Comparative Characterization and Identification of Poly-3-hydroxybutyrate Producing Bacteria with Subsequent Optimization of Polymer Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Morphological and Biochemical Characterization of P(3HB) Positive Bacterial Isolates
2.2. Molecular Identification of Selected P(3HB) Producing Bacterial Isolates
2.3. Molecular Characterization of Selected Bacteria Producing P(3HB) by Amplification of the phbCGene
2.4. Screening of P(3HB) Producing Bacteria on a Light Microscope
2.5. Optimization of the Nutrient Medium and Cultivation Conditions
2.6. Extraction and Quantification of P(3HB) by Chemical Means
2.7. Obtaining an Effective Producer under Ultraviolet Irradiation
2.8. Analysis of Selected P(3HB) Producing Bacterial Isolates in Transmission Electron Microscope (TEM)
2.9. Extraction of P(3HB) from Biomass
2.10. Fourier Transformn Infrared (FTIR) Characterization of Extracted P(3HB)
3. Results and Discussion
3.1. Characterization of Selected Bacteria
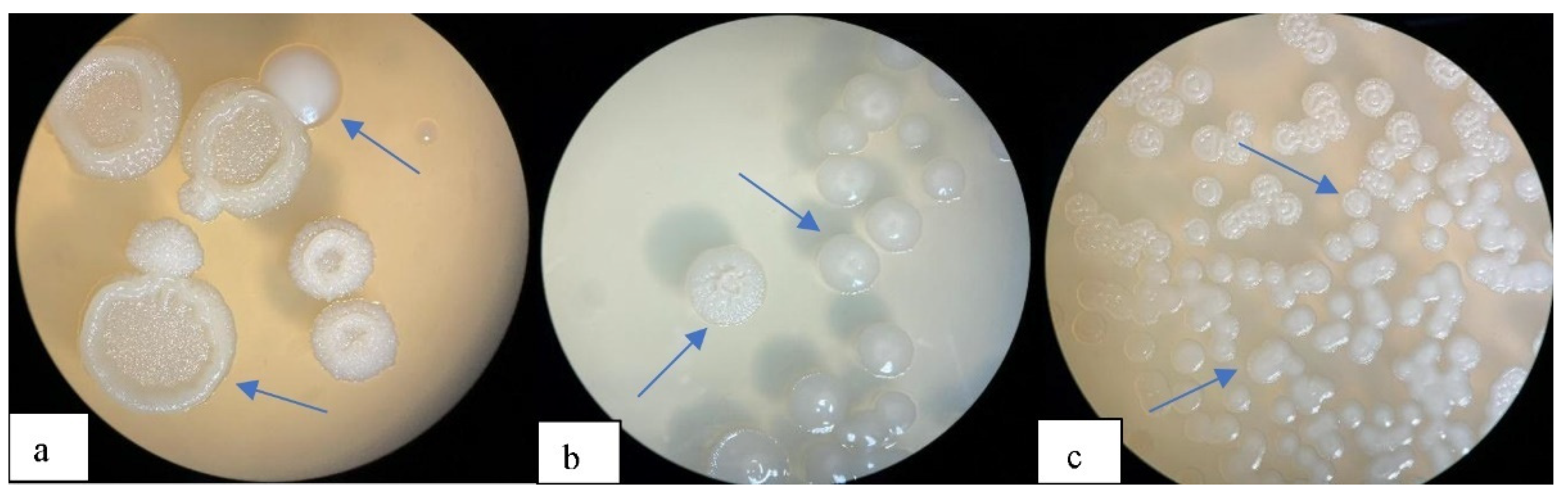
3.1.1. Morphological and Biochemical Characterization of P(3HB) Positive Bacterial Isolates
3.1.2. The Effect of the Concentration of Bean Broth on the Concentration of P(3HB) during Cultivation in Flasks
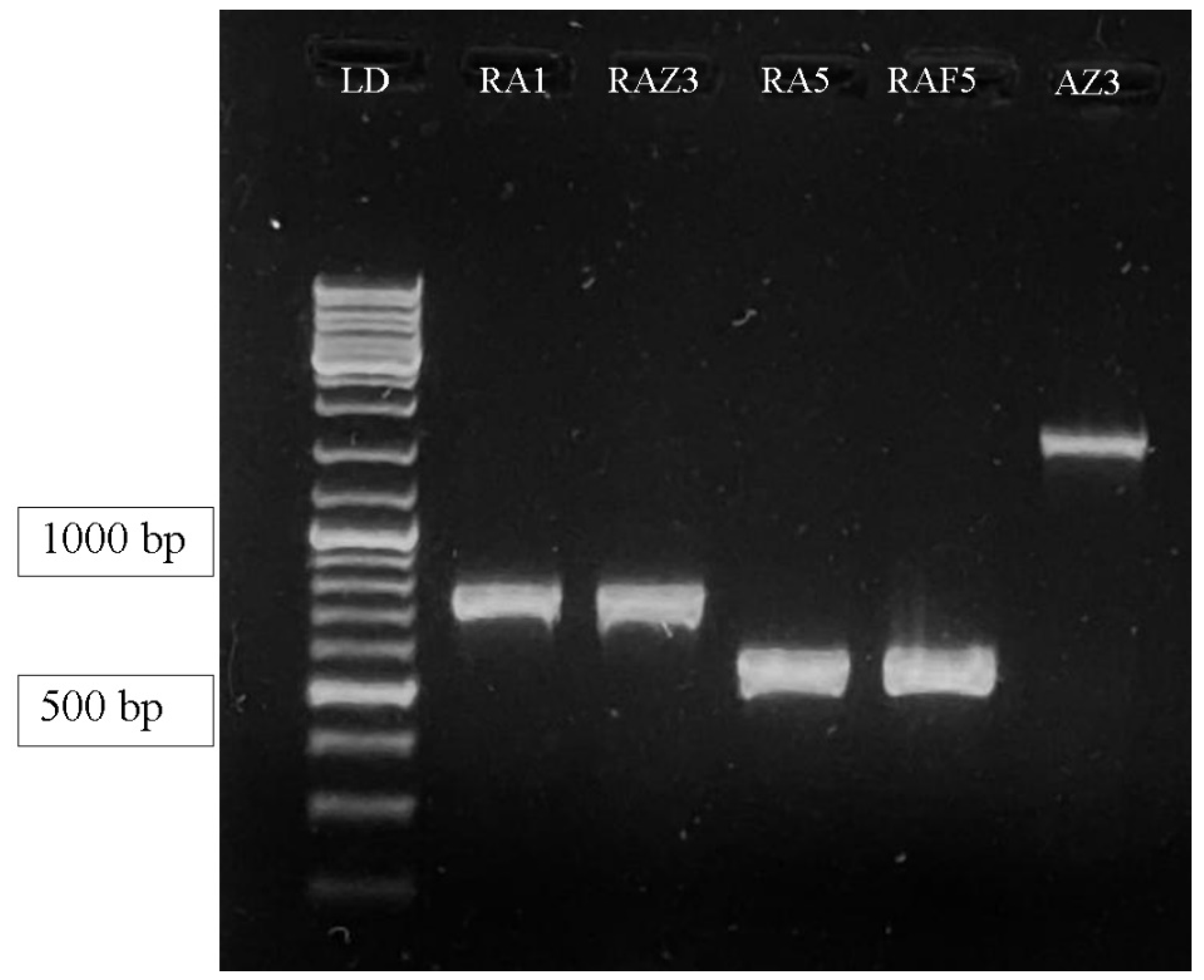
3.1.3. The Molecular Identification of Selected P(3HB) Positive Bacterial Isolates
3.1.4. Molecular Characterization of Selected P(3HB) Producing Isolates
3.1.5. Transmission Electron Microscopy (TEM) Analysis of Selected Bacterial Isolates Producing P(3HB)
3.2. Characterization of Film
3.2.1. The Melting Point of the P(3HB) Samples
3.2.2. FTIR Characterization of PHAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bengali, S. The COVID-19 Pandemic Is Unleashing a Tidal Wave of Plastic Waste. Los Angeles Times. 13 June 2020. Available online: https://www.latimes.com/world-nation/story/2020-06-13/coronavirus-pandemic-plastic-waste-recycling (accessed on 18 November 2020).
- Livia, A.-R. The ‘Great Pacific Garbage Patch’ Is Ballooning, 87,000 Tons of Plastic and Counting”. The New York Times, 22 March 2018; ISSN 0362-4331. [Google Scholar]
- UNCTAD. Post—COVID-19: Investment Promotion Agencies and the “New Normal”. Available online: https://unctad.org/system/files/official-document/diaepcbinf2020d5_en.pdf (accessed on 18 November 2020).
- Masanov, A.Y. Biodegradable Plastics: Current State of Markets and Prospects. Available online: http://vestkhimprom.ru/posts/biorazlagaemye-plastiki-tekushchee-sostoyanie-rynkov-i-perspektivy (accessed on 7 October 2017).
- United Nations Environment Programme (UNEP). Neglected—Environmental Justice Impacts of Marine Litter and Plastic Pollution; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2021; ISBN 978-92-807-3852-0. [Google Scholar]
- Page, W.J. Bacterial polyhydroxyalkanoates, natural biodegradable plastics with a great future. Can. J. Microbiol. 1995, 41, 1–3. [Google Scholar] [CrossRef]
- Cesário, M.T.; Raposo, R.S.; de Almeida, M.C.M.D.; van Keulen, F.; Ferreira, B.S.; da Fonseca, M.M.R. Enhanced bioproduction of poly-3-hydroxybutyrate from wheat straw lignocellulosic hydrolysates. New Biotechnol. 2014, 31, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrom, D. Polymer synthesis by microorganisms: Technology and economics. Trends Biotechnol. 1987, 5, 246–250. [Google Scholar] [CrossRef]
- Mongili, B.; Abdel Azim, A.; Fraterrigo Garofalo, S.; Batuecas, E.; Re, A.; Bocchini, S.; Fino, D. Novel insights in dimethyl carbonate-based extraction of polyhydroxybutyrate (P(3HB)). Biotechnol. Biofuels 2021, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Sedlacek, P.; Mravec, F.; Krzyzanek, V.; Nebesarova, J.; Samek, P.; Benesova, K.; Hrubanova, M.; Milerova, O.; Marova, I. The presence of PHB granules in cytoplasm protects non-halophilic bacterial cells against the harmful impact of hypertonic environments. New Biotechnol. 2017, 39, 68–80. [Google Scholar] [CrossRef]
- Kadouri, D.; Jurkevitch, E.; Okon, Y. Involvement of the reserve material poly-beta-hydroxybutyrate in Azospirillumbrasilense stress endurance and root colonization. Appl. Environ. Microbiol. 2003, 69, 3244–3250. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.K.; Lee, S.Y.; Tam, T.T. Towards Systems Metabolic Engineering of PHA Producers. In Plastics from Bacteria; Microbiology Monographs; Chen, G.Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 14. [Google Scholar] [CrossRef]
- Livshits, V.A.; Bonartsev, A.P.; Iordanskii, A.L.; Ivanov, E.A.; Makhina, T.A.; Myshkina, V.L.; Bonartseva, G.A. Microspheres Based on poly(3-hydroxy) butyrate for prolonged drug release. Polym. Sci. Ser. B 2009, 51, 256–263. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Bonartseva, G.A.; Voinova, V.V.; Kirpichnikov, M.P.; Shaitan, K.V. Bulletin of the Russian State Medical University. Scimago J. Ctry. Rank 2018, 6, 130–134. [Google Scholar]
- Bonartsev, A.P.; Shaitan, K.V.; Kirpichnikov, M.P.; Bonartseva, G.A. Biomedical Chemistry Series B. Biochem. Suppl. 2011, 5, 10–21. [Google Scholar]
- Holt, J.G.; Krieg, N.R.; Sneath, P.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; Williamsons and Wilkins: Baltimore, MA, USA, 1994; pp. 45–68. [Google Scholar]
- Tepper, E.Z.; Shilnikova, V.K.; Pereverzeva, G.I. Practicum on Microbiology; Shilnikova, V.K., Ed.; Bustard: Moscow, Russia, 2004; p. 256. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSIBLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legat, A.; Gruber, C.; Zangger, K.; Wanner, G.; Stan-Lotter, H. Identification of polyhydroxyalkanoates in Halococcus and other haloarchaeal species. Appl. Microbiol. Biotechnol. 2010, 87, 1119–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lathwal, P.; Nehra, K.; Singh, M.; Rana, J.S. Characterization of Novel and Efficient Poly-3-hydroxybutyrate (P(3HB)) Producing Bacteria Isolated from Rhizospheric Soils. J. Polym. Environ. 2018, 26, 1–14. [Google Scholar] [CrossRef]
- Williamson, D.H.; Wilkinson, J.F. The isolation and estimation of the poly-β-hydroxy- butyrate inclusions of Bacillus species. J. Gen. Microbiol. 1958, 19, 198–209. [Google Scholar] [CrossRef] [Green Version]
- Law, J.H.; Slepecky, R.A. Assay of polybetahydroxybutyric acid. J. Bacteriol. 1961, 82, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Huang, H.; Hu, G.; Chen, J.; Ho, K.P.; Chen, G. Production of poly-3-hydroxybutyrate by Bacillus sp. JMa5 cultivated in molasses media. Antonie Van Leeuwenhoek 2001, 80, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Aslım, B.; Çalışkan, F.; Beyatlı, Y.; Gündüz, U. Poly-β-hydroxybutyrate production by lactic acid bacteria. FEMS Microbiol. Lett. 1998, 159, 293–297. [Google Scholar] [CrossRef]
- Kosmachevskaya, O.V.; Osipova, E.V.; Van Chib, T.; Tuyet, P.T.; Topunov, A.F. The effect of cultivation conditions on the synthesis of Poly-3-hydroxybutyrate by nodule bacteria Rhizobium phaseoli. Appl. Biochem. Microbiol. 2020, 56, 60–68. [Google Scholar] [CrossRef]
- Bhuwal, A.K.; Singh, G.; Aggarwa, L.N.K.; Goyal, V.; Yadav, A. Poly-β-hydroxybutyrate production and management of cardboard industry effluent by new Bacillus sp. NA10. Bioresour. Bioprocess. 2014, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, S.; Mohanta, P.R.; Sarkar, B.; Davar, A.; Kumar, K.; Samantarai, D.P. Production of polyhydroxyalkanoates (PHA) by Bacillus strain isolated from wastewater and its biochemical characteristics. Proc. Natl. Acad. Sci. India Sec. B Biol. Sci. 2015, 87, 459–466. [Google Scholar] [CrossRef]
- Schlegel, H.G.; Gottschalk, G.; Von Bartha, R. Formation and utilization of poly-β-hydroxybutyric acid by knallgas bacteria (Hydrogenomonas). Nature 1961, 191, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.; Woldesenbet, F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low-cost agricultural waste material. BMC Res. Notes 2016, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehm, B.H. Polyester synthases: Natural catalysts for plastics. Biochem. J. 2003, 37, 15–33. [Google Scholar] [CrossRef] [Green Version]
- Qi, Q.; Rehm, B.H.A. Polyhydroxybutyrate biosynthesis in Caulobacter crescentus: Molecular characterization of the polyhydroxybutyrate synthase. Microbiology 2001, 147, 3353–3358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kung, S.S.; Chuang, Y.C.; Chen, C.H.; Chien, C.C. Isolation of polyhydroxyalkanoates-producing bacteria using a combination of phenotypic and genotypic approach. Lett. Appl. Microbiol. 2007, 44, 364–371. [Google Scholar] [CrossRef]
- Apparao, U.; Krishnaswamy, V.G. Production of polyhydroxyalkanoate (PHA) by a moderately halotolerant bacterium Klebsiella pneumonia isolated from rubber plantation area. Int. J. Environ. Bioremed. Biodegrad. 2015, 3, 54–61. [Google Scholar] [CrossRef]
- Łabuzek, S.; Radecka, I. Biosynthesis of PHB tercopolymer by Bacillus cereus UW85. J. Appl. Microbiol. 2001, 90, 353–357. [Google Scholar] [CrossRef]
- Singh, S.; Sithole, B.; Lekha, P.; Permaul, K.; Govinden, R. Optimization of cultivation medium and cyclic fed-batch fermentation strategy for enhanced polyhydroxyalkanoate production by Bacillus thuringiensis using a glucose-rich hydrolyzate. Bioresour. Bioprocess. 2021, 8, 11. [Google Scholar] [CrossRef]





| P(3HB) Primers | Sequence | GC Content (%) |
|---|---|---|
| BaRv | 5′TCACCGGCATTGCCATT ACCATTACCATTTCCG 3′ | 72 |
| BaFw | 5′GTAACAGGCGGATCT AAAGGTATCGGGG 3′ | 70 |
| AzRv | 5′ TCAACCCTTTTACGTA GCGTCCTGGTGCAG 3′ | 69 |
| AzFw | 5′ATGGATCAAGCCACCT CCTTCGCAAGTTTCTG 3′ | 74 |
| BmRv | 5′TCAGCAACCCACTTTT GCATTAGCTTCCAG 3′ | 71 |
| BmFw | 5′GTGGCAATTCCTTAC GTGCAAGAGTGGG 3′ | 72 |
| Strains | Color Sudanese Black | P(3HB) Yield (mg/mL) |
|---|---|---|
| Ra 1 | +++ * | 108.6 ± 0.88 |
| Ra 2 | +++ | 109.3 ± 0.87 |
| Ra 3 | ++- | 14.0 ± 0.17 |
| Ra 4 | +++ | 108.0 ± 0.89 |
| Ra 5 | +++ | 108.6 ± 0.88 |
| Ra 6 | +++ | 93.6 ± 0.72 |
| Ra 7 | ++- | 8.36 ± 0.57 |
| Ra1(2) | +-- | 08.0 ± 0.02 |
| Ra 1(1) | ++- | 108.6 ± 0.88 |
| AZ 3 | ++- | 138.3 ± 0.83 |
| The Content of Bean Decoction in a Mineral Environment, (g·L−1) | DCW*, (g·L−1) | P(3HB), (g·L−1) | Yield Specific P(3HB), (%) | Biomass Productivity, (g·L−1h−1) | P(3HB) Productivity, (g·L−1h−1) |
|---|---|---|---|---|---|
| 0 | 5 | 0.71 ± 0.02 | 14.20 | 0.104 | 0.015 |
| 5.0 | 7.44 | 1.05 ± 0.07 | 14.11 | 0.155 | 0.022 |
| 20.0 | 25.73 | 13.83 ± 0.83 | 53.75 | 0.536 | 0.288 |
| 35.0 | 26.14 | 13.95 ± 0.20 | 53.37 | 0.545 | 0.291 |
| 40.0 | 28.34 | 14.78 ± 0.35 | 52.15 | 0.590 | 0.308 |
| Strain | Strain Number http://www.ncbi. Accessed 20 April 2020 | Name of the Strain | Similarity, % |
|---|---|---|---|
| AZ 3 | MH763851.1 | Azotobacter chrocococcum | 99.25 |
| RAZ 3 | KT441079.1 | Bacillus megaterium | 100 |
| Ra 5 | LC430037.1 KT441079.1 | Bacillus aryabhattai | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rysbek, A.; Ramankulov, Y.; Kurmanbayev, A.; Richert, A.; Abeldenov, S. Comparative Characterization and Identification of Poly-3-hydroxybutyrate Producing Bacteria with Subsequent Optimization of Polymer Yield. Polymers 2022, 14, 335. https://doi.org/10.3390/polym14020335
Rysbek A, Ramankulov Y, Kurmanbayev A, Richert A, Abeldenov S. Comparative Characterization and Identification of Poly-3-hydroxybutyrate Producing Bacteria with Subsequent Optimization of Polymer Yield. Polymers. 2022; 14(2):335. https://doi.org/10.3390/polym14020335
Chicago/Turabian StyleRysbek, Aidana, Yerlan Ramankulov, Askar Kurmanbayev, Agnieszka Richert, and Sailau Abeldenov. 2022. "Comparative Characterization and Identification of Poly-3-hydroxybutyrate Producing Bacteria with Subsequent Optimization of Polymer Yield" Polymers 14, no. 2: 335. https://doi.org/10.3390/polym14020335
APA StyleRysbek, A., Ramankulov, Y., Kurmanbayev, A., Richert, A., & Abeldenov, S. (2022). Comparative Characterization and Identification of Poly-3-hydroxybutyrate Producing Bacteria with Subsequent Optimization of Polymer Yield. Polymers, 14(2), 335. https://doi.org/10.3390/polym14020335







