Fluorinated Ethylene Propylene Coatings Deposited by a Spray Process: Mechanical Properties, Scratch and Wear Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
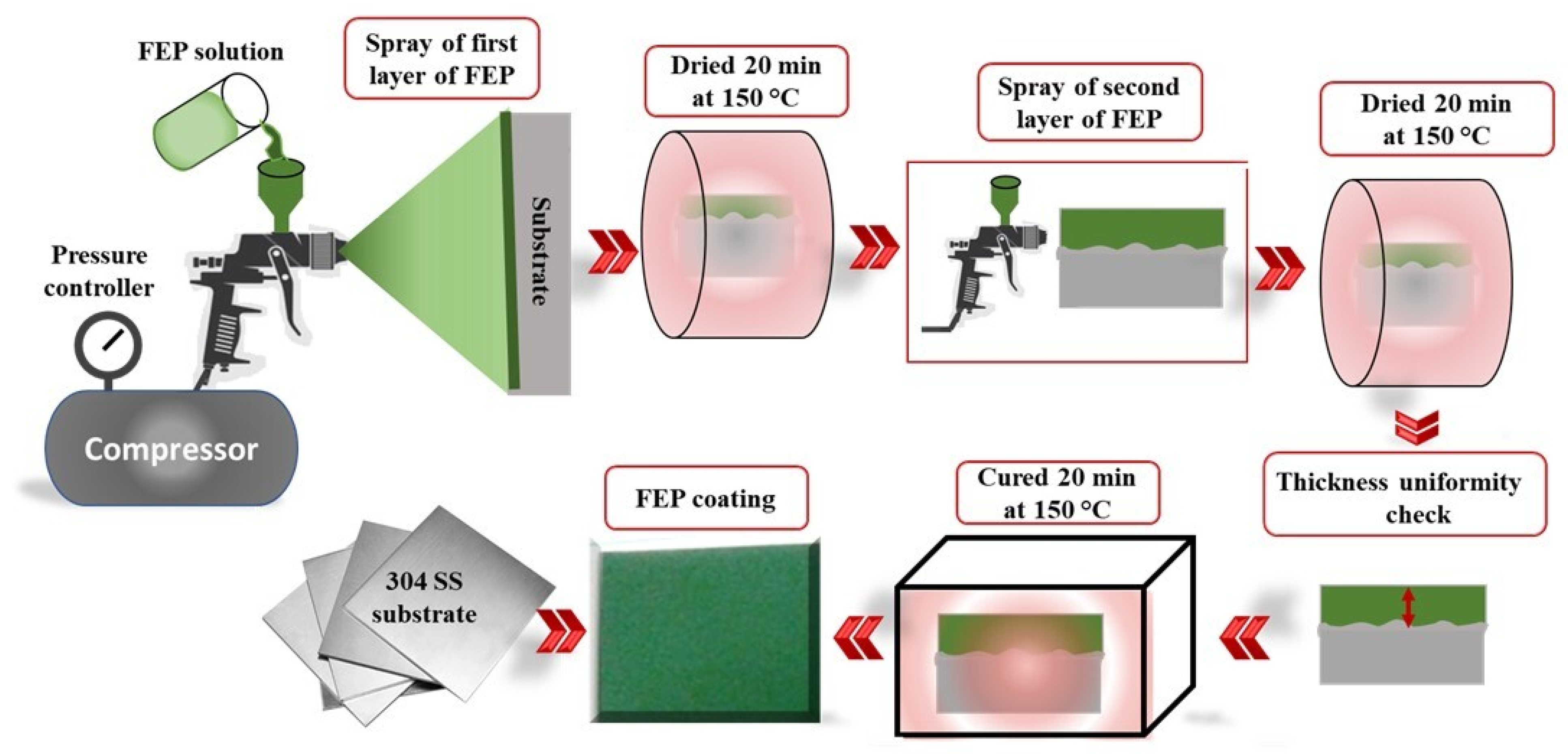
2.2. Coating Deposition
2.3. Structural Characterization
2.4. Nanoindentation Testing
2.5. Scratch Testing
2.6. Tribological Characterization
2.7. Corrosion Test
3. Results and Discussion
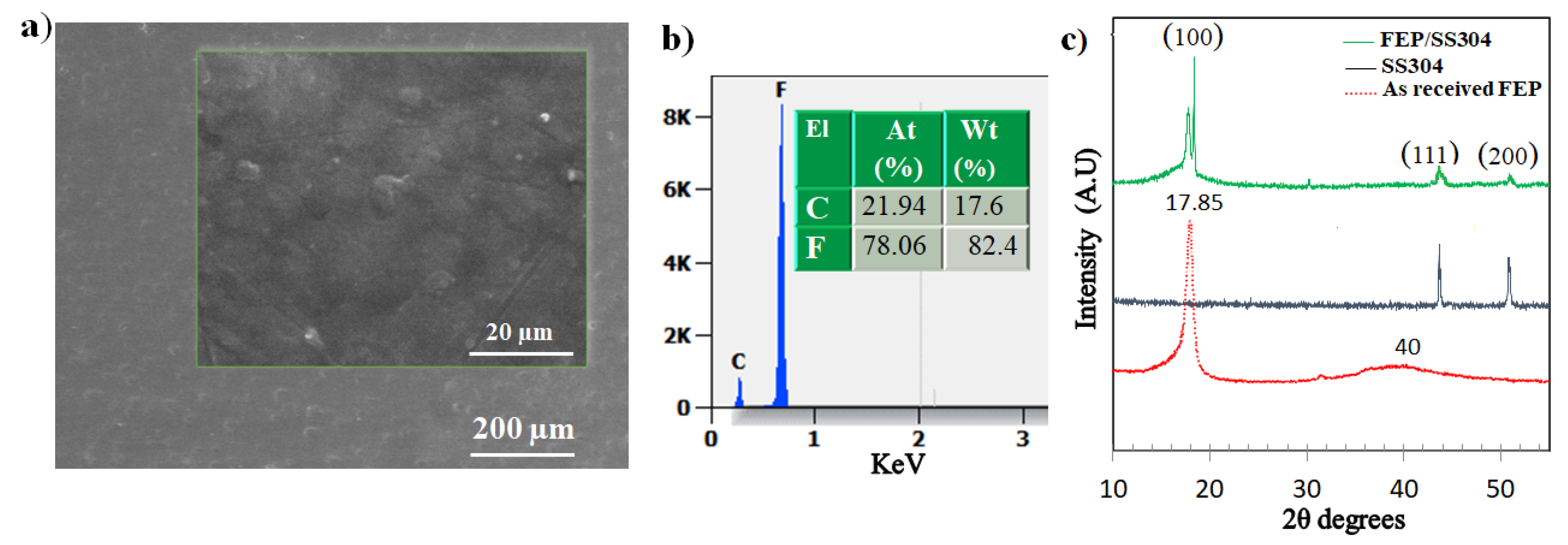
3.1. Microstructural Properties
3.2. Mechanical Properties
3.3. Scratch Behavior
3.4. Friction and Wear Resistance
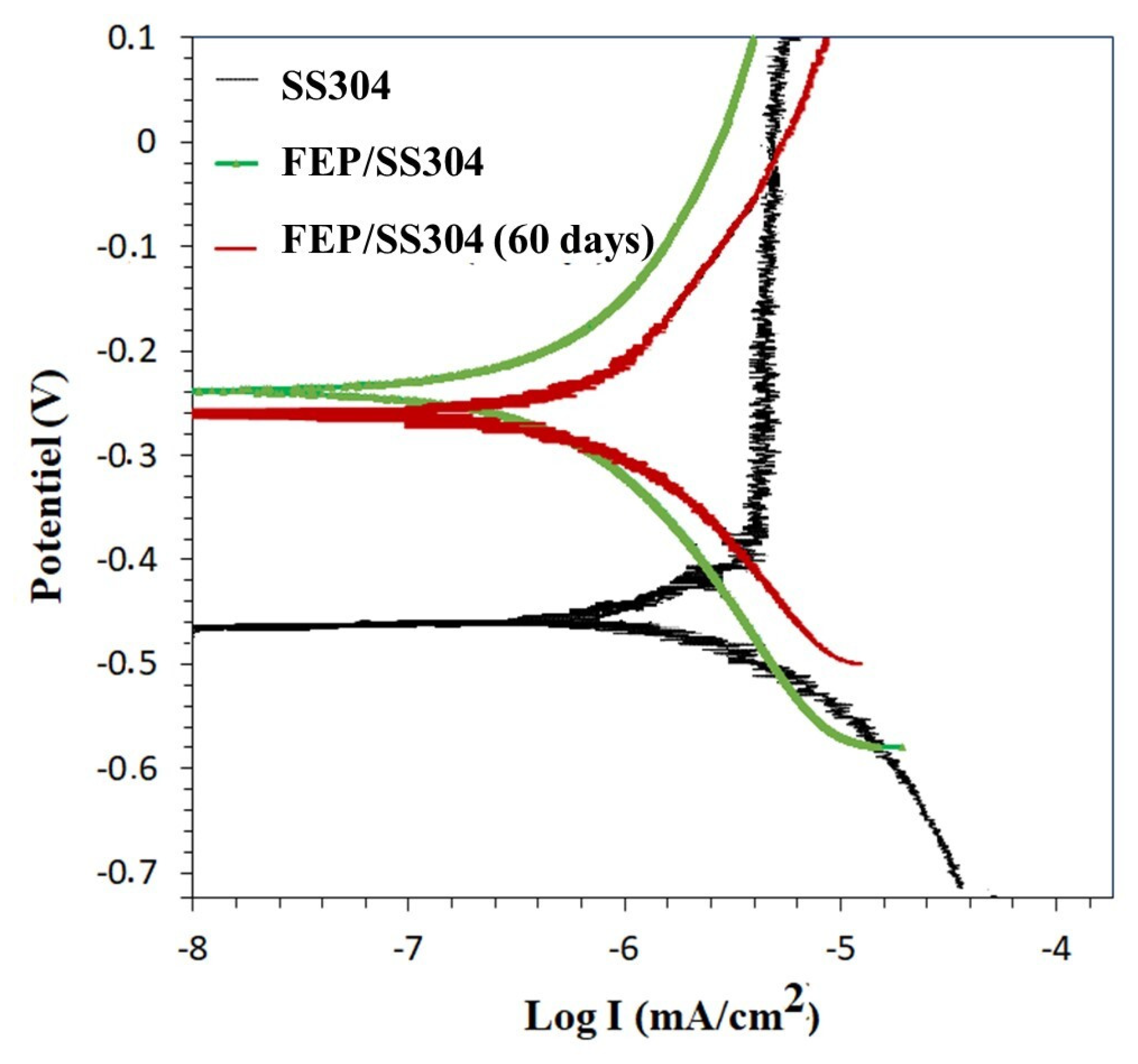
3.5. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boillot, P.; Peultier, J. Use of Stainless Steels in the Industry: Recent and Future Developments. Procedia Eng. 2014, 83, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Bodur, T.; Cagri-Mehmetoglu, A. Removal of Listeria monocytogenes, Staphylococcus aureus and Escherichia coli O157:H7 biofilms on stainless steel using scallop shell powder. Food Control. 2012, 25, 1–9. [Google Scholar] [CrossRef]
- Ismaïl, R.; Aviat, F.; Michel, V.; Le Bayon, I.; Gay-Perret, P.; Kutnik, M.; Fédérighi, M. Methods for Recovering Microorganisms from Solid Surfaces Used in the Food Industry: A Review of the Literature. Int. J. Environ. Res. Public Health 2013, 10, 6169–6183. [Google Scholar] [CrossRef]
- Cooper, I.; Tice, P. Food contact coatings—European legislation and future predictions. Surf. Coat. Int. Part B Coat. Trans. 2001, 84, 105–112. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Reduced wear and friction enabled by graphene layers on sliding steel surfaces in dry nitrogen. Carbon 2013, 59, 167–175. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Jiang, Z. Effects of temperature on the corrosion behavior of 13Cr martensitic stainless steel during exposure to CO2 and Cl− environment. Mater. Lett. 2005, 59, 3370–3374. [Google Scholar] [CrossRef]
- Ziemniak, S.; Hanson, M. Corrosion behavior of 304 stainless steel in high temperature, hydrogenated water. Corros. Sci. 2002, 44, 2209–2230. [Google Scholar] [CrossRef] [Green Version]
- Peter, I.; Rosso, M.; Gobber, F.S. Study of protective coatings for aluminum die casting molds. Appl. Surf. Sci. 2015, 358, 563–571. [Google Scholar] [CrossRef]
- Rodríguez-Alabanda, Ó.; Romero, P.E.; Soriano, C.; Sevilla, L.; Guerrero-Vaca, G. Study on the Main Influencing Factors in the Removal Process of Non-Stick Fluoropolymer Coatings Using Nd:YAG Laser. Polymers 2019, 11, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barhoumi, N.; Dhiflaoui, H.; Kaouther, K.; Ben Rhouma, A.; Hamdi, F. Study of Wear and Corrosion Performance of Fluoropolymer PFA Electrostatically Deposited on 304 Steel. In Advances in Mechanical Engineering and Mechanics II; Lecture Notes in Mechanical Engineering; Bouraoui, T., Ed.; Springer: Cham, Switzerland, 2021; pp. 103–110. [Google Scholar] [CrossRef]
- Primc, G. Recent Advances in Surface Activation of Polytetrafluoroethylene (PTFE) by Gaseous Plasma Treatments. Polymers 2020, 12, 2295. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, Y.; Okazaki, Y.; Shibahara, M.; Nishino, M.; Seto, Y.; Endo, K.; Yamamura, K. Effects of He and Ar Heat-Assisted Plasma Treatments on the Adhesion Properties of Polytetrafluoroethylene (PTFE). Polymers 2021, 13, 4266. [Google Scholar] [CrossRef] [PubMed]
- Shim, E.; Jang, J.-P.; Moon, J.-J.; Kim, Y. Improvement of Polytetrafluoroethylene Membrane High-Efficiency Particulate Air Filter Performance with Melt-Blown Media. Polymers 2021, 13, 4067. [Google Scholar] [CrossRef] [PubMed]
- Ebnesajjad, S.; Khaladkar, P.R. Fluoropolymer Applications in the Chemical Processing Industries: The Definitive User’s Guide and Handbook; Elsevier Science: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Guerrero-Vaca, G.; Carrizo-Tejero, D.; Rodríguez-Alabanda, Ó.; Romero, P.E.; Molero, E. Experimental Study for the Stripping of PTFE Coatings on Al-Mg Substrates Using Dry Abrasive Materials. Materials 2020, 13, 799. [Google Scholar] [CrossRef] [Green Version]
- Olifirov, L.K.; Stepashkin, A.A.; Sherif, G.; Tcherdyntsev, V.V. Tribological, Mechanical and Thermal Properties of Fluorinated Ethylene Propylene Filled with Al-Cu-Cr Quasicrystals, Polytetrafluoroethylene, Synthetic Graphite and Carbon Black. Polymers 2021, 13, 781. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Correia, D.M.; Ribeiro, C.; Fernandes, M.M.; Lanceros-Méndez, S. Fluorinated Polymers as Smart Materials for Advanced Biomedical Applications. Polymers 2018, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Teng, H. Overview of the Development of the Fluoropolymer Industry. Appl. Sci. 2012, 2, 496–512. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Yan, F. Friction and Wear Behaviors of Several Polymers Sliding Against GCr15 and 316 Steel Under the Lubrication of Sea Water. Tribol. Lett. 2011, 42, 17–25. [Google Scholar] [CrossRef]
- Akram, W.; Rafique, A.F.; Maqsood, N.; Khan, A.; Badshah, S.; Khan, R.U. Khan Characterization of PTFE Film on 316L Stainless Steel Deposited through Spin Coating and Its Anticorrosion Performance in Multi Acidic Mediums. Materials 2020, 13, 388. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yuan, J.; Huang, J.; Ren, K.; Liu, Y.; Lu, S.; Li, H. Large-scale fabrication of superhydrophobic polyurethane/nano-Al2O3 coatings by suspension flame spraying for anti-corrosion applications. Appl. Surf. Sci. 2014, 311, 864–869. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Giacomelli, C.; Spinelli, A. Evaluation of the inhibitor effect of l-ascorbic acid on the corrosion of mild steel. Mater. Chem. Phys. 2004, 83, 129–134. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, J.; Shen, J.; Guo, S.; Lamnawar, K. Scratch behavior and mechanical properties of alternating multi-layered PMMA/PC materials. Wear 2021, 486–487, 204069. [Google Scholar] [CrossRef]
- Panin, S.V.; Luo, J.; Buslovich, D.G.; Alexenko, V.O.; Kornienko, L.A.; Bochkareva, S.A.; Byakov, A.V. Experimental—FEM Study on Effect of Tribological Load Conditions on Wear Resistance of Three-Component High-Strength Solid-Lubricant PI-Based Composites. Polymers 2021, 13, 2837. [Google Scholar] [CrossRef]
- Moon, S.W.; Seo, J.; Seo, J.-H.; Choi, B.-H. Scratch Properties of Clear Coat for Automotive Coating Comprising Molecular Necklace Crosslinkers with Silane Functional Groups for Various Environmental Factors. Polymers 2021, 13, 3933. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, K.; Dhiflaoui, H.; Ben Aissa, C.; Barhoumi, N.; Larbi, A.B.C. Friction and Wear Behavior of a Physical Vapor Deposition Coating Studied Using a Micro-Scratch Technique. J. Eng. Mater. Technol. 2022, 144, 1–26. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Mendibide, C.; Fontaine, J.; Steyer, P.; Esnouf, C. Dry Sliding Wear Model of Nanometer Scale Multilayered TiN/CrN PVD Hard Coatings. Tribol. Lett. 2004, 17, 779–789. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H. Water-induced shape memory behavior of poly (vinyl alcohol) and p-coumaric acid-modified water-soluble chitosan blended membrane. Carbohydr. Polym. 2021, 257, 117633. [Google Scholar] [CrossRef]
- Tcherdyntsev, V.V.; Olifirov, L.K.; Kaloshkin, S.D.; Zadorozhnyy, M.Y.; Danilov, V.D. Thermal and mechanical properties of fluorinated ethylene propylene and polyphenylene sulfide-based composites obtained by high-energy ball milling. J. Mater. Sci. 2018, 53, 13701–13712. [Google Scholar] [CrossRef]
- Sulen, W.L.; Ravi, K.; Bernard, C.; Ichikawa, Y.; Ogawa, K. Deposition Mechanism Analysis of Cold-Sprayed Fluoropolymer Coatings and Its Wettability Evaluation. J. Therm. Spray Technol. 2020, 29, 1643–1659. [Google Scholar] [CrossRef]
- Ordoñez, M.F.C.; Paruma, J.S.R.; Osorio, F.S.; Farias, M.C.M. The Effect of Counterpart Material on the Sliding Wear of TiAlN Coatings Deposited by Reactive Cathodic Pulverization. Sci. Cum Ind. 2015, 3, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Nemati, N.; Emamy, M.; Yau, S.; Kim, J.-K.; Kim, D.-E. High temperature friction and wear properties of graphene oxide/polytetrafluoroethylene composite coatings deposited on stainless steel. RSC Adv. 2016, 6, 5977–5987. [Google Scholar] [CrossRef]
- Wang, H.; Sun, A.; Qi, X.; Dong, Y.; Fan, B. Experimental and Analytical Investigations on Tribological Properties of PTFE/AP Composites. Polymers 2021, 13, 4295. [Google Scholar] [CrossRef]
- Sidebottom, M.A.; Pitenis, A.A.; Junk, C.P.; Kasprzak, D.J.; Blackman, G.S.; Burch, H.E.; Harris, K.L.; Sawyer, W.G.; Krick, B. Ultralow wear Perfluoroalkoxy (PFA) and alumina composites. Wear 2016, 362–363, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Ghosh, P.K.; Yadav, K.L.; Kumar, K. Thermo-mechanical and anti-corrosive properties of MWCNT/epoxy nanocomposite fabricated by innovative dispersion technique. Compos. Part B Eng. 2017, 113, 291–299. [Google Scholar] [CrossRef]
- Husain, E.; Nazeer, A.A.; Alsarraf, J.; Al-Awadi, K.; Murad, M.; Al-Naqi, A.; Shekeban, A. Corrosion behavior of AISI 316 stainless steel coated with modified fluoropolymer in marine condition. J. Coat. Technol. Res. 2018, 15, 945–955. [Google Scholar] [CrossRef]
- Delimi, A.; Galopin, E.; Coffinier, Y.; Pisarek, M.; Boukherroub, R.; Talhi, B.; Szunerits, S. Investigation of the cor-rosion behavior of carbon steel coated with fluoropolymer thin films. Surf. Coat. Technol. 2011, 205, 4011–4017. [Google Scholar] [CrossRef]
- Liang, J.; Azhar, U.; Men, P.; Chen, J.; Liu, Y.; He, J.; Geng, B. Fluoropolymer/SiO2 encapsulated aluminum pigments for enhanced corrosion protection. Appl. Surf. Sci. 2019, 487, 1000–1007. [Google Scholar] [CrossRef]
- Leivo, E.; Wilenius, T.; Kinos, T.; Vuoristo, P.; Mäntylä, T. Properties of thermally sprayed fluoropolymer PVDF, ECTFE, PFA and FEP coatings. Prog. Org. Coat. 2004, 49, 69–73. [Google Scholar] [CrossRef]




| Element | Fe | C | Si | Mn | P | S | N | Cr | Mo | Ni | Cu | Co |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wt (%) | 70.594 | 0.049 | 0.42 | 1.9 | 0.024 | 0.025 | 0.078 | 18.1 | 0.35 | 8.06 | 0.35 | 0.05 |
| Chemical Composition (wt%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| C | F | Si | S | Mn | Cr | Fe | Ni | |
| Track of 0.3 N | 21.10 | 78.90 | ||||||
| Track of 1 N | 20.09 | 77.62 | 0.125 | 0.015 | 0.40 | 1.75 | ||
| Track of 2 N | 14.45 | 63.56 | 0.80 | 0.020 | 0.38 | 4.90 | 14.3 | 1.59 |
| Track of 3 N | 10.58 | 54.6 | 0.86 | 0.02 | 0.46 | 6.86 | 24.17 | 2.45 |
| Immersion Time | Samples | Ecorr (mV) | Icorr (µA/cm−2) | Inhibition Efficiency (%) |
|---|---|---|---|---|
| 1 h | SS304 | −450 | 33.1 × 10−4 | - |
| FEP/SS304 | −240 | 1.58 × 10−4 | 95.22 | |
| 60 days | FEP/SS304 | −264 | 2.51 × 10−4 | 92.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barhoumi, N.; Khlifi, K.; Maazouz, A.; Lamnawar, K. Fluorinated Ethylene Propylene Coatings Deposited by a Spray Process: Mechanical Properties, Scratch and Wear Behavior. Polymers 2022, 14, 347. https://doi.org/10.3390/polym14020347
Barhoumi N, Khlifi K, Maazouz A, Lamnawar K. Fluorinated Ethylene Propylene Coatings Deposited by a Spray Process: Mechanical Properties, Scratch and Wear Behavior. Polymers. 2022; 14(2):347. https://doi.org/10.3390/polym14020347
Chicago/Turabian StyleBarhoumi, Najoua, Kaouther Khlifi, Abderrahim Maazouz, and Khalid Lamnawar. 2022. "Fluorinated Ethylene Propylene Coatings Deposited by a Spray Process: Mechanical Properties, Scratch and Wear Behavior" Polymers 14, no. 2: 347. https://doi.org/10.3390/polym14020347
APA StyleBarhoumi, N., Khlifi, K., Maazouz, A., & Lamnawar, K. (2022). Fluorinated Ethylene Propylene Coatings Deposited by a Spray Process: Mechanical Properties, Scratch and Wear Behavior. Polymers, 14(2), 347. https://doi.org/10.3390/polym14020347








