Microwave-Assisted Lignin Extraction—Utilizing Deep Eutectic Solvents to Their Full Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bark Preparation
2.3. Moisture Content of Larch Bark
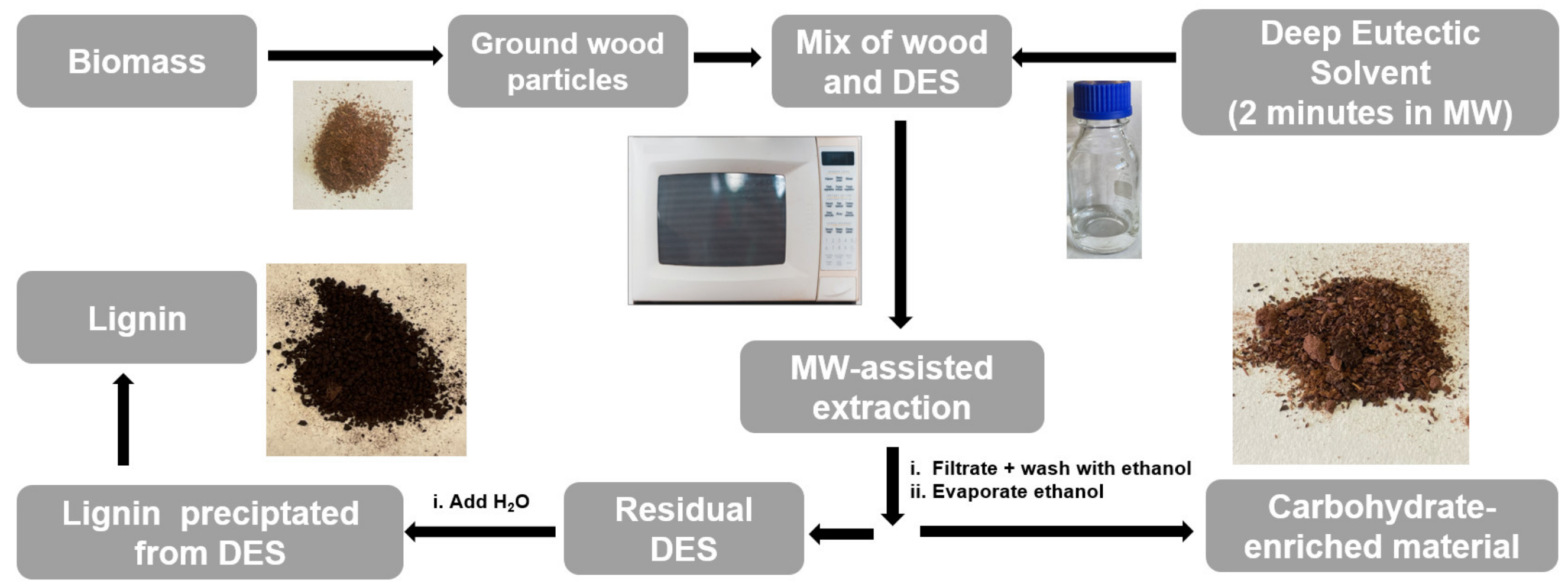
2.4. Biopolymer Extraction
2.5. Lignin Characterization
3. Results
3.1. Optimization of the DES Preparation
3.2. Optimization of the Reaction Time
3.3. Comparison to Traditional DES Extraction and Organosolv Process
3.4. Spectroscopic Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Studer, M.H.; Demartini, J.D.; Davis, M.F.; Sykes, R.W.; Davison, B.; Keller, M.; Tuskan, G.A.; Wyman, C.E. Lignin content in natural Populus variants affects sugar release. Proc. Natl. Acad. Sci. USA 2011, 108, 6300–6305. [Google Scholar] [CrossRef] [PubMed]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Meister, J.J. Chemical modification of lignin. In Chemical Modification of Lignocellulosic Materials; Hon, D.N.-S., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 129–157. [Google Scholar]
- Yadav, P.; Athanassiadis, D.; Antonopoulou, I.; Rova, U.; Christakopoulos, P.; Tysklind, M.; Matsakas, L. Environmental impact and cost assessment of a novel lignin production method. J. Clean. Prod. 2021, 279, 123515–123522. [Google Scholar] [CrossRef]
- Zakzeski, J.; Jongerius, A.L.; Bruijnincx, P.C.; Weckhuysen, B.M. Catalytic lignin valorization process for the production of aromatic chemicals and hydrogen. ChemSusChem 2012, 5, 1602–1609. [Google Scholar] [CrossRef]
- Pieratti, E.; Paletto, A.; Atena, A.; Bernardi, S.; Palm, M.; Patzelt, D.; Romagnoli, M.; Teston, F.; Voglar, G.E.; Grebenc, T.; et al. Environmental and climate change impacts of eighteen biomass-based plants in the alpine region: A comparative analysis. J. Clean. Prod. 2020, 242, 118449–118460. [Google Scholar] [CrossRef]
- Himmel, M.E. Biomass Recalcitrance: Deconstructing the Plant Cell Wall for Bioenergy; Wiley-Blackwell: Oxford, UK, 2009. [Google Scholar] [CrossRef]
- Matsushita, Y.; Yasuda, S. Preparation and evaluation of lignosulfonates as a dispersant for gypsum paste from acid hydrolysis lignin. Bioresour. Technol. 2005, 96, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.V.; Oliet, M.; Rodriguez, F.; Garcia, J.; Gilarranz, M.A.; Rodríguez, J.J. Modification of ammonium lignosulfonate by phenolation for use in phenolic resins. Bioresour. Technol. 2005, 96, 1013–1018. [Google Scholar] [CrossRef]
- Mansouri, N.-E.E.; Salvado, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, sodaanthraquinone, organosolv and ethanol process lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Monteil-Rivera, F.; Huang, G.H.; Paquet, L.; Deschamps, S.; Beaulieu, C.; Hawari, J. Microwave-assisted extraction of lignin from triticale straw: Optimization and microwave effects. Bioresour. Technol. 2012, 104, 775–782. [Google Scholar] [CrossRef]
- Gierer, J. Chemistry of delignification. Wood Sci. Technol. 1986, 19, 289–312. [Google Scholar] [CrossRef]
- Pye, E.K.; Lora, J.H. The Alcell process: A proven alternative to kraft pulping. Tappi J. 1991, 74, 113–118. [Google Scholar]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123–126146. [Google Scholar] [CrossRef]
- Ho, K.K.; Soo, K.C. Recent Efforts to Prevent Undesirable Reactions From Fractionation to Depolymerization of Lignin: Toward Maximizing the Value From Lignin. Front. Energy Res. 2018, 6, 92. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Qu, Y.; Gao, P. Xylanase pretreatment leads to enhanced soda pulping of wheat straw. Enzyme Microb. Technol. 2002, 30, 734–740. [Google Scholar] [CrossRef]
- El Hage, R.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- Sheldon, R. Catalytic reactions in ionic liquids. Chem. Commun. 2001, 23, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S.S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef]
- Pu, Y.; Jiang, N.; Ragauskas, A.J. Ionic liquid as a green solvent for lignin. J. Wood Chem. Technol. 2007, 27, 23–33. [Google Scholar] [CrossRef]
- Sun, N.; Rahman, M.; Qin, Y.; Maxim, M.L.; Rodriguez, H.; Rogers, R.D. Complete dissolution and partial delignification of woodmin the ionic liquid 1-ethyl-3-methylimidazolium acetate. Green Chem. 2009, 11, 646–655. [Google Scholar] [CrossRef]
- Oh, Y.; Park, S.; Yoo, E.; Jo, S.; Hong, J.; Kim, H.J.; Kim, K.J.; Oh, K.K.; Lee, S.H. Dihydrogenbonding deep eutectic solvents as reaction media for lipase-catalyzed transesterification. Biochem. Eng. J. 2019, 142, 34–40. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Mbous, Y.P.; Hayyan, M.; Hayyan, A.; Wong, W.F.; Hashim, M.A.; Looi, C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering-promises and challenges. Biotechnol. Adv. 2017, 35, 105–134. [Google Scholar] [CrossRef]
- Fu, D.; Mazza, G.; Tamaki, Y. Lignin extraction from straw by ionic liquids and enzymatic hydrolysis of the cellulosic residues. J. Agric. Food Chem. 2010, 58, 2915–2922. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biofuels Bioprod. Biorefin. 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Chen, Z.; Ragauskas, A.; Wan, C. Lignin extraction and upgrading using deep eutectic solvents. Ind. Crops Prod. 2020, 147, 112241–112272. [Google Scholar] [CrossRef]
- Kingston, H.M.; Haswell, S.J. Microwave-Enhanced Chemistry–Fundamentals, Sample Preparation, and Applications; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar] [CrossRef]
- De la Hoz, A.; Díaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Strauss, C.R.; Varma, R.S. Microwaves in green and sustainable chemistry. Top. Curr. Chem. 2006, 266, 199–232. [Google Scholar] [CrossRef]
- Betancourt, A.; Yezza, A.; Halasz, A.; Van Tra, H.; Hawari, J. Rapid microwave assisted esterification method for the analysis of poly-3-hydroxybutyrate in Alcaligenes latus by gas chromatography. J. Chromatogr. A 2007, 1154, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. Microwave dielectric heating in synthetic organic chemistry. Chem. Soc. Rev. 2008, 37, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, D.S.; de Korte, J.; de Vries, E.P.C.; Hameleers, L.; Wilbers, E.; Jurak, E.; Deuss, P.J. Highly Efficient Semi-Continuous Extraction and In-Line Purification of High β-O-4 Butanosolv Lignin. Front. Chem. 2021, 9, 655983–655996. [Google Scholar] [CrossRef] [PubMed]
- Eskicioglu, C.; Terzian, N.; Kennedy, K.J.; Droste, R.L.; Hamoda, M. A thermal microwave effects for enhancing digestibility of waste activated sludge. Water Res. 2007, 41, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Eskicioglu, C.; Kennedy, K.J.; Droste, R.L. Enhancement of batch waste activated sludge digestion by microwave pretreatment. Water Environ. Res. 2007, 79, 2304–2317. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Shi, J.; Pu, Y.; Yang, B.; Ragauskas, A.; Wyman, C.E. Comparison of microwaves to fluidized sand baths for heating tubular reactors for hydrothermal and dilute acid batch pretreatment of corn stover. Bioresour. Technol. 2011, 102, 5952–5961. [Google Scholar] [CrossRef]
- Jackowiak, D.; Frigon, J.C.; Ribeiro, T.; Pauss, A.; Guiot, G. Enhancing solubilisation and methane production kinetic of switchgrass by microwave pretreatment. Bioresour. Technol. 2011, 102, 3535–3540. [Google Scholar] [CrossRef]
- ISO/DIS Standard No. 3130; Wood-Determination of Water Content for Physical and Mechanical Testing. International Organization for Standardization: Geneva, Switzerland, 1994.
- Taylor, K.M.; Taylor, Z.E.; Hnady, S.T. Rapid synthesis of aurones under mild conditions using a combination of microwaves and deep eutectic solvents. Tetrahedron Lett. 2017, 58, 240–241. [Google Scholar] [CrossRef]
- Hammond, O.S.; Eslava, S.; Smith, A.J.; Zhang, J.; Edler, K.J. Microwave-assisted deep eutectic-solvothermal preparation of iron oxide nanoparticles for photoelectrochemical solar water splitting. J. Mater. Chem. A 2017, 5, 16189–16199. [Google Scholar] [CrossRef]
- Oh, Y.; Park, S.; Jung, D.; Oh, K.K.; Le, S.H. Effect of hydrogen bond donor on the choline chloride-based deep eutectic solvent-mediated extraction of lignin from pine wood. Int. J. Biol. Macromol. 2020, 165, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Grzybek, J.; Sepprer, T.; Petutschnigg, A.; Schnabel, T. Organosolv Lignin from European Tree Bark: Influence of Bark Pretreatment. Materials 2021, 14, 7774. [Google Scholar] [CrossRef] [PubMed]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; van Dam, J.E.G. Characterisation of structuredependent functional properties of lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Rashid, T.; Kait, C.F.; Murugesan, T.A. “Fourier transformed infrared” compound study of lignin recovered from a formic acid process. Procedia Eng. 2016, 148, 312–1319. [Google Scholar] [CrossRef]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Faix, O.; Beinhoff, O. FTIR spectra of milled wood lignins and lignin polymer models (DHP’s) with enhanced resolution obtained by deconvolution. J. Wood Chem. Technol. 1988, 8, 505–522. [Google Scholar] [CrossRef]
- Fernandes, C.; Melro, E.; Magalhães, S.; Alves, L.; Craveiro, R.; Filipe, A.; Valente, A.J.M.; Martins, G.; Antunes, F.E.; Romano, A.; et al. New deep eutectic solvent assisted extraction of highly pure lignin from maritime pine sawdust (Pinus pinaster Ait.). Int. J. Biol. Macromol. 2021, 177, 294–305. [Google Scholar] [CrossRef]
- Li, S.; Lundquist, K. A New Method for the Analysis of Phenolic Groups in Lignins by 1 H-NMR. Nord. Pulp Pap. Res. J. 1994, 3, 191–195. [Google Scholar] [CrossRef]
- Sun, R.; Tomkinson, J.; Mao, F.C.; Sun, X.F. Physicochemical characterization of lignins from rice straw by hydrogen peroxide treatment. J. Appl. Polym. Sci. 2001, 79, 719–732. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; Abächerli, A.; Semke, H.; Malherbe, R.; Käuper, P.; Nadif, A.; Van Dam, J.E.G. Analytical protocols for characterisation of sulphur-free lignin. Ind. Crops Prod. 2004, 19, 271–281. [Google Scholar] [CrossRef]
- Pan, X.J.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: Relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, W.; Yang, L.; Zu, Y.; Zu, B.; Zhu, M.; Zhang, Y.; Zhang, X.; Zhang, R.; Sun, Z. Investigation of the effects of different organosolv pulping methods on antioxidant capacity and extraction efficiency of lignin. Food Chem. 2012, 131, 313–317. [Google Scholar] [CrossRef]
- Sun, S.-N.; Cao, X.-F.; Xu, F.; Sun, R.-C.; Jones, G.L. Structural Features and Antioxidant Activities of Lignins from Steam-Exploded Bamboo (Phyllostachys pubescens). J. Agric. Food Chem. 2014, 62, 5939–5947. [Google Scholar] [CrossRef] [PubMed]



| Time (Minutes) | Yield (mg) | Yield (%) |
|---|---|---|
| 2 | 60 | 24 |
| 5 | 55 | 22 |
| 10 | 134 | 53 |
| 15 | 177 | 70 |
| 20 | 225 | 89 |
| 25 | 201 | 80 |
| 30 | 243 | 96 |
| DES | Yield (mg) | Yield (%) |
|---|---|---|
| CHCl/AA | 243 | 96 |
| CHCl/CA | 190 | 75 |
| CHCl/DG | 186 | 74 |
| CHCl/U | 165 | 65 |
| Wavenumber (cm−1) | Band Assignment |
|---|---|
| 3400–3300 | O−H Stretch |
| 2935–2918 and 2859–2850 | C–H stretching in methyl and methylene groups and in aromatic methoxyl groups |
| 1712–1686 | C=O Stretch in unconjugated ketone, carbonyl and in ester groups (frequently of carbohydrate origin) |
| 1656–1650 | C=O stretching in conjugated p-subst. Arylketones |
| 1616–1593 | Aromatic skeletal vibrations plus C=O stretching |
| 1518–1505 | Aromatic skeletal vibrations |
| 1463–1422 | C–H deformations in methyl and methylene and aromatic skeletal vibrations combined with C–H in-plane deformation |
| 1373–1353 | Aliphatic C–H stretching in methyl and phenolic OH |
| 1274–1262 | G ring breathing plus C=O stretching |
| 1229–1200 | C–C plus C–O plus C=O stretching |
| 1168–1150 | C=O in ester groups (conjugated) (typical for HGS lignins) |
| 1164–1154 | Aromatic C–H in-plane deformation plus secondary alcohols plus C–O stretch |
| 1126–1107 | C–O deformation in secondary alcohols and aliphatic ethers |
| 1040–1020 | Aromatic C–H in-plane deformation, plus C–O deformation in primary alcohols, plus C=O Stretch (unconjugated) |
| 965–952 | –HC=CH-out-of-plane deformations (trans) |
| 879–851/824–802 | C–H out-of-plane in positions 2,5, and 6 of G units |
| Total Phenolic Content | Antioxidant Activity | |||
|---|---|---|---|---|
| µg GAE/mg | Abs. (A.U.) | A15 (A.U.) | A0 (A.U.) | Inhibition (%) |
| 590 | 2.432 | 0.155 | 0.532 | 71 |
| 590 | 2.431 | 0.149 | 0.532 | 72 |
| 588 | 2.424 | 0.140 | 0.532 | 74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meindl, A.; Petutschnigg, A.; Schnabel, T. Microwave-Assisted Lignin Extraction—Utilizing Deep Eutectic Solvents to Their Full Potential. Polymers 2022, 14, 4319. https://doi.org/10.3390/polym14204319
Meindl A, Petutschnigg A, Schnabel T. Microwave-Assisted Lignin Extraction—Utilizing Deep Eutectic Solvents to Their Full Potential. Polymers. 2022; 14(20):4319. https://doi.org/10.3390/polym14204319
Chicago/Turabian StyleMeindl, Alina, Alexander Petutschnigg, and Thomas Schnabel. 2022. "Microwave-Assisted Lignin Extraction—Utilizing Deep Eutectic Solvents to Their Full Potential" Polymers 14, no. 20: 4319. https://doi.org/10.3390/polym14204319
APA StyleMeindl, A., Petutschnigg, A., & Schnabel, T. (2022). Microwave-Assisted Lignin Extraction—Utilizing Deep Eutectic Solvents to Their Full Potential. Polymers, 14(20), 4319. https://doi.org/10.3390/polym14204319








