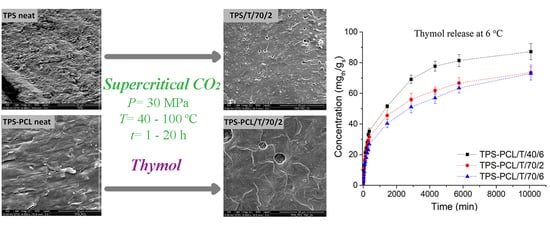
Supercritical CO2 Impregnation of Thymol in Thermoplastic Starch-Based Blends: Chemico-Physical Properties and Release Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Thermoplastic Starch-Based Pellets
2.2.2. Impregnation of Polymers with Thymol
2.3. Characterization of TPS-Based Samples
2.3.1. Attenuated Total Reflection Fourier Transform Infrared (FTIR-ATR) Spectroscopy
2.3.2. Thermal Analysis
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Mercury Intrusion Porosimetry
2.4. Thymol Release Tests
3. Results and Discussion
3.1. Impregnation of TPS and TPS–PCL with Thymol
3.2. Characterization of TPS and TPS–PCL Samples
3.2.1. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy on TPS-Based Materials
3.2.2. Surface Texture and Morphology Studies on TPS-Based Materials
3.2.3. Thermal Properties of TPS-Based Materials
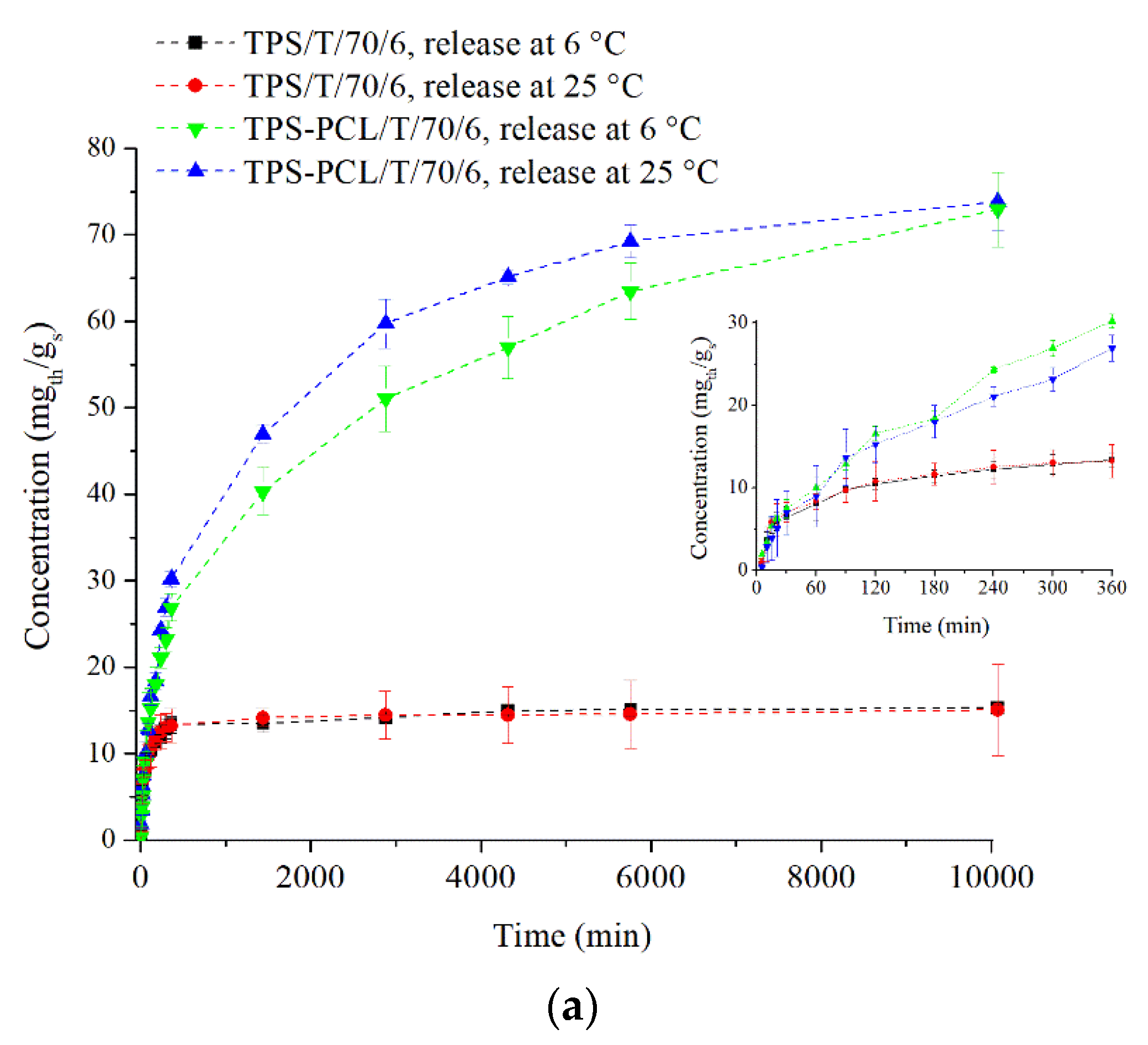
3.3. Thymol Release from TPS-Based Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| scCO2 | supercritical carbon dioxide |
| TPS | thermoplastic starch |
| TPS–PCL | thermoplastic starch blended with poly (ε-caprolactone) |
References
- De Souza, A.C.; Dias, A.A.M.; Sousa, H.C.; Tadini, C.C. Impregnation of cinnamaldehyde into cassava starch biocomposite films using supercritical fluid technology for the development of food active packaging. Carbohydr. Polym. 2014, 102, 830–837. [Google Scholar] [CrossRef]
- Uddin, S.; Fowler, S.W.; Saeed, T. Microplastic particles in the Persian/Arabian Gulf—A review on sampling and identification. Mar. Pollut. Bull. 2020, 154, 111100. [Google Scholar] [CrossRef] [PubMed]
- Diyana, Z.; Jumaidin, R.; Selamat, M.; Ghazali, I.; Julmohammad, N.; Huda, N.; Ilyas, R. Physical Properties of Thermoplastic Starch Derived from Natural Resources and Its Blends: A Review. Polymers 2021, 13, 1396. [Google Scholar] [CrossRef] [PubMed]
- Todhanakasem, T.; Jaiprayat, C.; Sroysuwan, T.; Suksermsakul, S.; Suwapanich, R.; Maleenont, K.K.; Koombhongse, P.; Young, B.M. Active Thermoplastic Starch Film with Watermelon Rind Extract for Future Biodegradable Food Packaging. Polymers 2022, 14, 3232. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Whiteside, W.S.; Ashogbon, A.O.; Kumar, M. Recent advances in thermoplastic starches for food packaging: A review. Food Packag. Shelf Life 2021, 30, 100743. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Santagata, G.; D’Ayala, G.G.; Cerruti, P.; Oliag, P.T.; Boix, M.A.C.; Malinconico, M. Enhancement of interfacial adhesion between starch and grafted poly(ε-caprolactone). Carbohydr. Polym. 2016, 147, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly(Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Galindo, E.P.; Nesic, A.; Cabrera-Barjas, G.; Mardones, C.; von Baer, D.; Bautista-Baños, S.; Garcia, O.D. Physical-Chemical Evaluation of Active Food Packaging Material Based on Thermoplastic Starch Loaded with Grape cane Extract. Molecules 2020, 25, 1306. [Google Scholar] [CrossRef] [PubMed]
- Guarás, M.P.; Alvarez, V.A.; Ludueña, L.N. Processing and characterization of thermoplastic starch/polycaprolactone/compatibilizer ternary blends for packaging applications. J. Polym. Res. 2015, 22, 165. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Castillo, L.A.; Farenzena, S.; Pintos, E.; Rodríguez, M.S.; Villar, M.A.; García, M.A.; López, O.V. Active films based on thermoplastic corn starch and chitosan oligomer for food packaging applications. Food Packag. Shelf Life 2017, 14, 128–136. [Google Scholar] [CrossRef]
- López, O.V.; Villanueva, M.E.; Copello, G.J.; Villar, M.A. Flexible thermoplastic starch films functionalized with copper particles for packaging of food products. Funct. Compos. Mater. 2020, 1, 6. [Google Scholar] [CrossRef]
- Cocero, M.J.; Martín, Á.; Mattea, F.; Varona, S. Encapsulation and co-precipitation processes with supercritical fluids: Fundamentals and applications. J. Supercrit. Fluids 2009, 47, 546–555. [Google Scholar] [CrossRef]
- Weidner, E. Impregnation via supercritical CO2–What we know and what we need to know. J. Supercrit. Fluids 2018, 134, 220–227. [Google Scholar] [CrossRef]
- Varona, S.; Rodríguez-Rojo, S.; Martín, Á.; Cocero, M.J.; Duarte, C.M. Supercritical impregnation of lavandin (Lavandula hybrida) essential oil in modified starch. J. Supercrit. Fluids 2011, 58, 313–319. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.-M.; Tassaing, T.; Jérôme, C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: A review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef]
- Milovanovic, S.; Jankovic-Castvan, I.; Ivanovic, J.; Zizovic, I. Effect of starch xero- and aerogels preparation on the supercritical CO2 impregnation of thymol. Starch 2015, 67, 174–182. [Google Scholar] [CrossRef]
- Dias, A.; Braga, M.; Seabra, I.; Ferreira, P.; Gil, M.; de Sousa, H. Development of natural-based wound dressings impregnated with bioactive compounds and using supercritical carbon dioxide. Int. J. Pharm. 2011, 408, 9–19. [Google Scholar] [CrossRef]
- Marković, D.; Milovanović, S.; De Clerck, K.; Zizovic, I.; Stojanović, D.; Radetić, M. Development of material with strong antimicrobial activity by high pressure CO2 impregnation of polyamide nanofibers with thymol. J. CO2 Util. 2018, 26, 19–27. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. Generating phytosterol nanoparticles in nanoporous bioaerogels via supercritical carbon dioxide impregnation: Effect of impregnation conditions. J. Food Eng. 2017, 207, 99–107. [Google Scholar] [CrossRef]
- García-González, C.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, S.; Markovic, D.; Aksentijevic, K.; Stojanovic, D.B.; Ivanovic, J.; Zizovic, I. Application of cellulose acetate for controlled release of thymol. Carbohydr. Polym. 2016, 147, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Radetic, M.; Zizovic, I. Solubility of thymol in supercritical carbon dioxide and its impregnation on cotton gauze. J. Supercrit. Fluids 2013, 84, 173–181. [Google Scholar] [CrossRef]
- Ivanovic, J.; Knauer, S.; Fanovich, A.; Milovanovic, S.; Stamenic, M.; Jaeger, P.; Zizovic, I.; Eggers, R. Supercritical CO2 sorption kinetics and thymol impregnation of PCL and PCL-HA. J. Supercrit. Fluids 2016, 107, 486–498. [Google Scholar] [CrossRef]
- Kuska, R.; Milovanovic, S.; Frerich, S.; Ivanovic, J. Thermal analysis of polylactic acid under high CO2 pressure applied in supercritical impregnation and foaming process design. J. Supercrit. Fluids 2019, 144, 71–80. [Google Scholar] [CrossRef]
- Pajnik, J.; Milovanovic, S.; Stojanovic, D.; Dimitrijevic-Brankovic, S.; Jankovic-Častvan, I.; Uskokovic, P. Utilization of supercritical carbon dioxide for development of antibacterial surgical sutures. J. Supercrit. Fluids 2022, 181, 105490. [Google Scholar] [CrossRef]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Ivanovic, J.; Zizovic, I. Supercritical impregnation of cellulose acetate with thymol. J. Supercrit. Fluids 2015, 97, 107–115. [Google Scholar] [CrossRef]
- Milovanovic, S.; Hollermann, G.; Errenst, C.; Pajnik, J.; Frerich, S.; Kroll, S.; Rezwan, K.; Ivanovic, J. Supercritical CO2 impregnation of PLA/PCL films with natural substances for bacterial growth control in food packaging. Food Res. Int. 2018, 107, 486–495. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Ivanovic, J. Added-value porous materials for controlled thymol release obtained by supercritical CO2 impregnation process. Cell. Polym. 2019, 38, 153–166. [Google Scholar] [CrossRef]
- Chang, C.-J.; Venkatesan, M.; Cho, C.-J.; Chung, P.-Y.; Chandrasekar, J.; Lee, C.-H.; Wang, H.-T.; Wong, C.-M.; Kuo, C.-C. Thermoplastic Starch with Poly(butylene adipate-co-terephthalate) Blends Foamed by Supercritical Carbon Dioxide. Polymers 2022, 14, 1952. [Google Scholar] [CrossRef] [PubMed]
- Kamaruddin, Z.H.; Jumaidin, R.; Ilyas, R.A.; Selamat, M.Z.; Alamjuri, R.H.; Yusof, F.A. Biocomposite of Cassava Starch-Cymbopogan Citratus Fibre: Mechanical, Thermal and Biodegradation Properties. Polymers 2022, 14, 514. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Othman, S.H.; Rashid, S.A.; Basha, R.K. Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll. 2020, 106, 105884. [Google Scholar] [CrossRef]
- Nazri, M.S.M.; Tawakkal, I.S.M.A.; Khairuddin, N.; Talib, R.A.; Othman, S.H. Characterization of jackfruit straw-based films: Effect of starch and plasticizer contents. Pertanika J. Sci. Technol. 2019, 27, 1–14. [Google Scholar]
- Liu, D.; Tang, W.; Xin, Y.; Yang, J.; Yuan, L.; Huang, X.; Yin, J.; Nie, S.; Xie, M. Comparison on structure and physicochemical properties of starches from adzuki bean and dolichos bean. Food Hydrocoll. 2020, 105, 105784. [Google Scholar] [CrossRef]
- Waheed, S.; Ahmad, A.; Khan, S.M.; Gul, S.-E.; Jamil, T.; Islam, A.; Hussain, T. Synthesis, characterization, permeation and antibacterial properties of cellulose acetate/polyethylene glycol membranes modified with chitosan. Desalination 2014, 351, 59–69. [Google Scholar] [CrossRef]
- Rexach, E.G.S.; de Arenaza, I.M.; Sarasua, J.-R.; Meaurio, E. Antimicrobial poly(ε-caprolactone)/thymol blends: Phase behavior, interactions and drug release kinetics. Eur. Polym. J. 2016, 83, 288–299. [Google Scholar] [CrossRef]
- Moskala, E.; Varnell, D.; Coleman, M. Concerning the miscibility of poly(vinyl phenol) blends—FTi.r. study. Polymer 1985, 26, 228–234. [Google Scholar] [CrossRef]
- Lerma-Canto, A.; Gomez-Caturla, J.; Herrero-Herrero, M.; Garcia-Garcia, D.; Fombuena, V. Development of Polylactic Acid Thermoplastic Starch Formulations Using Maleinized Hemp Oil as Biobased Plasticizer. Polymers 2021, 13, 1392. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Li, D.; Wang, L.-J.; Adhikari, B. Temperature thresholds and time-temperature dependence of gelatinization for heat-moisture treated corn starch. J. Food Eng. 2018, 217, 43–49. [Google Scholar] [CrossRef]
- Kamaruddin, Z.H.; Jumaidin, R.; Ilyas, R.A.; Selamat, M.Z.; Alamjuri, R.H.; Yusof, F.A.M. Influence of Alkali Treatment on the Mechanical, Thermal, Water Absorption, and Biodegradation Properties of Cymbopogan citratus Fiber-Reinforced, Thermoplastic Cassava Starch–Palm Wax Composites. Polymers 2022, 14, 2769. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.C.; Carmona, V.B.; Simão, J.A.; Mattoso, L.H.C.; Marconcini, J.M. Biodegradable blends of urea plasticized thermoplastic starch (UTPS) and poly(ε-caprolactone) (PCL): Morphological, rheological, thermal and mechanical properties. Carbohydr. Polym. 2017, 167, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Chen, C.; Gao, L.; Jiao, L.; Xu, H.; Guo, W. Physical and degradation properties of binary or ternary blends composed of poly (lactic acid), thermoplastic starch and GMA grafted POE. Polym. Degrad. Stab. 2011, 96, 175–182. [Google Scholar] [CrossRef]
- Wang, G.; Yang, S.; Wei, Z.; Dong, X.; Wang, H.; Qi, M. Facile preparation of poly(ε-caprolactone)/Fe3O4@graphene oxide superparamagnetic nanocomposites. Polym. Bull. 2013, 70, 2359–2371. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Patil, S.; Mishra, R.K.; Jana, S. Structural and Physical Properties of Biofield Treated Thymol and Menthol. J. Mol. Pharm. Org. Process. Res. 2015, 3, 1000127. [Google Scholar] [CrossRef]
- Turco, R.; Ortega-Toro, R.; Tesser, R.; Mallardo, S.; Collazo-Bigliardi, S.; Boix, A.C.; Malinconico, M.; Rippa, M.; Di Serio, M.; Santagata, G. Poly (Lactic Acid)/Thermoplastic Starch Films: Effect of Cardoon Seed Epoxidized Oil on Their Chemicophysical, Mechanical, and Barrier Properties. Coatings 2019, 9, 574. [Google Scholar] [CrossRef]
- Liu, P.; Yu, L.; Liu, H.; Chen, L.; Li, L. Glass transition temperature of starch studied by a high-speed DSC. Carbohydr. Polym. 2009, 77, 250–253. [Google Scholar] [CrossRef]
- Oshani, B.N.; Davachi, S.M.; Hejazi, I.; Seyfi, J.; Khonakdar, H.A.; Abbaspourrad, A. Enhanced compatibility of starch with poly(lactic acid) and poly(ɛ-caprolactone) by incorporation of POSS nanoparticles: Study on thermal properties. Int. J. Biol. Macromol. 2019, 141, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Release kinetics and antimicrobial properties of carvacrol encapsulated in electrospun poly-(ε-caprolactone) nanofibres. Application in starch multilayer films. Food Hydrocoll. 2017, 79, 158–169. [Google Scholar] [CrossRef]
- Guo, Y.; Liang, K.; Ji, Y. New degradable composite elastomers of POC/PCL fabricated via in-situ copolymerization blending strategy. Eur. Polym. J. 2019, 110, 337–343. [Google Scholar] [CrossRef]
- Thomas, L.V.; Nair, P.D. (Citric acid–co–polycaprolactone triol) polyester. Biomatter 2011, 1, 81–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davoodi, M.; Kavoosi, G.; Shakeri, R. Preparation and characterization of potato starch-thymol dispersion and film as potential antioxidant and antibacterial materials. Int. J. Biol. Macromol. 2017, 104, 173–179. [Google Scholar] [CrossRef]
- Hubackova, J.; Dvorackova, M.; Svoboda, P.; Mokrejs, P.; Kupec, J.; Pozarova, I.; Alexy, P.; Bugaj, P.; Machovsky, M.; Koutny, M. Influence of various starch types on PCL/starch blends anaerobic biodegradation. Polym. Test. 2013, 32, 1011–1019. [Google Scholar] [CrossRef]
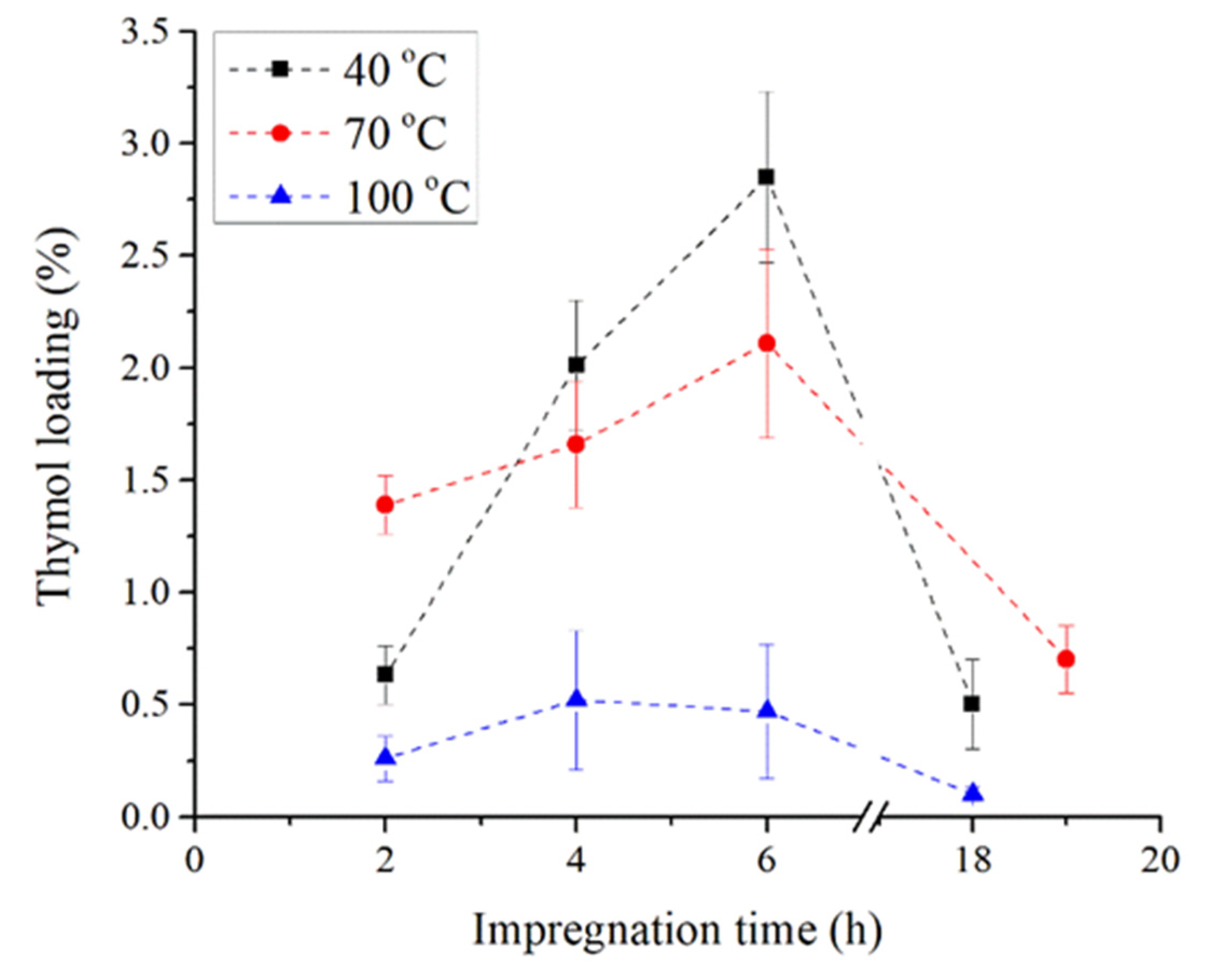

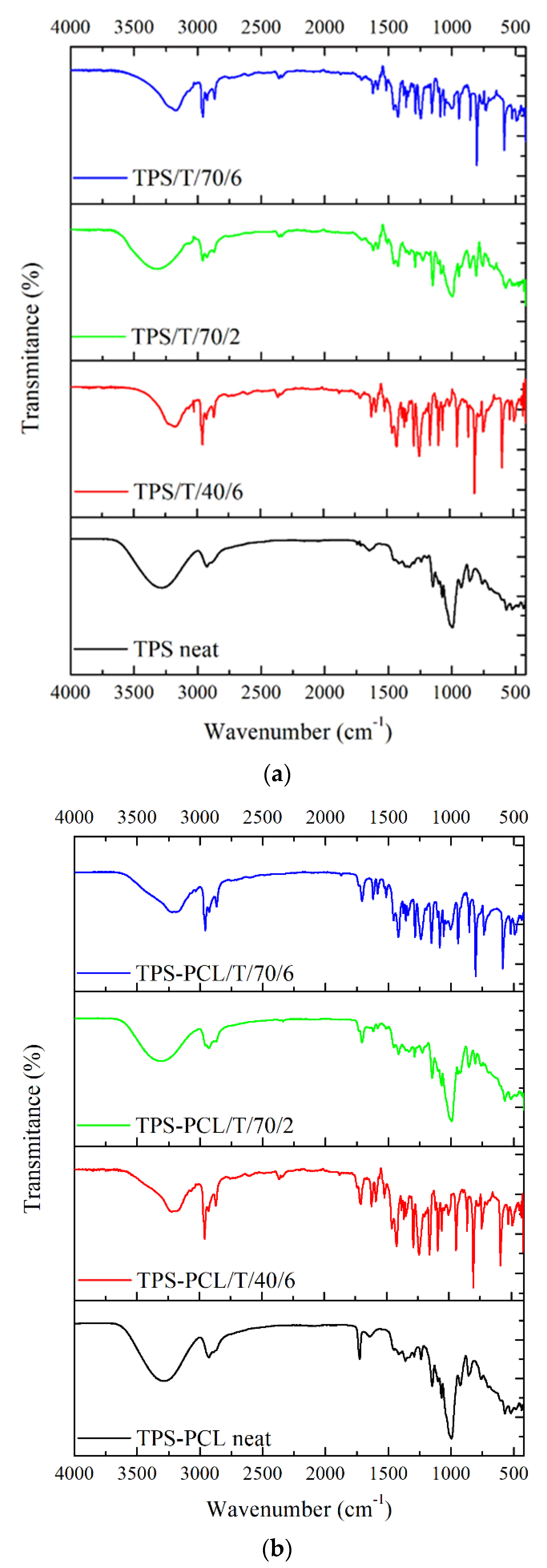
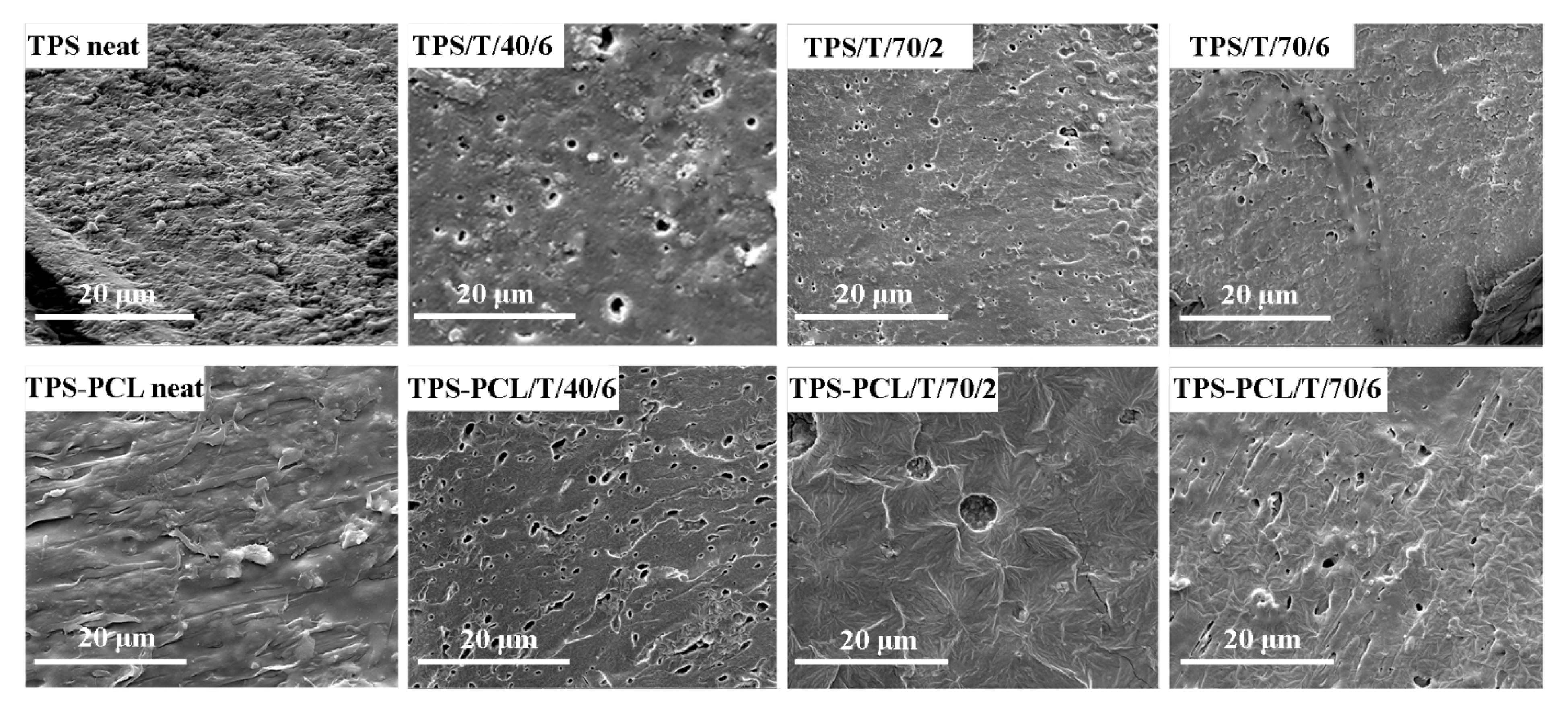



| Polymer | Temperature, °C | CO2 Density, kg/m3 | Time, h | Sample Abbreviation |
|---|---|---|---|---|
| TPS | 40 | 910 | 2 | TPS/T/40/2 |
| 4 | TPS/T/40/4 | |||
| 6 | TPS/T/40/6 | |||
| 18 | TPS/T/40/18 | |||
| 70 | 788 | 2 | TPS/T/70/2 | |
| 4 | TPS/T/70/4 | |||
| 6 | TPS/T/70/6 | |||
| 19 | TPS/T/70/19 | |||
| 100 | 662 | 2 | TPS/T/100/2 | |
| 4 | TPS/T/100/4 | |||
| 6 | TPS/T/100/6 | |||
| 18 | TPS/T/100/18 | |||
| TPS–PCL | 40 | 910 | 1 | TPS-PCL/T/40/1 |
| 2 | TPS-PCL/T/40/2 | |||
| 4 | TPS-PCL/T/40/4 | |||
| 6 | TPS-PCL/T/40/6 | |||
| 18 | TPS-PCL/T/40/18 | |||
| 70 | 788 | 1 | TPS-PCL/T/70/1 | |
| 2 | TPS-PCL/T/70/2 | |||
| 4 | TPS-PCL/T/70/4 | |||
| 6 | TPS-PCL/T/70/6 | |||
| 20 | TPS-PCL/T/70/20 | |||
| 100 | 662 | 1 | TPS-PCL/T/100/1 | |
| 2 | TPS-PCL/T/100/2 | |||
| 4 | TPS-PCL/T/100/4 | |||
| 6 | TPS-PCL/T/100/6 | |||
| 19 | TPS-PCL/T/100/19 |
| Sample | Vp | Ss | Dp,average | P |
|---|---|---|---|---|
| (mm3/g) | (m2/g) | (nm) | (vol%) | |
| TPS/T/40/6 | 8.5 | 0.9 | 8 | 0.4 |
| TPS/T/70/2 | 12.2 | 3.1 | 8 | 1.9 |
| TPS/T/70/6 | 12.9 | 0.5 | 74 | 1.7 |
| TPS-PCL/T/40/6 | 15.0 | 1.9 | 40 | 2.0 |
| TPS-PCL/T/70/2 | 25.7 | 4.3 | 36 | 3.2 |
| TPS-PCL/T/70/6 | 40.6 | 2.1 | 148 | 5.6 |
| Sample | WL (%) (25-150 °C) | Starch | PCL | MR (%) at 600 °C | 2nd Heating | |||
|---|---|---|---|---|---|---|---|---|
| Tonset (°C) | Tpeak (°C) | Tonset (°C) | Tpeak (°C) | Tg TPS (°C) | Tg PCL (°C) | |||
| TPS neat | 6.6 | 293.2 | 319.7 | 9.0 | 149 | / | ||
| TPS/T/40/6 | 6.2 | 294.2 | 318.3 | 8.1 | 160 | |||
| TPS/T/70/2 | 8.8 | 296.6 | 319.2 | 10.4 | 162 | |||
| TPS/T/70/6 | 7.1 | 294.7 | 318.2 | 10.6 | 163 | |||
| TPS–PCL neat | 7.4 | 296.9 | 319.0 | 382.3 | 401.6 | 8.4 | 152 | −74 |
| TPS–PCL/T/40/6 | 12.5 | 300.6 | 316.6 | 381.9 | 400.9 | 9.8 | 154 | −73 |
| TPS–PCL/T/70/2 | 9.9 | 300.8 | 316.6 | 383.6 | 404.3 | 9.1 | 156 | −72 |
| TPS–PCL/T/70/6 | 15.5 | 297.2 | 318.0 | 378.4 | 402.4 | 8.1 | 160 | −73 |
| Sample | 2nd Heating Scan | |||
|---|---|---|---|---|
| Tg TPS (°C) | Tg PCL (°C) | ΔHm (J/g) | Tm PCL (°C) | |
| TPS neat | 149 | / | / | / |
| TPS/T/40/6 | 160 | |||
| TPS/T/70/2 | 162 | |||
| TPS/T/70/6 | 163 | |||
| TPS–PCL neat | 152 | −74 | 2.017 | 54 |
| TPS–PCL/T/40/6 | 154 | −73 | no peak | |
| TPS–PCL/T/70/2 | 156 | −72 | 1.583 | 55 |
| TPS–PCL/T/70/6 | 160 | −73 | no peak | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucic Skoric, M.; Milovanovic, S.; Zizovic, I.; Ortega-Toro, R.; Santagata, G.; Malinconico, M.; Kalagasidis Krusic, M. Supercritical CO2 Impregnation of Thymol in Thermoplastic Starch-Based Blends: Chemico-Physical Properties and Release Kinetics. Polymers 2022, 14, 4360. https://doi.org/10.3390/polym14204360
Lucic Skoric M, Milovanovic S, Zizovic I, Ortega-Toro R, Santagata G, Malinconico M, Kalagasidis Krusic M. Supercritical CO2 Impregnation of Thymol in Thermoplastic Starch-Based Blends: Chemico-Physical Properties and Release Kinetics. Polymers. 2022; 14(20):4360. https://doi.org/10.3390/polym14204360
Chicago/Turabian StyleLucic Skoric, Marija, Stoja Milovanovic, Irena Zizovic, Rodrigo Ortega-Toro, Gabriella Santagata, Mario Malinconico, and Melina Kalagasidis Krusic. 2022. "Supercritical CO2 Impregnation of Thymol in Thermoplastic Starch-Based Blends: Chemico-Physical Properties and Release Kinetics" Polymers 14, no. 20: 4360. https://doi.org/10.3390/polym14204360
APA StyleLucic Skoric, M., Milovanovic, S., Zizovic, I., Ortega-Toro, R., Santagata, G., Malinconico, M., & Kalagasidis Krusic, M. (2022). Supercritical CO2 Impregnation of Thymol in Thermoplastic Starch-Based Blends: Chemico-Physical Properties and Release Kinetics. Polymers, 14(20), 4360. https://doi.org/10.3390/polym14204360