline apatite glass: Preparation, characterization and in vitro evaluation of re-mineralizing effect. Carbohydr. Polym. Technol. Appl. 2021, 2, 100035. [Google Scholar] [CrossRef]
- Hamdy, T.M.; Mousa, S.M.A.; Sherief, M.A. Effect of Incorporation of Lanthanum and Cerium-doped Hydroxyapatite on Acrylic Bone Cement Produced from Phosphogypsum Waste. Egypt. J. Chem. 2019, 63, 1823–1832. [Google Scholar] [CrossRef]
- Abdelnabi, A.; Hamza, M.K.; El-Borady, O.M.; Hamdy, T.M. Effect of Different Formulations and Application Methods of Coral Calcium on its Remineralization Ability on Carious Enamel. Open Access Maced. J. Med. Sci. 2020, 8, 94–99. [Google Scholar] [CrossRef]
- Hamdy, T.M. Polymerization shrinkage in contemporary resin-based dental composites: A Review Article. Egypt. J. Chem. 2021, 64, 3087–3092. [Google Scholar] [CrossRef]
- Fonseca, R.B.; Kasuya, A.V.B.; Favarão, I.N.; Naves, L.Z.; Hoeppner, M.G. The influence of polymerization type and reinforcement method on flexural strength of acrylic resin. Sci. World J. 2015, 2015, 919142. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef]
- Hamdy, T.M.; Saniour, S.H.; Sherief, M.A.; Zaki, D.Y. Effect of incorporation of 20 wt% amorphous nano-hydroxyapatite fillers in poly methyl methacrylate composite on the compressive strength. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1136–1141. [Google Scholar] [CrossRef]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Flexural strength and hardness of filler-reinforced pmma targeted for denture base application. Materials 2021, 14, 2659. [Google Scholar] [CrossRef]
- Tandra, E.; Wahyuningtyas, E.; Sugiatno, E. The effect of nanoparticles TiO2 on the flexural strength of acrylic resin denture plate. Padjadjaran J. Dent. 2018, 30, 35. [Google Scholar] [CrossRef]
- Alqahtani, M. Mechanical properties enhancement of self-cured PMMA reinforced with zirconia and boron nitride nanopowders for high-performance dental materials. J. Mech. Behav. Biomed. Mater. 2020, 110, 103937. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.V.S.; Nigam, A.; Naeem, A.; Gaur, A.; Pandey, K.K.; Deora, A. Reinforcing heat-cured poly-methyl-methacrylate resins using fibers of glass, polyaramid, and nylon: An in vitro study. J. Contemp. Dent. Pract. 2016, 17, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Prabhu, N.; Balakrishnan, D.; Narayan, A.I. Comparative study of the flexural strength of high impact denture base resins reinforced by silver nanoparticles and e-glass fibres: An in-vitro study. J. Clin. Diagn. Res. 2018, 12, 22–26. [Google Scholar] [CrossRef]
- Faot, F.; Panza, L.H.V.; Garcia, R.C.M.R.; Cury, A.A.D.B. Impact and Flexural Strength, and Fracture Morphology of Acrylic Resins With Impact Modifiers. Open Dent. J. 2009, 3, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.P.; Cecchin, D.; Soares, R.G.; Botelho, A.L.; Takahashi, J.M.F.K.; Mazzetto, M.O.; Mesquita, M.F. Evaluation of Vickers hardness of different types of acrylic denture base resins with and without glass fibre reinforcement. Gerodontology 2012, 29, e155–e160. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, M.; Bagheri, R.; Khaledi, A.A.R. Effects of aluminum oxide addition on the flexural strength, surface hardness, and roughness of heat-polymerized acrylic resin. J. Dent. Sci. 2012, 7, 238–244. [Google Scholar] [CrossRef]
- Hamdy, T.M.; Galal, M.; Ismail, A.G.; Abdelraouf, R.M. Evaluation of flexibility, microstructure and elemental analysis of some contemporary nickel-titanium rotary instruments. Open Access Maced. J. Med. Sci. 2019, 7, 3647–3654. [Google Scholar] [CrossRef]
- Ashour Ahmed, M.; El-Shennawy, M.; Althomali, Y.M.; Omar, A.A. Effect of Titanium Dioxide Nano Particles Incorporation on Mechanical and Physical Properties on Two Different Types of Acrylic Resin Denture Base. World J. Nano Sci. Eng. 2016, 6, 111–119. [Google Scholar] [CrossRef]
- Nevarez-Rascón, A.; Hurtado-Macías, A.; Esparza-Ponce, H.E.; Nevarez-Rascón, M.M.; González-Hernández, J.; Yacamán, M.J. Nano-structured hydroxyapatite and titanium dioxide enriching PENTA /UDMA adhesive as aesthetic coating for tooth enamel. Dent. Mater. 2021, 37, e290–e299. [Google Scholar] [CrossRef]
- Yadav, N.S.; Elkawash, H. Flexural strength of denture base resin reinforced with aluminum oxide and processed by different processing techniques. J. Adv. Oral Res. 2011, 2, 33–36. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, C.J.; Asaoka, K. Correlation in the mechanical properties of acrylic denture base resins. Dent. Mater. J. 2012, 31, 157–164. [Google Scholar] [CrossRef]
- Bahrani, F.; Vojdani, M.; Safari, A.; Karampoor, G. Comparison of Hardness and Surface Roughness of Two Denture bases Polymerized by Different Methods. World J. Dent. 2012, 3, 171–175. [Google Scholar] [CrossRef]
- Kumar, G.V.; Devi, R.; Anto, N. Evaluation and Comparison of the Surface Roughness and Porosity of Different Provisional Restorative Materials: An in vitro Study. CODS J. Dent. 2016, 8, 39–45. [Google Scholar] [CrossRef]
- Tolga Demirtaş, T.; Kaynak, G.; Gümüşderelioğlu, M. Bone-like hydroxyapatite precipitated from 10×SBF-like solution by microwave irradiation. Mater. Sci. Eng. C 2015, 49, 713–719. [Google Scholar] [CrossRef]
- Fonseca, R.B.; Favarão, I.N.; Kasuya, A.V.B.; Abrão, M.; da Luz, N.F.M.; Naves, L.Z. Influence of Glass Fiber wt% and Silanization on Mechanical Flexural Strength of Reinforced Acrylics. J. Mater. Sci. Chem. Eng. 2014, 2, 11–15. [Google Scholar] [CrossRef][Green Version]
- Shah, J.; Bulbule, N.; Kulkarni, S.; Shah, R.; Kakade, D. Comparative evaluation of sorption, solubility and microhardness of heat cure polymethylmethacrylate denture base resin & flexible (thermoplastic polyamide nylon) denture base resin. J. Clin. Diagn. Res. 2014, 8, ZF01. [Google Scholar] [CrossRef]
- Gad, M.M.; Rahoma, A.; Al-Thobity, A.M.; ArRejaie, A.S. Influence of incorporation of ZrO2 nanoparticles on the repair strength of polymethyl methacrylate denture bases. Int. J. Nanomed. 2016, 11, 5633–5643. [Google Scholar] [CrossRef]
- Al-Kheraif, A.A.A. The effect of mechanical and chemical polishing techniques on the surface roughness of heat-polymerized and visible light-polymerized acrylic denture base resins. Saudi Dent. J. 2014, 26, 56–62. [Google Scholar] [CrossRef]
- Yahya, N.A.; Gonzalez, M.A.; Ibrahim, M.S.; Wen, Y.K. Surface Roughness of Tooth Coloured Restorative Materials. Ann. Dent. 2020, 27, 41–49. [Google Scholar] [CrossRef]
- Paluszyński, J.; Slówko, W. Measurements of the surface microroughness with the scanning electron microscope. J. Microsc. 2009, 233, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Van Pham, K.; Vo, C.Q. A new method for assessment of nickel-titanium endodontic instrument surface roughness using field emission scanning electronic microscope. BMC Oral Health 2020, 20, 240. [Google Scholar] [CrossRef]
- Martelo, J.B.; Andersson, M.; Liguori, C.; Lundgren, J. Three-dimensional scanning electron microscopy used as a profilometer for the surface characterization of polyethylene-coated paperboard. Nord. Pulp Pap. Res. J. 2021, 36, 276–283. [Google Scholar] [CrossRef]
- Tekale, R.; Mowade, T.; Radke, U. Comparative evaluation of water sorption of heat-polymerized polymethyl methacrylate denture base resin reinforced with different concentrations of silanized titanium dioxide nanoparticles: An in vitro study. Contemp. Clin. Dent. 2019, 10, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Abualsaud, R. Behavior of PMMA denture base materials containing titanium dioxide nanoparticles: A literature review. Int. J. Biomater. 2019, 2019, 6190610. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A. Properties improvement of PMMA using nano TiO2. J. Appl. Polym. Sci. 2010, 118, 2890–2897. [Google Scholar] [CrossRef]
- Kreve, S.; Dos Reis, A.C. Denture Liners: A Systematic Review Relative to Adhesion and Mechanical Properties. Sci. World J. 2019, 2019, 6913080. [Google Scholar] [CrossRef]
- Hashem, M.; Al Rez, M.F.; Fouad, H.; Elsarnagawy, T.; Elsharawy, M.A.; Umar, A.; Assery, M.; Ansari, S.G. Influence of titanium oxide nanoparticles on the physical and thermomechanical behavior of poly methyl methacrylate (pmma): A denture base resin. Sci. Adv. Mater. 2017, 9, 938–944. [Google Scholar] [CrossRef]
- Tham, W.L.; Chow, W.S.; Ishak, Z.A.M. Simulated body fluid and water absorption effects on poly(methyl methacrylate)/hydroxyapatite denture base composites. Express Polym. Lett. 2010, 4, 517–528. [Google Scholar] [CrossRef]
- ISO 1567:1999; Dentistry—Denture Base Polymers. ISO: Geneva, Switzerland, 1999. Available online: https://www.iso.org/standard/20266.html (accessed on 26 August 2022).
- Alwan, S.A.; Alameer, S.S. The Effect of the Addition of Silanized Nano Titania Fillers on Some Physical and Mechanical Properties of Heat Cured Acrylic Denture Base Materials. J. Baghdad Coll. Dent. 2015, 27, 86–91. [Google Scholar] [CrossRef]
| Test/Group | Control Group | Treated Group | p Value |
|---|---|---|---|
| Flexural strength (MPa) | 75.4 ± 2.1 | 137.6 ± 3.2 | ≤0.001 * |
| Micro-hardness (VHN) | 15 ± 1.3 | 15.7 ± 0.8 | 0.385 |
| Surface roughness (Ra) | 16 ± 2.8 | 13.7 ± 3.2 | 0.269 |
| Water sorption (μg/mm3) | 0.63 ± 0.01 | 0.36 ± 0.01 | ≤0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelraouf, R.M.; Bayoumi, R.E.; Hamdy, T.M. Influence of Incorporating 5% Weight Titanium Oxide Nanoparticles on Flexural Strength, Micro-Hardness, Surface Roughness and Water Sorption of Dental Self-Cured Acrylic Resin. Polymers 2022, 14, 3767. https://doi.org/10.3390/polym14183767
Abdelraouf RM, Bayoumi RE, Hamdy TM. Influence of Incorporating 5% Weight Titanium Oxide Nanoparticles on Flexural Strength, Micro-Hardness, Surface Roughness and Water Sorption of Dental Self-Cured Acrylic Resin. Polymers. 2022; 14(18):3767. https://doi.org/10.3390/polym14183767
Chicago/Turabian StyleAbdelraouf, Rasha M., Rania E. Bayoumi, and Tamer M. Hamdy. 2022. "Influence of Incorporating 5% Weight Titanium Oxide Nanoparticles on Flexural Strength, Micro-Hardness, Surface Roughness and Water Sorption of Dental Self-Cured Acrylic Resin" Polymers 14, no. 18: 3767. https://doi.org/10.3390/polym14183767
APA StyleAbdelraouf, R. M., Bayoumi, R. E., & Hamdy, T. M. (2022). Influence of Incorporating 5% Weight Titanium Oxide Nanoparticles on Flexural Strength, Micro-Hardness, Surface Roughness and Water Sorption of Dental Self-Cured Acrylic Resin. Polymers, 14(18), 3767. https://doi.org/10.3390/polym14183767








