Characterization and Modification of Red Mud and Ferrosilicomanganese Fines and Their Application in the Synthesis of Hybrid Hydrogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Inorganic Phases
2.2.1. Neutralization with Synthetic Seawater
2.2.2. Acidification with HCl
2.3. Determination of Cation Exchange Capacity
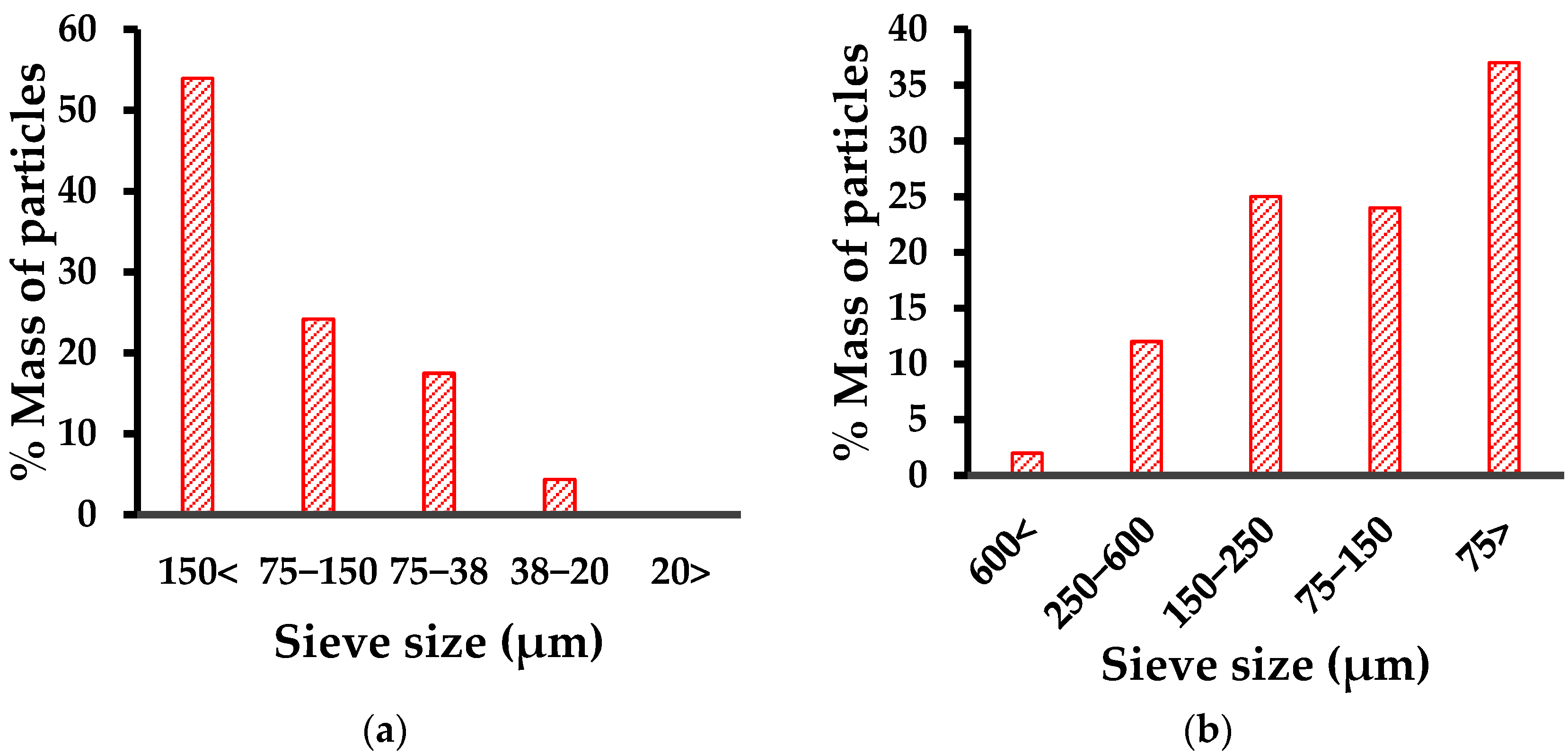
2.4. Granulometry
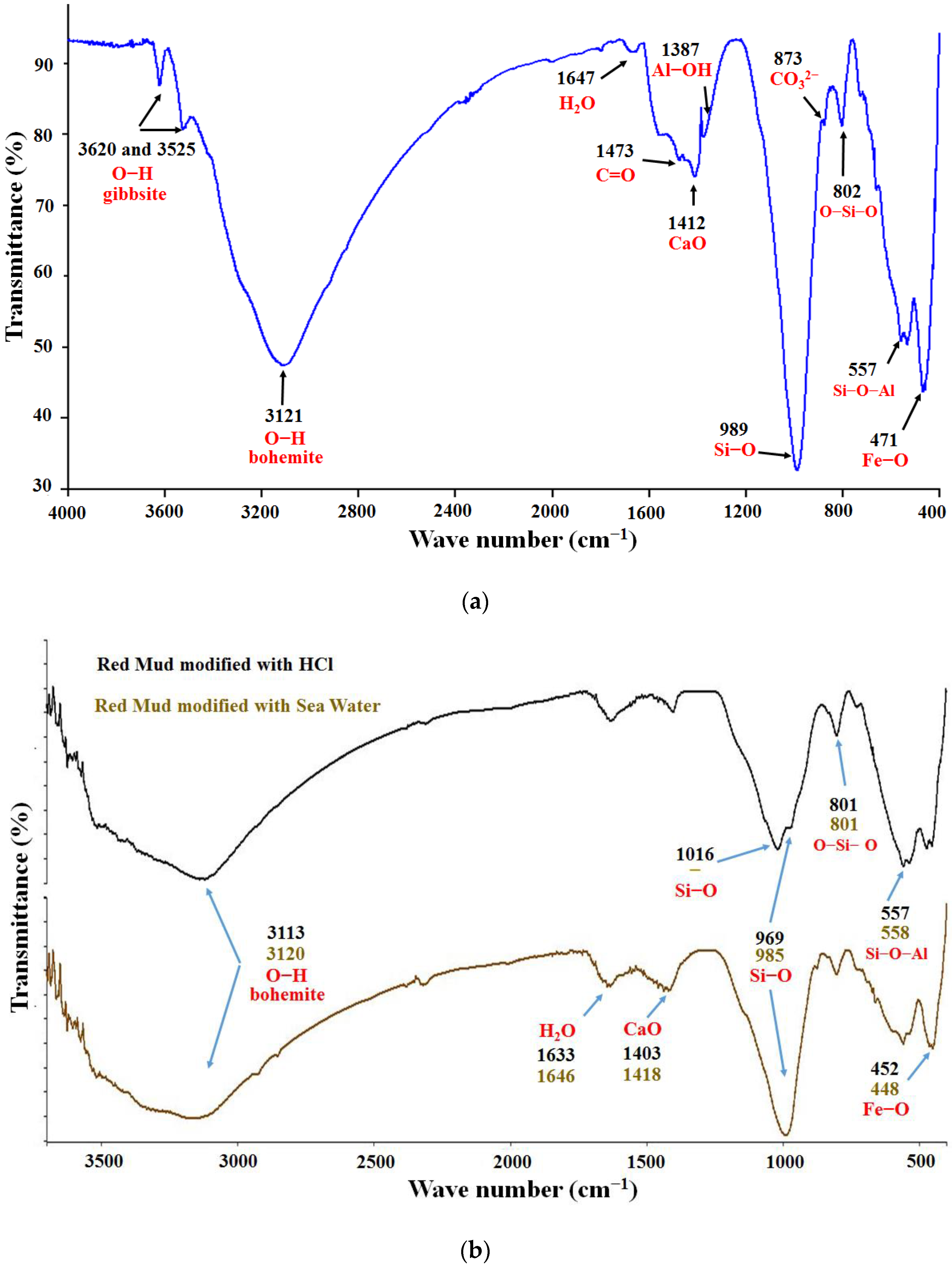
2.5. Analysis by Fourier Transform Infrared Spectroscopy (FTIR)
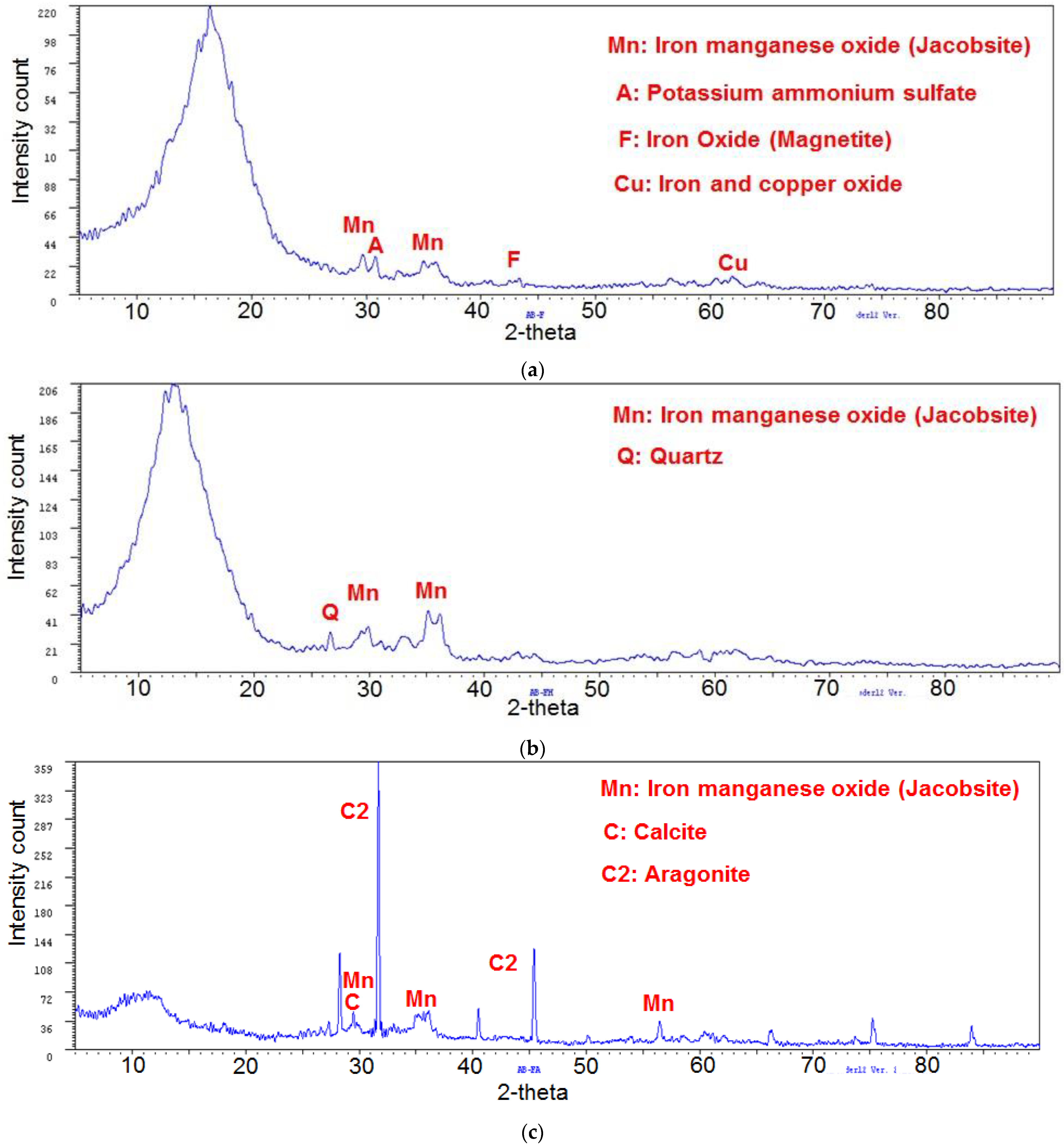
2.6. Analysis by Wide Angle X-ray Diffraction
2.7. Analysis by Optical Emission Spectroscopy with Inductively Coupled Plasma (ICP-OES)
2.8. Scanning Electron Microscopy Analysis (SEM)
2.9. Transmission Electron Microscopy Analysis (TEM)
2.10. Synthesis of Hybrid Hydrogels
2.11. Water Absorption in Hydrogels
2.12. Dynamic Rheology of Hydrogels
2.13. Evaluation of the Microhardness of Hydrogels
3. Results and Discussion
3.1. Determination of Cation Exchange Capacity (CEC)
3.2. Granulometry
3.3. Analysis by Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Analysis by Wide Angle X-ray Diffraction (XRD)
3.5. Analysis by Optical Emission Spectroscopy with Inductively Coupled Plasma (ICP-OES)
3.6. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) Analysis
3.7. Synthesis of Hybrid Hydrogels
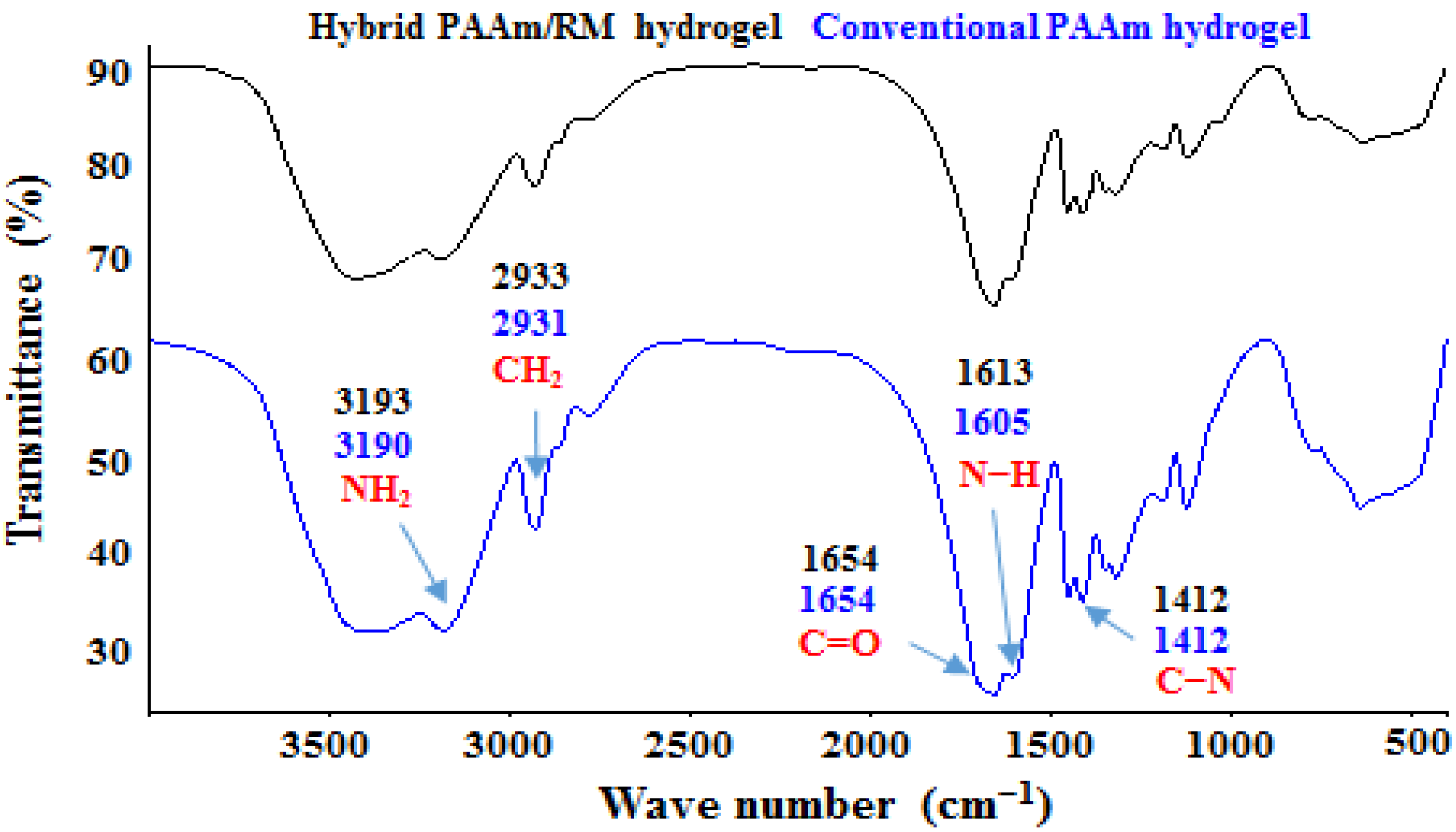
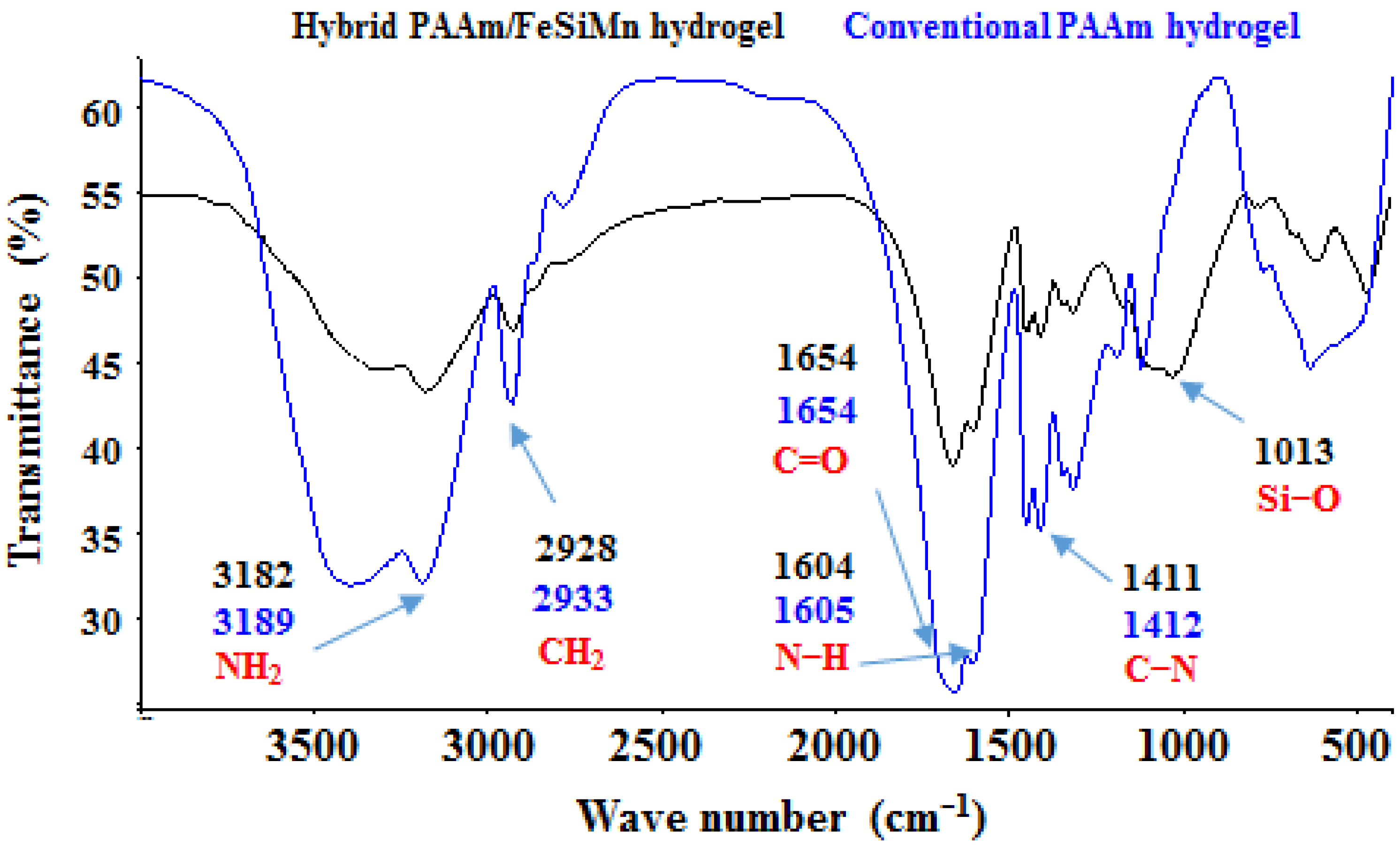
3.8. FTIR Analysis of Hybrid Hydrogels
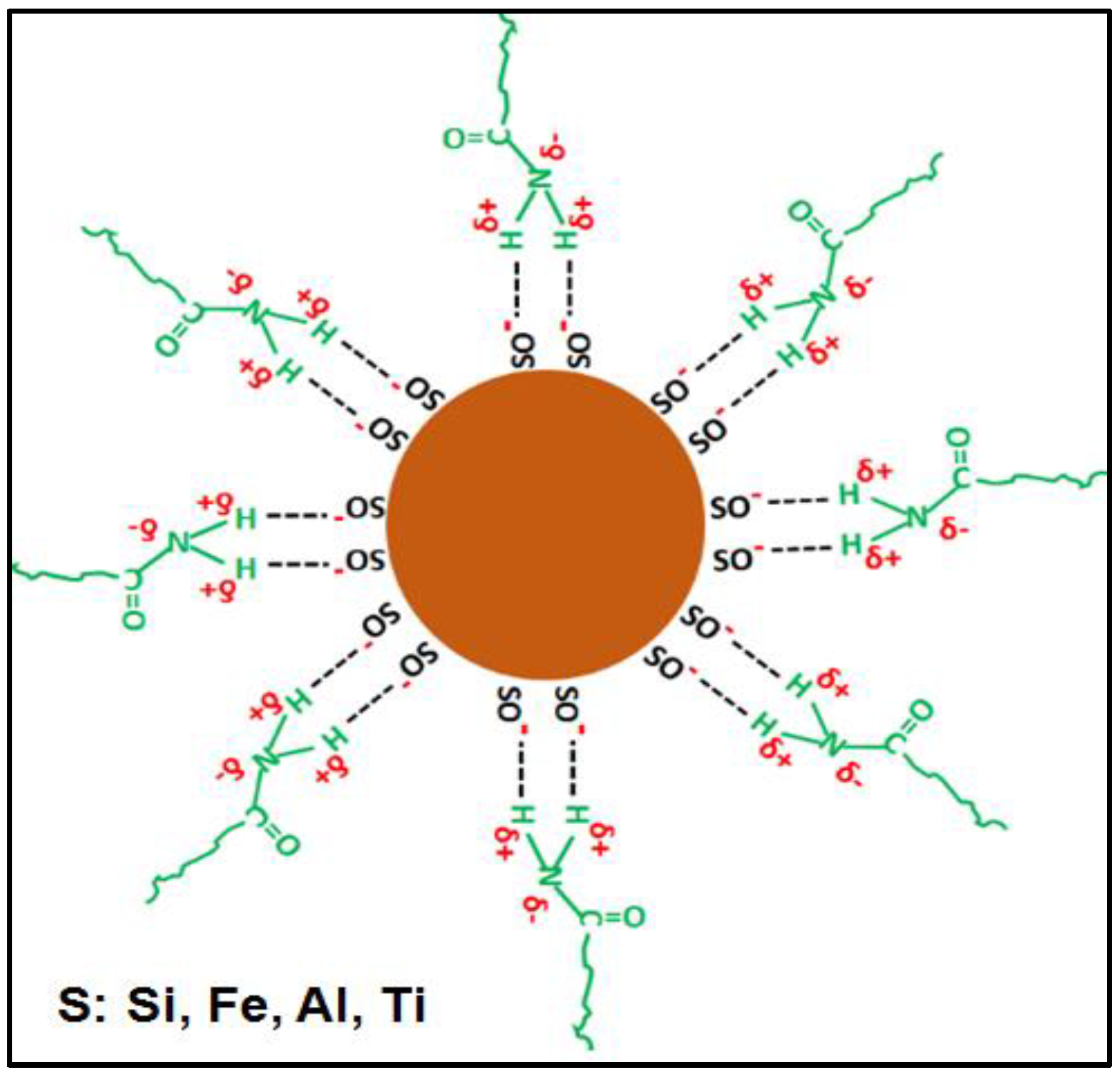
- Formation of hydrogen bonds between the surface hydroxyl groups of the clay and the amide group;
- Rupture of intermolecular hydrogen bonds between polyacrylamide chains, due to their separation;
- Ion–dipole interactions or coordination bonds, which occur between the exchangeable cations that the clay has and the amide group, for example, between a Ca2+ and the free electron pair of the nitrogen atom or the oxygen atom (RM has Ca2+ as an exchangeable cation);
- Formation of hydrogen bonds between the amide group and the interlayer water molecules present in the clay (RM has interlayer water).
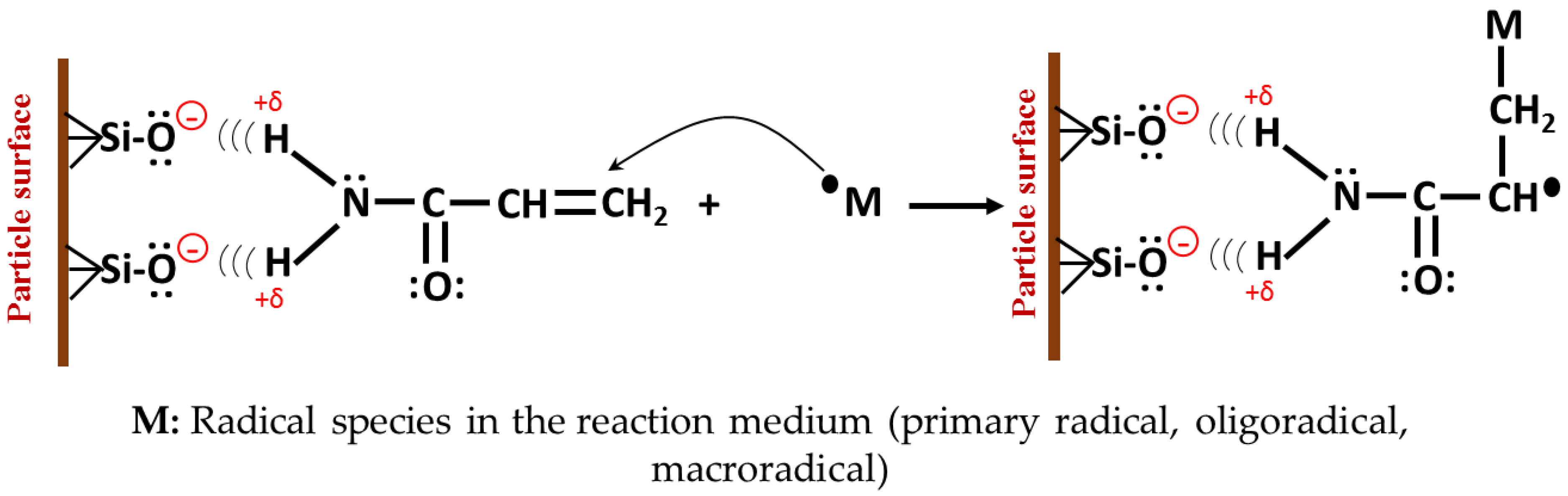
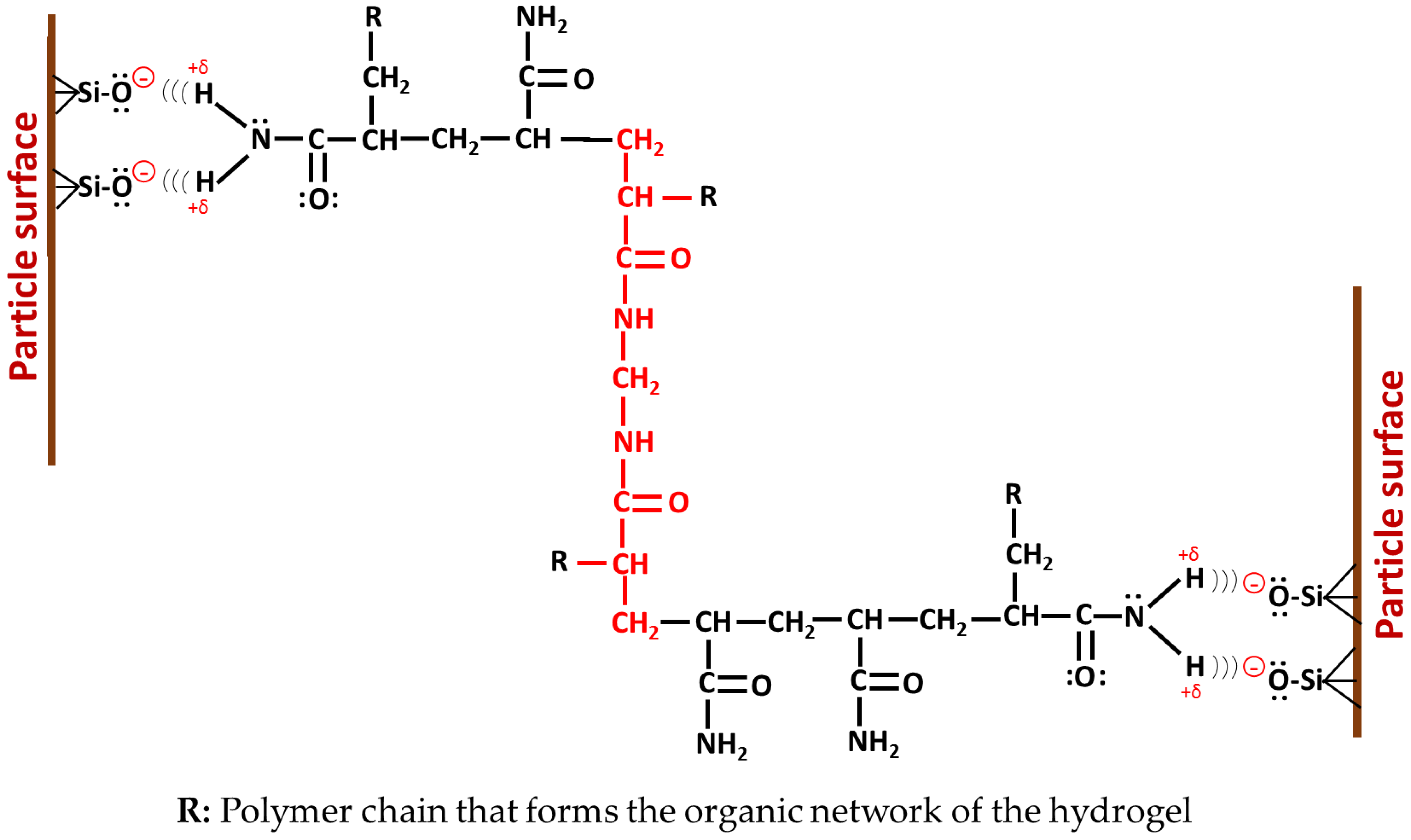
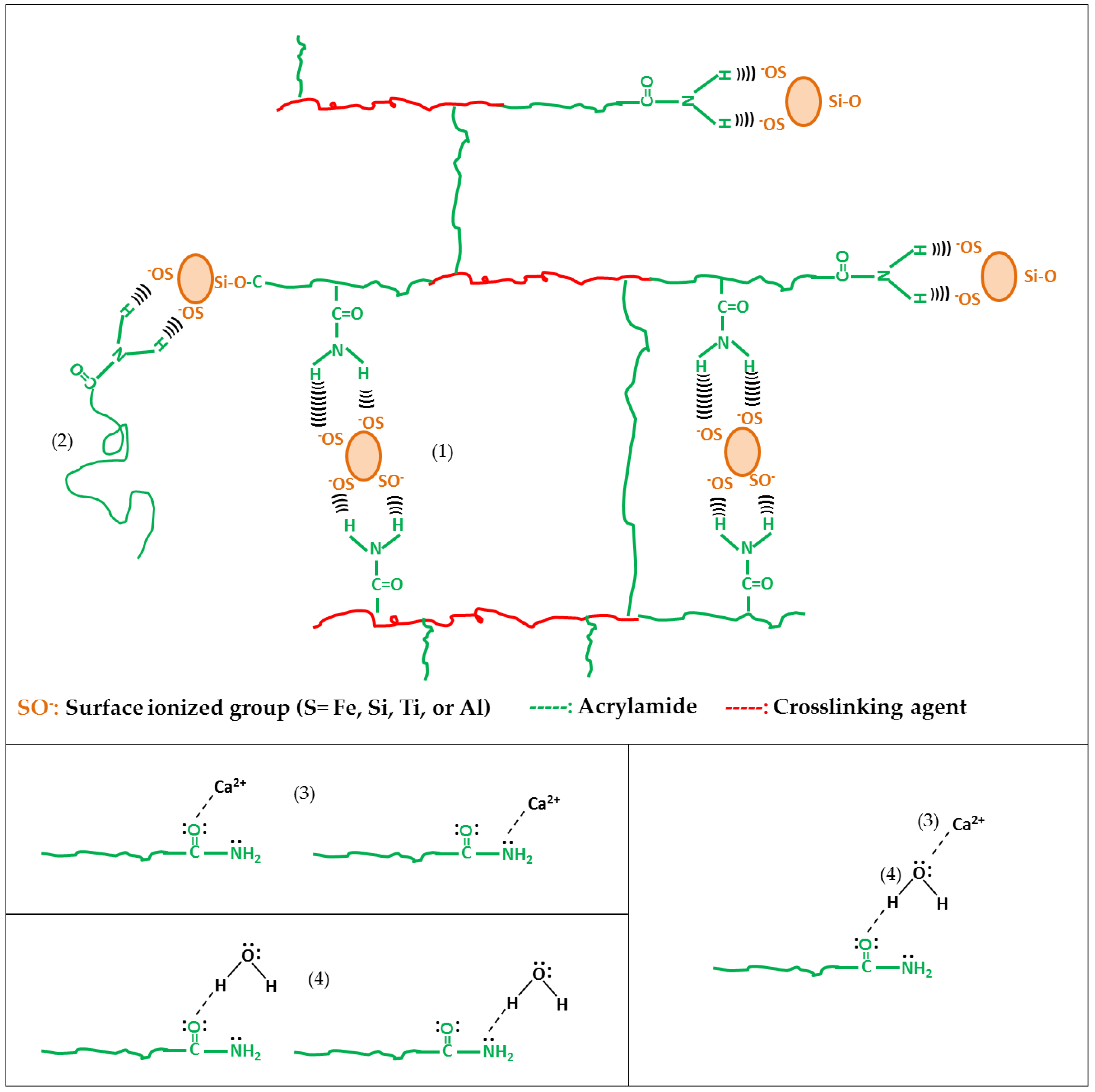
3.9. Mechanism of Formation of Hybrid Hydrogels
3.9.1. Pre-Synthesis Stage
3.9.2. Synthesis Stage
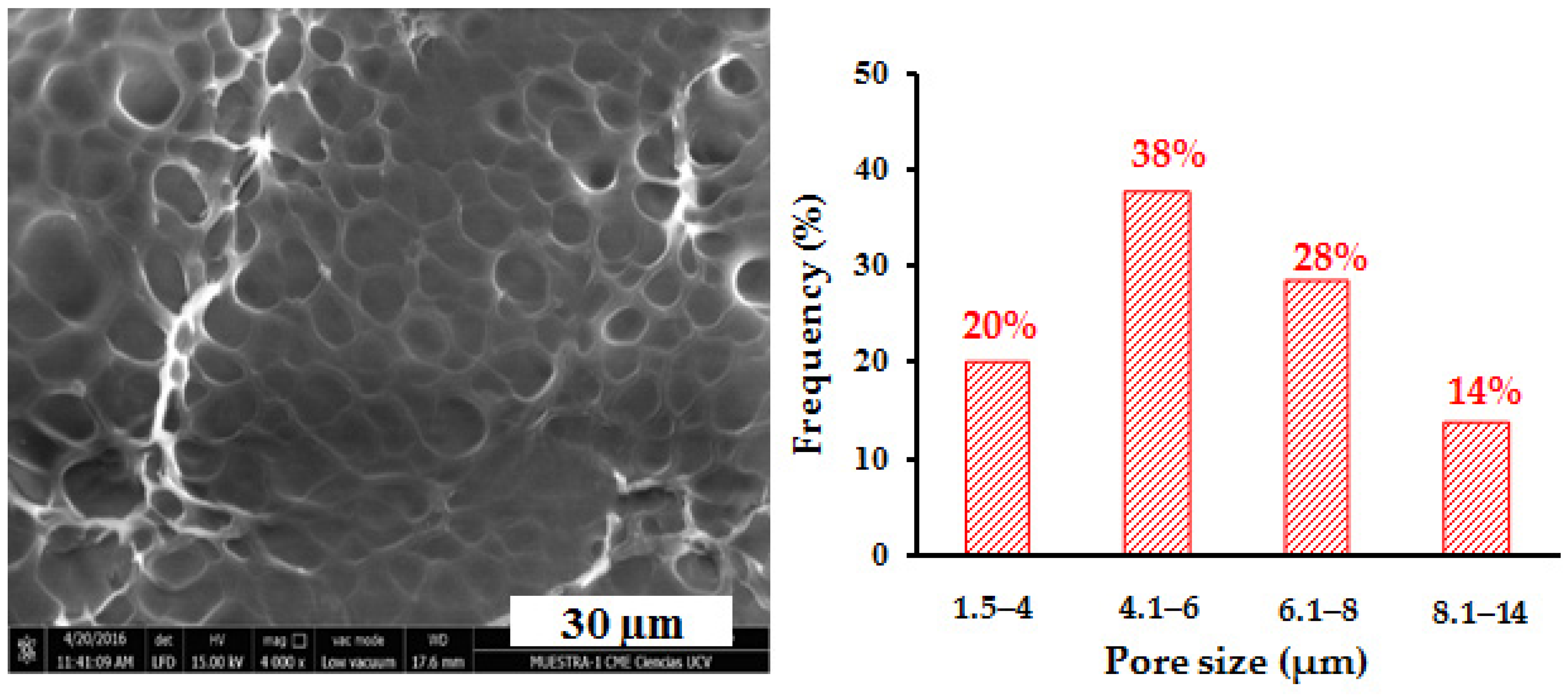
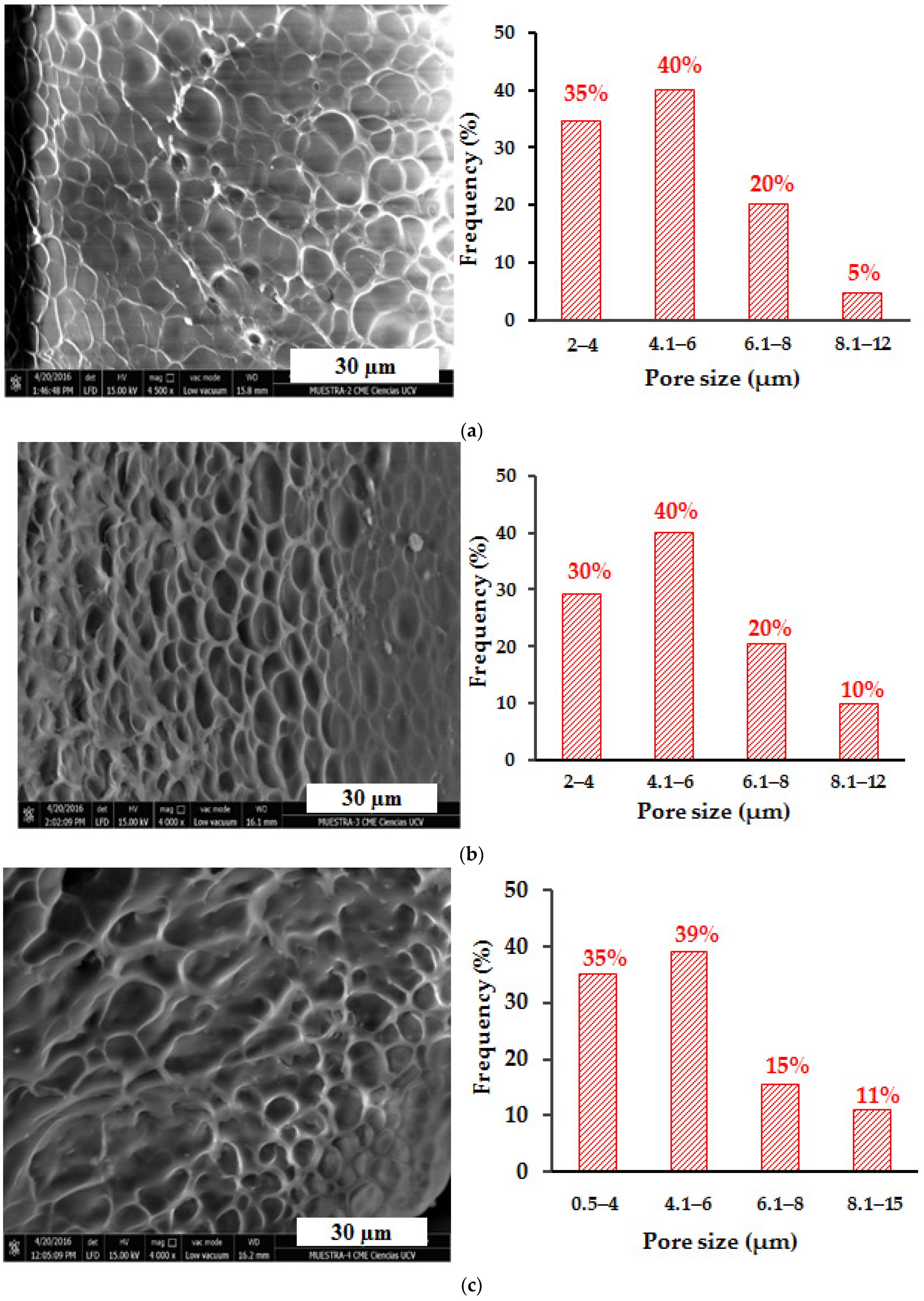
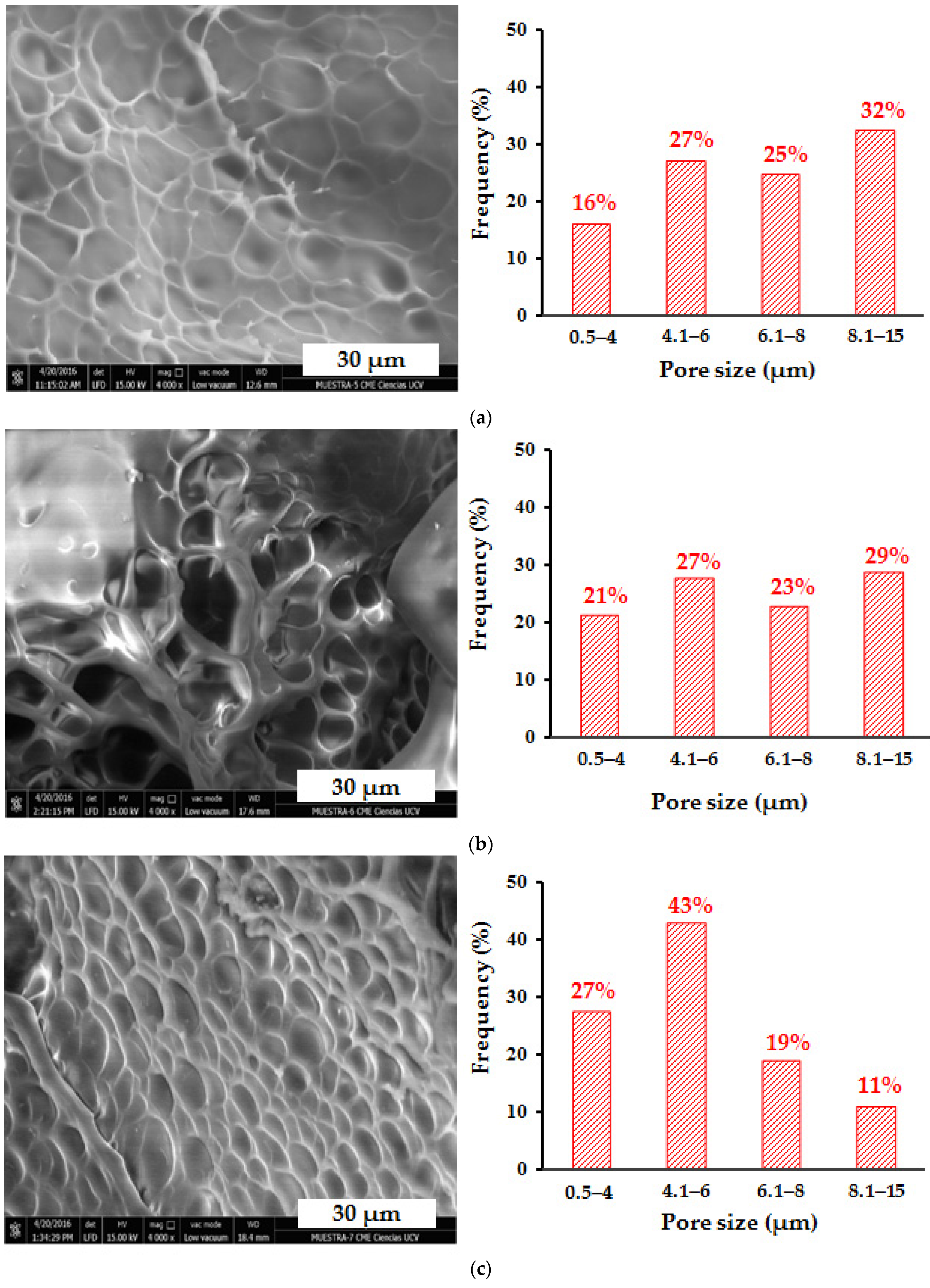
3.10. Scanning Electron Microscopy (SEM) Analysis of Hydrogels
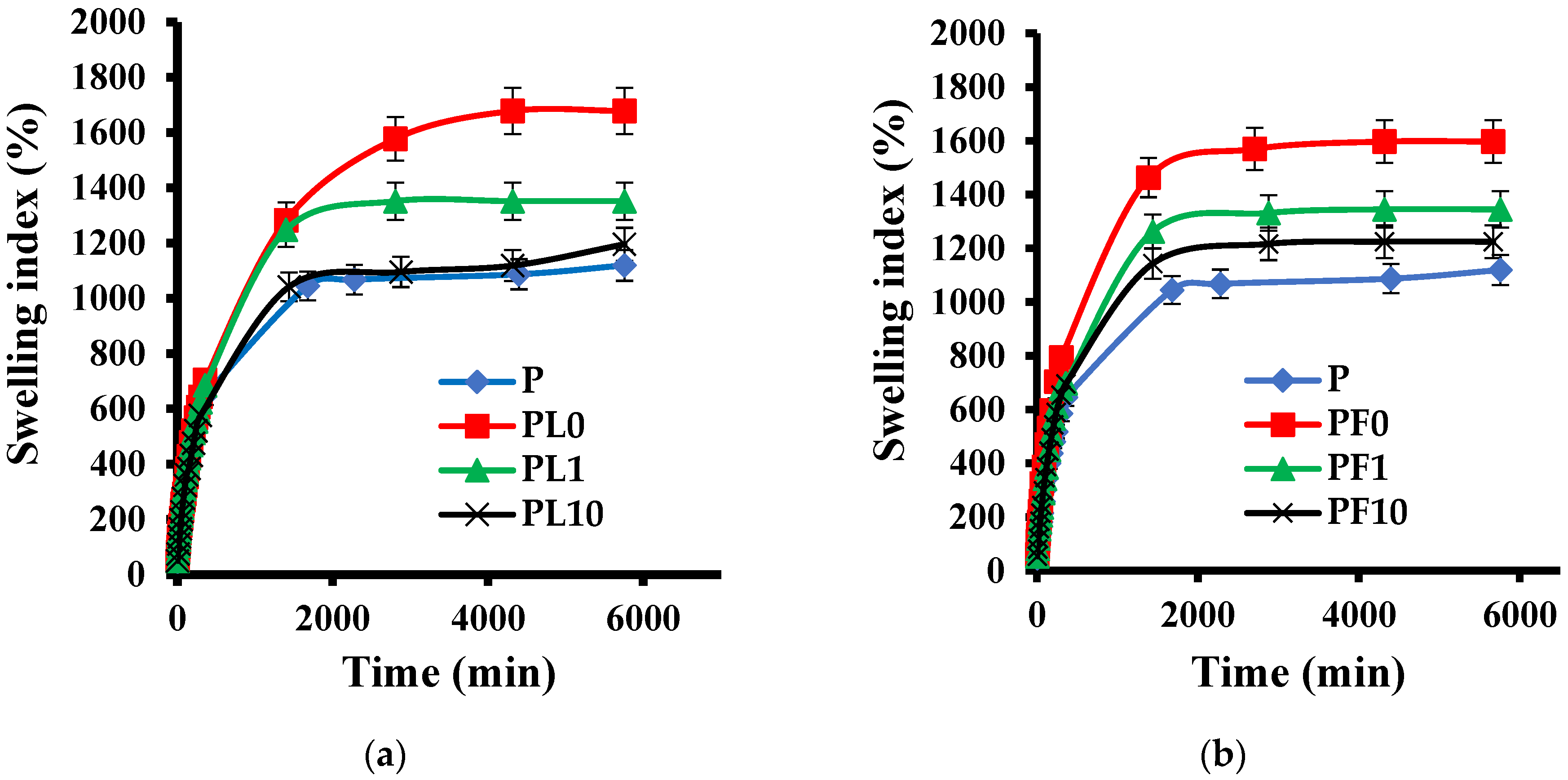
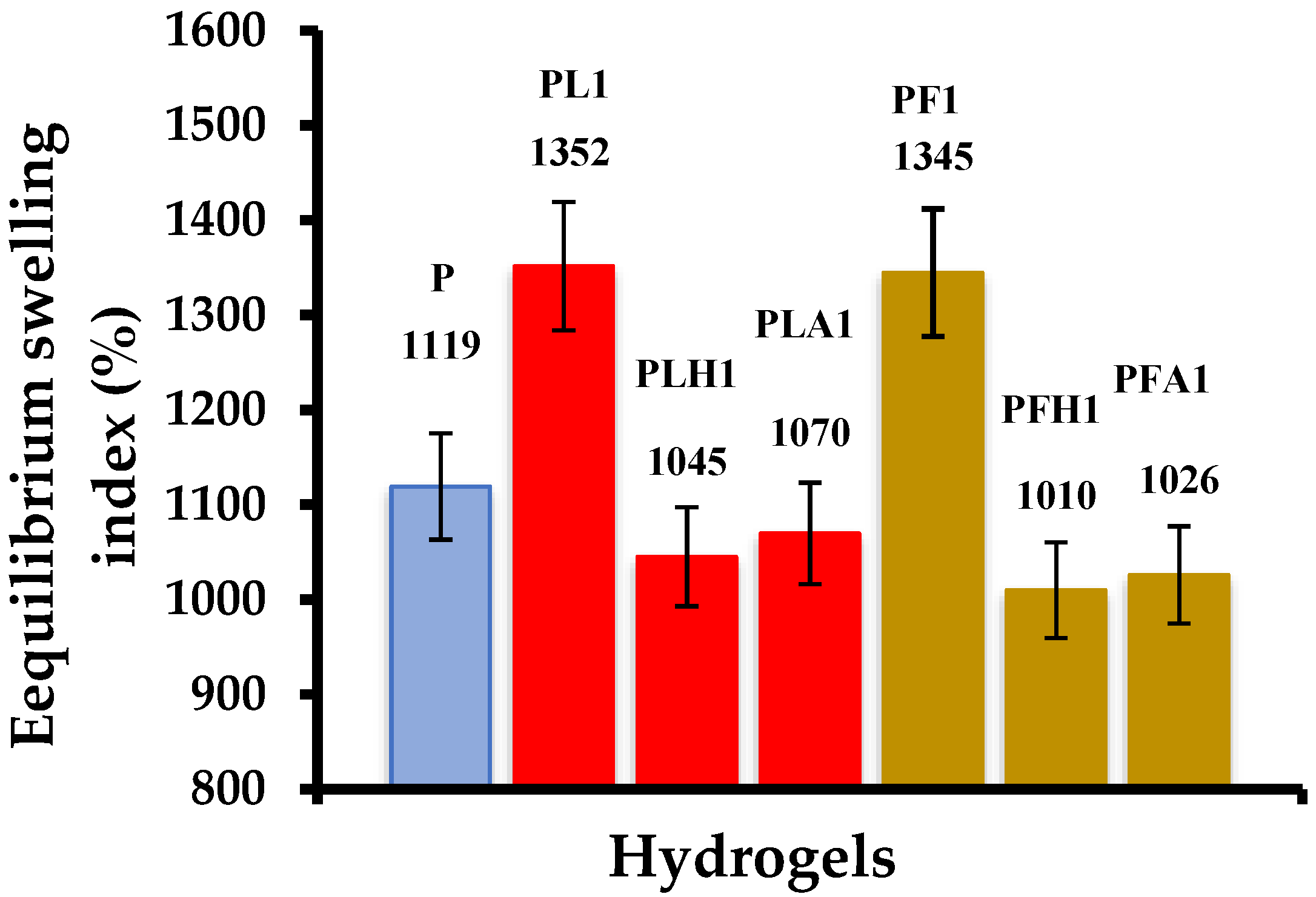
3.11. Swelling Index
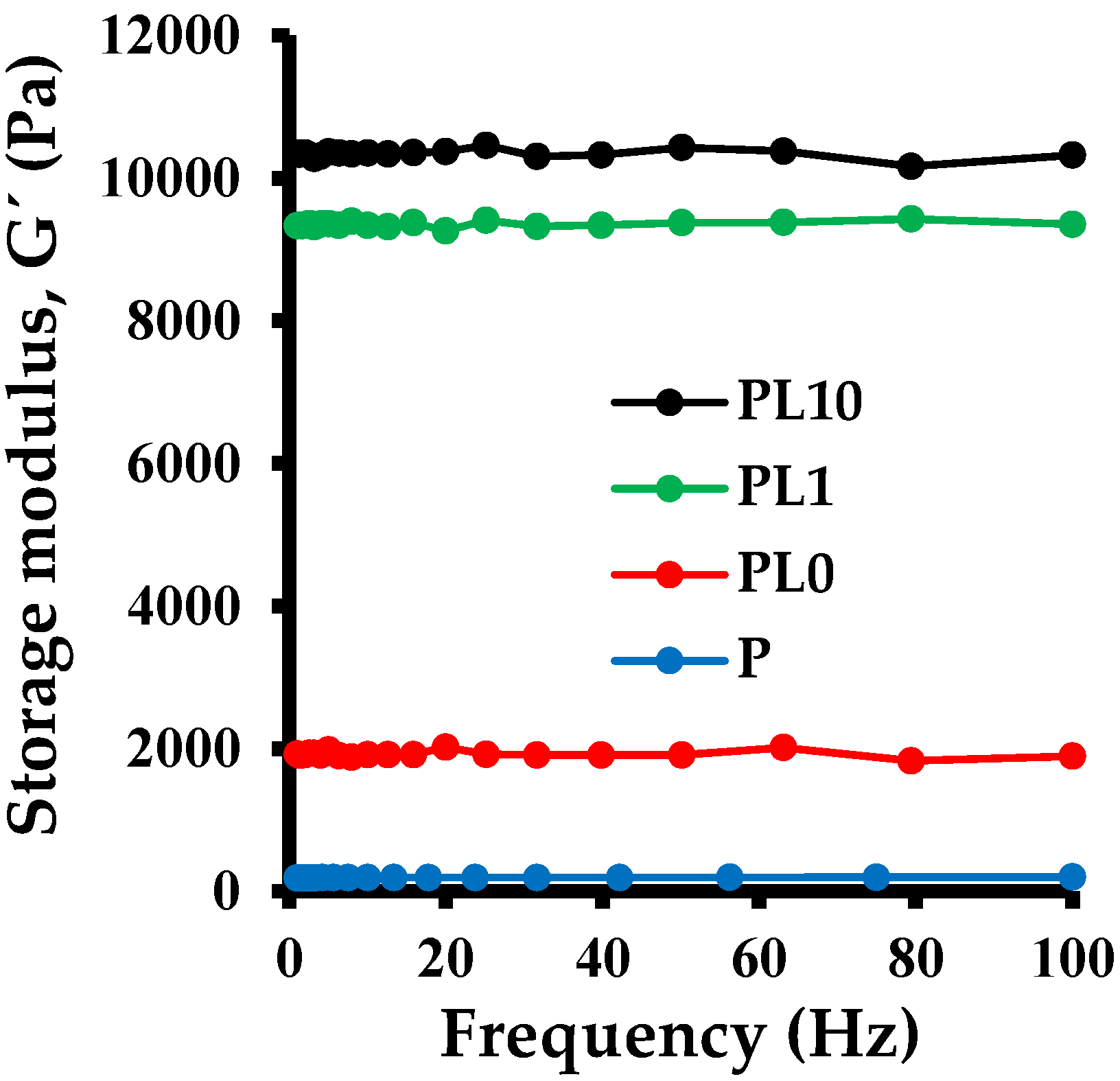
3.12. Dynamic Rheology Analysis
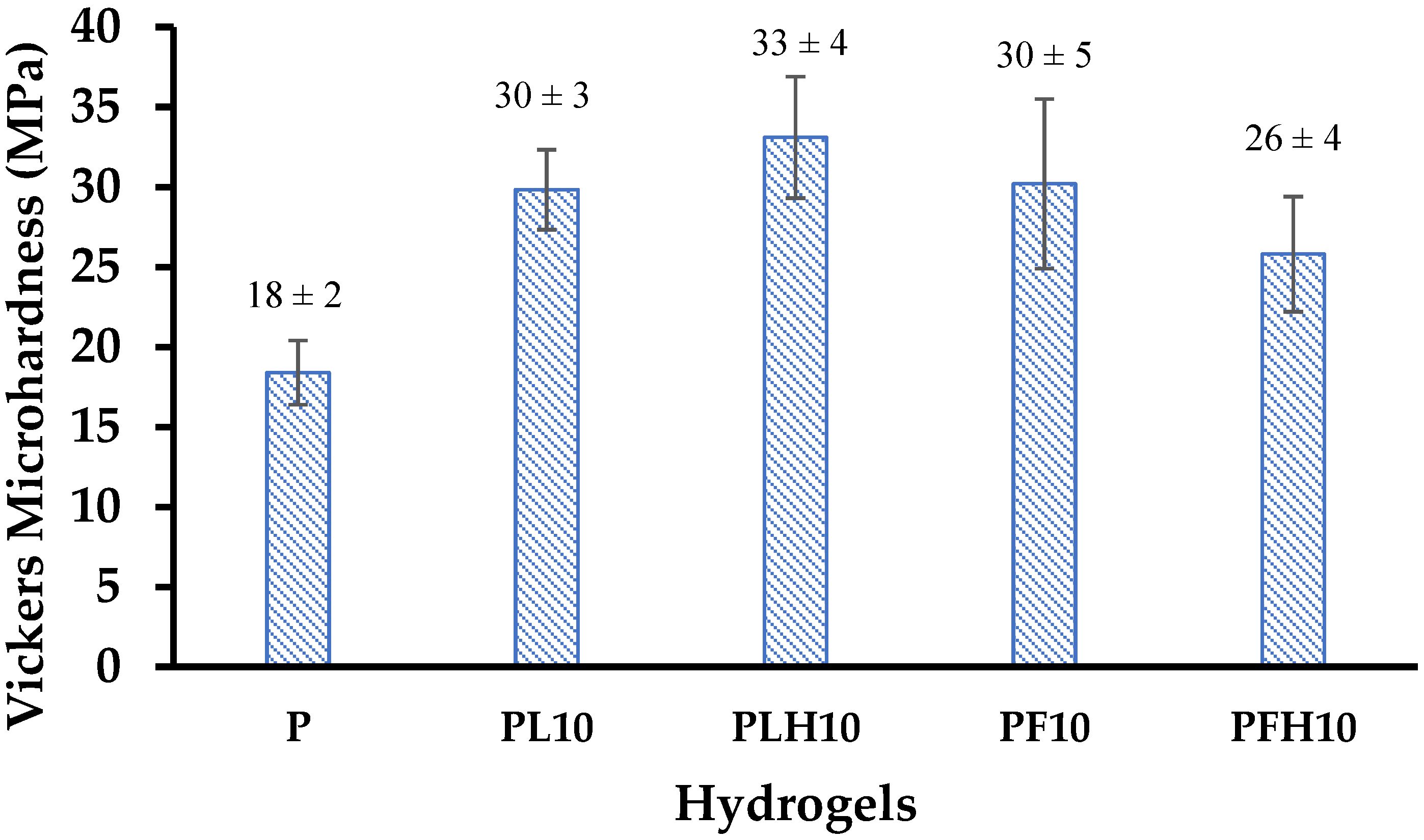
3.13. Microhardness Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2014, 34, 2662–2673. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, N.; Tang, H.; Wei, S. A Review on Comprehensive Utilization of Red Mud and Prospect Analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef]
- Gelencsér, A.; Kováts, N.; Turóczi, B.; Rostási, A.; Hoffer, A.; Imre, K.; Nyirő, I.; Csákberényi, D.; Tóth, A.; Czitrovszky, A.; et al. The Red Mud Accident in Ajka (Hungary): Characterization and Potential Health Effects of Fugitive Dust. Environ. Sci. Technol. 2011, 45, 1608–1615. [Google Scholar] [CrossRef]
- Ruyters, S.; Mertens, J.; Vassilieva, E.; Dehandschutter, B.; Poffijn, A.; Smolders, E. The Red Mud Accident in Ajka (Hungary): Plant Toxicity and Trace Metal Bioavailability in Red Mud Contaminated Soil. Environ. Sci. Technol. 2011, 45, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Burke, I.; Mayes, W.; Peacock, C.; Brown, P.; Jarvis, A.; Grui, K. Speciation of Arsenic, Chromium, and Vanadium in Red Mud Samples from the Ajka Spill Site, Hungary. Environ. Sci. Technol. 2012, 46, 3085–3092. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R.; Ming, H. Red mud as an amendment for pollutants in solid and liquid phases. Geoderma 2011, 163, 1–12. [Google Scholar] [CrossRef]
- Sutar, H.; Mishra, S.; Sahoo, S.; Chakraverty, A.; Maharana, H. Progress of Red Mud Utilization: An Overview. Amen. Chem. Sci. J. 2014, 4, 255–279. [Google Scholar] [CrossRef]
- Liang, W.; Couperthwaite, S.; Kaur, G.; Yan, C.; Jhonstone, D.; Millar, G. Effect of strong acids on red mud structural and fluoride adsorption properties. J. Colloid Interface Sci. 2014, 423, 158–165. [Google Scholar] [CrossRef]
- Ye, J.; Cong, X.; Zhanga, P.; Hoffmannc, E.; Zenga, G.; Liua, Y.; Fanga, W.; Wua, Y.; Zhang, H. Interaction between phosphate and acid-activated neutralized red mud during adsorption process. Appl. Surf. Sci. 2015, 356, 128–134. [Google Scholar] [CrossRef]
- Genç-Fuhrman, H.; Tjell, J.; McConchie, D.; Schuiling, O. Adsorption of arsenate from water using neutralized red mud. J. Colloid Interface Sci. 2003, 264, 327–334. [Google Scholar] [CrossRef]
- Smičiklas, I.; Smiljanić, S.; Perić-Grujić, A.; Šljivić-Ivanović, M.; Mitrić, M.; Antonović, D. Effect of acid treatment on red mud properties with implications. Chem. Eng. J. 2014, 242, 27–35. [Google Scholar] [CrossRef]
- Sahu, C.; Patel, R.; Ray, C. Neutralization of red mud using CO2 sequestration cycle. J. Hazard. Mater. 2010, 179, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Jollet, V.; Gissane, C.; Schlaf, M. Optimization of the neutralization of Red Mud by pyrolysis bio-oil using a design of experiments approach. Energy Environ. Sci. 2014, 7, 1125–1133. [Google Scholar] [CrossRef]
- Oliveira, A.; Costa, D.; Teixeira, I.; Moura, F. Gold nanoparticles supported on modified red mud for biphasicoxidation of sulfur compounds: A synergistic effect. Appl. Catal. B 2015, 162, 475–482. [Google Scholar] [CrossRef]
- Sushil, S.; Batra, V. Catalytic applications of red mud, an aluminium industry waste: A review. Appl. Catal. B 2008, 81, 64–77. [Google Scholar] [CrossRef]
- Rivas, J.; Gomez, L.; Ernandes, A.; Alves, A.; Romulo, S. Influencia del contenido de lodo rojo (residuo de bauxita) en las propiedades físico-mecánicas de materiales cerámicos conformados por extrusión. Rev. Latinoam. Metal. Mater. 2009, 29, 93–100. [Google Scholar]
- Bertocchi, A.; Ghiani, M.; Peretti, R.; Zucca, A. Red mud and fly ash for remediation of mine sites contaminated with As, Cd, Cu, Pb and Zn. J. Hazard. Mater. 2006, 134, 112–119. [Google Scholar] [CrossRef]
- Bhat, A.; Khalil, H.; Bhat, I.; Banthia, A. Dielectric and Material Properties of Poly (Vinyl Alcohol): Based Modified Red Mud Polymer Nanocomposites. J. Polym. Environ. 2012, 20, 395–403. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, C.; Luan, Z.; Peng, X.; Ren, X.; Wang, J. Arsenate removal from aqueous solutions using modified red mud. J. Hazard. Mater. 2008, 152, 486–492. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, C.; Wang, J.; Tong, D.; Yu, W.; Wang, Y. Recent Advances in Clay Mineral-Containing Nanocomposite Hydrogels. Soft Matter. 2015, 11, 9229–9246. [Google Scholar] [CrossRef]
- Rai, S.; Wasewar, K.; Lataye, D.; Mukhopadhyay, J.; Yoo, C. Feasibility of red mud neutralization with seawater using Taguchi’s methodology. Int. J. Environ. Sci. Technol. 2013, 10, 305–314. [Google Scholar] [CrossRef]
- Vasquez, A.; López, M.; Kortaberria, G.; Martin, L.; Mondragon, I. Modification of montmorillonite with cationic surfactants. Thermal and chemical analysis including CEC determination. Appl. Clay Sci. 2008, 41, 24–36. [Google Scholar] [CrossRef]
- EPA—United States Environmental Protection Agency. Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils; Method EPA 3051; United States Environmental Protection Agency: Washington, DC, USA, 2007.
- Genç-Fuhrman, H.; Tjell, C.; McConchie, D. Increasing the arsenate adsorption capacity of neutralized red mud (Bauxsol). J. Colloid Interface Sci. 2004, 271, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.; Lumsdon, D.; Hillier, S. Effect of pH on the cation exchange capacity of some halloysite nanotubes. Clay Miner. 2016, 51, 373–383. [Google Scholar] [CrossRef]
- EPA—United States Environmental Protection Agency. The National Primary Drinking Water Regulations (NPDWR); Table of Regulated Drinking Water Contaminants, Code of Federal Regulations (annual edition); United States Environmental Protection Agency: Washington, DC, USA, 2010.
- MARNR. Gaceta Oficial de la República Bolivariana de Venezuela nº 36395 13-02-1998; Normas sanitarias de calidad del agua potable; Ministerio del Ambiente y de los Recursos Naturales Renovables: Caracas, Venezuela, 1998. [Google Scholar]
- Ye, N.; Yang, J.; Ke, X.; Zhu, J.; Li, Y.; Xiang, C.; Wang, H.; Li, L.; Xiao, B. Synthesis and characterization of geopolymer from Bayer Red Mud with thermal pretreatment. J. Am. Ceram. Soc. 2014, 97, 1652–1660. [Google Scholar] [CrossRef]
- Gök, A.; Omastová, M.; Prokes, J. Synthesis and characterization of red mud/polyaniline composites: Electrical properties and thermal stability. Eur. Polym. J. 2007, 43, 2471–2480. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Boyjoo, A.; Choueib, Z.; Zhu, H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Xiao, B. Application of Bayer red mud for iron recovery and building material production from alumosilicate residues. J. Hazard. Mater. 2009, 161, 474–478. [Google Scholar] [CrossRef]
- Palmer, S.; Frost, R. Characterisation of bauxite and seawater neutralised bauxite residue using XRD and vibrational spectroscopic techniques. J. Mater. Sci. 2009, 44, 55–63. [Google Scholar] [CrossRef]
- Santona, L.; Castaldi, P.; Melis, P. Evaluation of the interaction mechanisms between red muds and heavy metals. J. Hazard. Mater. 2006, 136, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Atun, G.; Hisarli, G. A Study of Surface Properties of Red Mud by Potentiometric Method. J. Colloid Interface Sci. 2000, 228, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, Y.; Satokawa, S.; Kuroda, K.; Kato, C. Preparation of a kaolinite polyacrylamide intercalation compound. Clays Clay Miner. 1990, 38, 137–143. [Google Scholar] [CrossRef]
- Deng, Y.; Dixon, J.; White, G.; Loeppert, R.; Juo, A. Bonding between polyacrylamide and smectite. Colloids Surf. A Physicochem. Eng. Aspects 2006, 281, 82–91. [Google Scholar] [CrossRef]
- Jal, P.; Patel, S.; Mishra, B. Chemical modification of silica surface by immobilization of functional groups for extractive concentration of metal ions. Talanta 2004, 62, 1005–1028. [Google Scholar] [CrossRef]
- Ramírez, A.; Contreras, D.; Prin, J.L.; Rojas de Gáscue, B. Entrecruzamientos iónicos en hidrogeles de poli(acrilamida-co-ácido itacónico) postulados a partir del análisis de su morfología y cinética de absorción. Acta Microscópica 2013, 22, 205–209. [Google Scholar]
- Mahdavinia, G.R.; Marandi, G.B.; Pourjavadi, A.; Kiani, G. Semi-IPN carrageenan-based nanocomposite hydrogels: Synthesis and swelling behavior. J. Appl. Polym. Sci. 2010, 118, 2989–2997. [Google Scholar] [CrossRef]
- Okay, O.; Oppermann, W. Polyacrylamide-Clay Nanocomposite Hydrogels: Rheological and Light Scattering Characterization. Macromology 2007, 40, 3378–3387. [Google Scholar] [CrossRef]
- Sriya, D. Rheology and Morphology of Pristine Graphene/Polyacrylamide Gels. Acs Appl. Mater. Interfaces 2013, 5, 8633–8640. [Google Scholar] [CrossRef]
- Urbano, F.; Rivas, B. Synthesis, characterization, and sorption properties of water-insoluble poly(2-acrylamido-2-methyl-1-propane sulfonic acid)-montmorillonite composite. Polym. Bull. 2013, 70, 1143–1162. [Google Scholar] [CrossRef]
- Simeonov, M.S.; Apostolov, A.A.; Vassileva, E.D. In situ calcium phosphates deposition in hydrogels of poly(acrylic acid)-polyacrylamide interpenetrating polymer networks. RSC Adv. 2016, 6, 16274–16284. [Google Scholar] [CrossRef]







| Solution A | Solution B | ||
|---|---|---|---|
| Salts | Mass (g) | Salts | Mass (g) |
| NaCl | 23.9000 | Na2SO4·10H2O | 9.0600 |
| MgCl2·6H2O | 10.8300 | NaHCO3 | 0.2000 |
| CaCl2 anhydrous | 1.1500 | NaF | 0.0003 |
| SrCl2·6H2O | 0.0042 | H3BO3 | 0.0027 |
| KCl | 0.6820 | Distilled water | 100 mL |
| KBr | 0.0990 | ||
| Distilled water | 856 mL | ||
| Mass AAm (g) | Mass NMBA (g) | Mass PSA (g) | Inorganic Phase | Hydrogel Nomenclature | |
|---|---|---|---|---|---|
| Type | % | ||||
| 2 | 0.02 | 0.01 | – | P | |
| 2 | 0.02 | 0.01 | RM | 0.1 | PL0 |
| 2 | 0.02 | 0.01 | RM | 1 | PL1 |
| 2 | 0.02 | 0.01 | RM | 10 | PL10 |
| 2 | 0.02 | 0.01 | HCl-modified RM | 0.1 | PLH0 |
| 2 | 0.02 | 0.01 | HCl-modified RM | 1 | PLH1 |
| 2 | 0.02 | 0.01 | HCl-modified RM | 10 | PLH10 |
| 2 | 0.02 | 0.01 | Synthetic seawater-modified RM | 0.1 | PLA0 |
| 2 | 0.02 | 0.01 | Synthetic seawater-modified RM | 1 | PLA1 |
| 2 | 0.02 | 0.01 | Synthetic seawater-modified RM | 10 | PLA10 |
| 2 | 0.02 | 0.01 | FeSiMn | 0.1 | PF0 |
| 2 | 0.02 | 0.01 | FeSiMn | 1 | PF1 |
| 2 | 0.02 | 0.01 | FeSiMn | 10 | PF10 |
| 2 | 0.02 | 0.01 | HCl-modified FeSiMn | 0.1 | PFH0 |
| 2 | 0.02 | 0.01 | HCl-modified FeSiMn | 1 | PFH1 |
| 2 | 0.02 | 0.01 | HCl-modified FeSiMn | 10 | PFH10 |
| 2 | 0.02 | 0.01 | Synthetic seawater-modified FeSiMn | 0.1 | PFA0 |
| 2 | 0.02 | 0.01 | Synthetic seawater-modified FeSiMn | 1 | PFA1 |
| 2 | 0.02 | 0.01 | Synthetic seawater-modified FeSiMn | 10 | PFA10 |
| Element | Wavelength (nm) | RM (mg/kg) | FeSiMn (mg/kg) |
|---|---|---|---|
| Cu | 327.393 | 4.5 ± 0.7 | 168.6 ± 3.2 |
| Co | 228.616 | 3.5 ± 0.9 | ND * |
| Ni | 231.604 | 2.2 ± 0.4 | 127.1 ± 1.8 |
| Ti | 334.94 | 1013.9 ± 119 | – |
| Fe | 238.204 | 237,925 ± 15,328 | 12,015 ± 103 |
| Li | 670.784 | ND * | – |
| Cd | 214.44 | 7.0 ± 0.1 | 71.1 ± 1 |
| Mg | 285.213 | 1140 ± 122.7 | – |
| Ca | 213.933 | 18,453 ± 1568 | 20,260 ± 1061 |
| Cr | 267.716 | 7.7 ± 1.9 | 13.1 ± 1.2 |
| Mn | 257.61 | 134 ± 28.6 | 710,500 ± 35,115 |
| Pb | 220.353 | 37 ± 2.8 | 488.3 ± 4.4 |
| Mo | 202.031 | ND * | ND * |
| P | 213.617 | 15.6 ± 4.6 | 3.3 ± 0.9 |
| Se | 196.026 | ND * | 71.1±5.3 |
| Zn | 213.857 | 11.3 ± 8.5 | 35,445 ± 1959 |
| Sn | 189.927 | ND * | ND * |
| V | 290.88 | 81.2 ± 4.5 | 32 ± 0.9 |
| Suspension | pH as a Function of the Percentage of Inorganic Phase | |||
|---|---|---|---|---|
| 0% | 0.1% | 1% | 10% | |
| Aam + RM | 5.84 | 8.25 | 9.87 | 10.47 |
| Aam + FeSiMn | 5.84 | 7.93 | 9.21 | 10.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, A.; Gómez, L.; Müller, A.J.; Rojas de Gáscue, B. Characterization and Modification of Red Mud and Ferrosilicomanganese Fines and Their Application in the Synthesis of Hybrid Hydrogels. Polymers 2022, 14, 4330. https://doi.org/10.3390/polym14204330
Ramírez A, Gómez L, Müller AJ, Rojas de Gáscue B. Characterization and Modification of Red Mud and Ferrosilicomanganese Fines and Their Application in the Synthesis of Hybrid Hydrogels. Polymers. 2022; 14(20):4330. https://doi.org/10.3390/polym14204330
Chicago/Turabian StyleRamírez, Arnaldo, Leonir Gómez, Alejandro J. Müller, and Blanca Rojas de Gáscue. 2022. "Characterization and Modification of Red Mud and Ferrosilicomanganese Fines and Their Application in the Synthesis of Hybrid Hydrogels" Polymers 14, no. 20: 4330. https://doi.org/10.3390/polym14204330
APA StyleRamírez, A., Gómez, L., Müller, A. J., & Rojas de Gáscue, B. (2022). Characterization and Modification of Red Mud and Ferrosilicomanganese Fines and Their Application in the Synthesis of Hybrid Hydrogels. Polymers, 14(20), 4330. https://doi.org/10.3390/polym14204330







