Significantly Enhanced Crystallization of Poly(ethylene succinate-co-1,2-propylene succinate) by Cellulose Nanocrystals as an Efficient Nucleating Agent
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
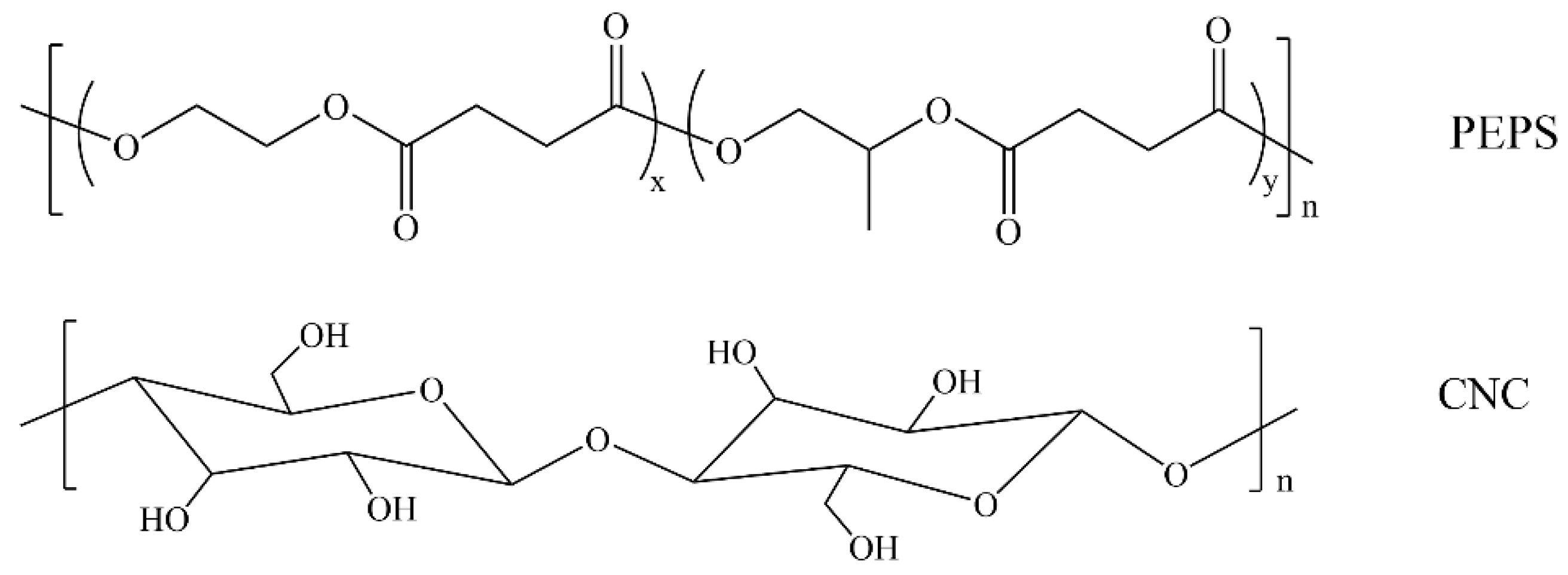
2.2. Preparation of PEPS/CNC Composites
2.3. Characterizations
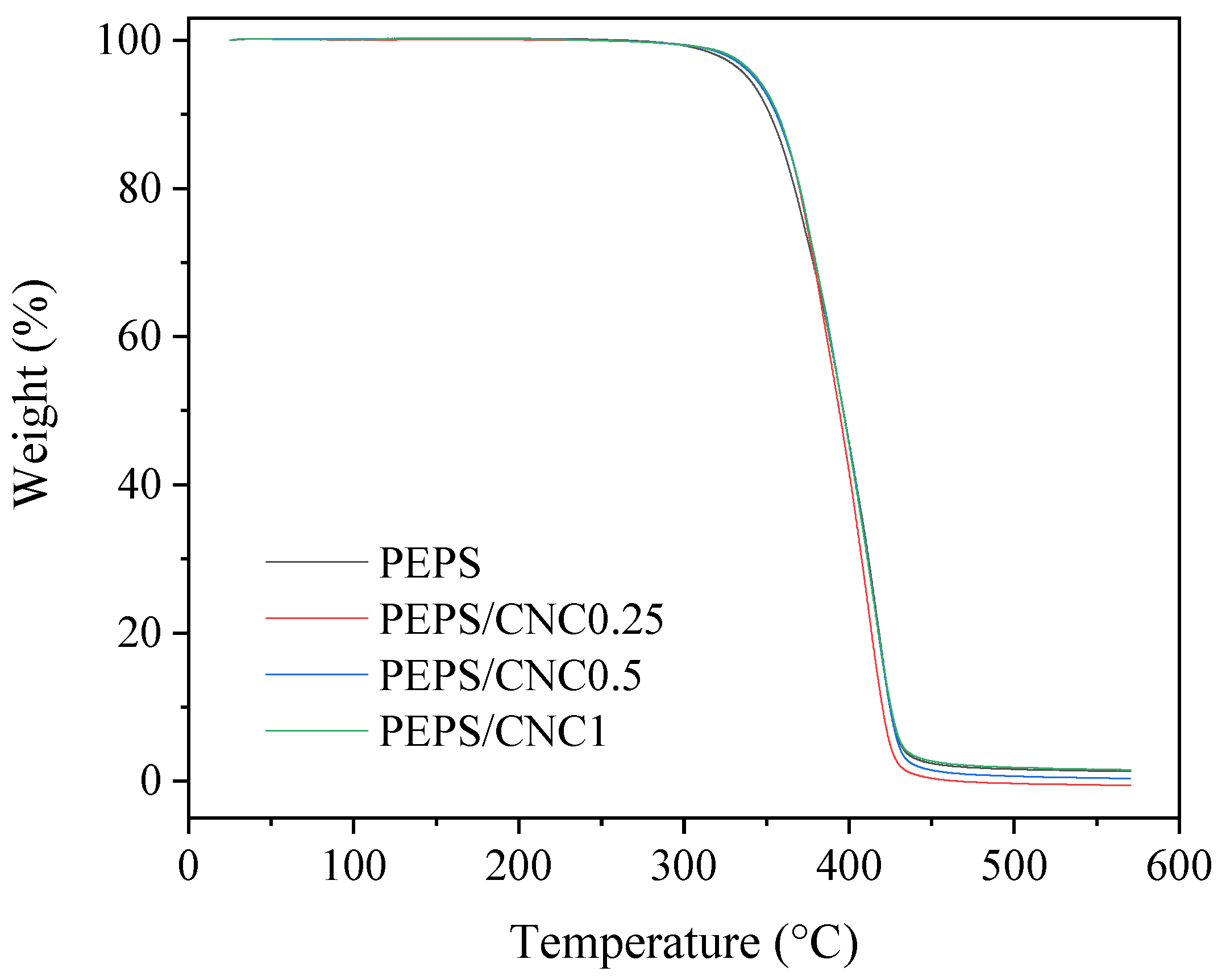
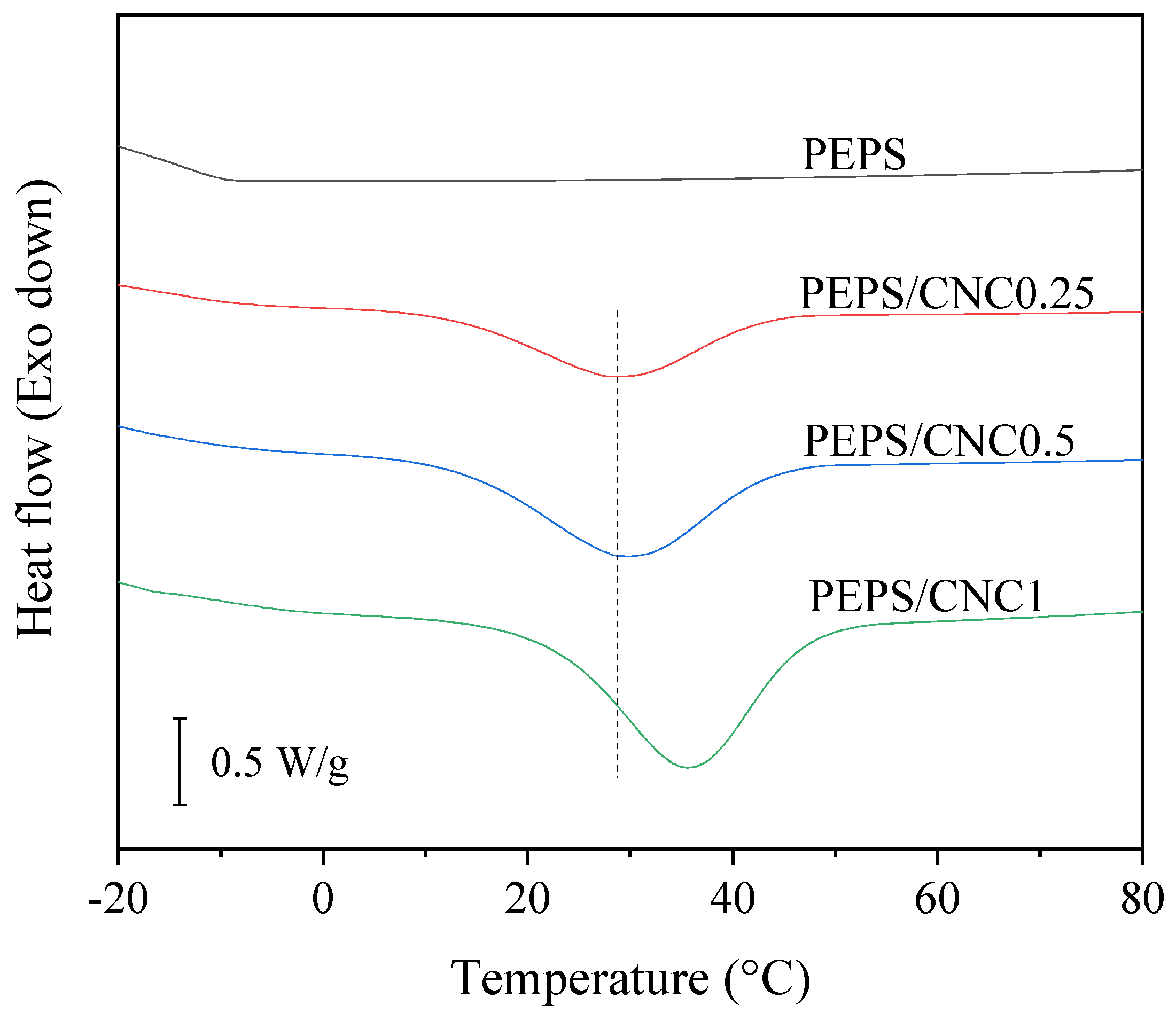
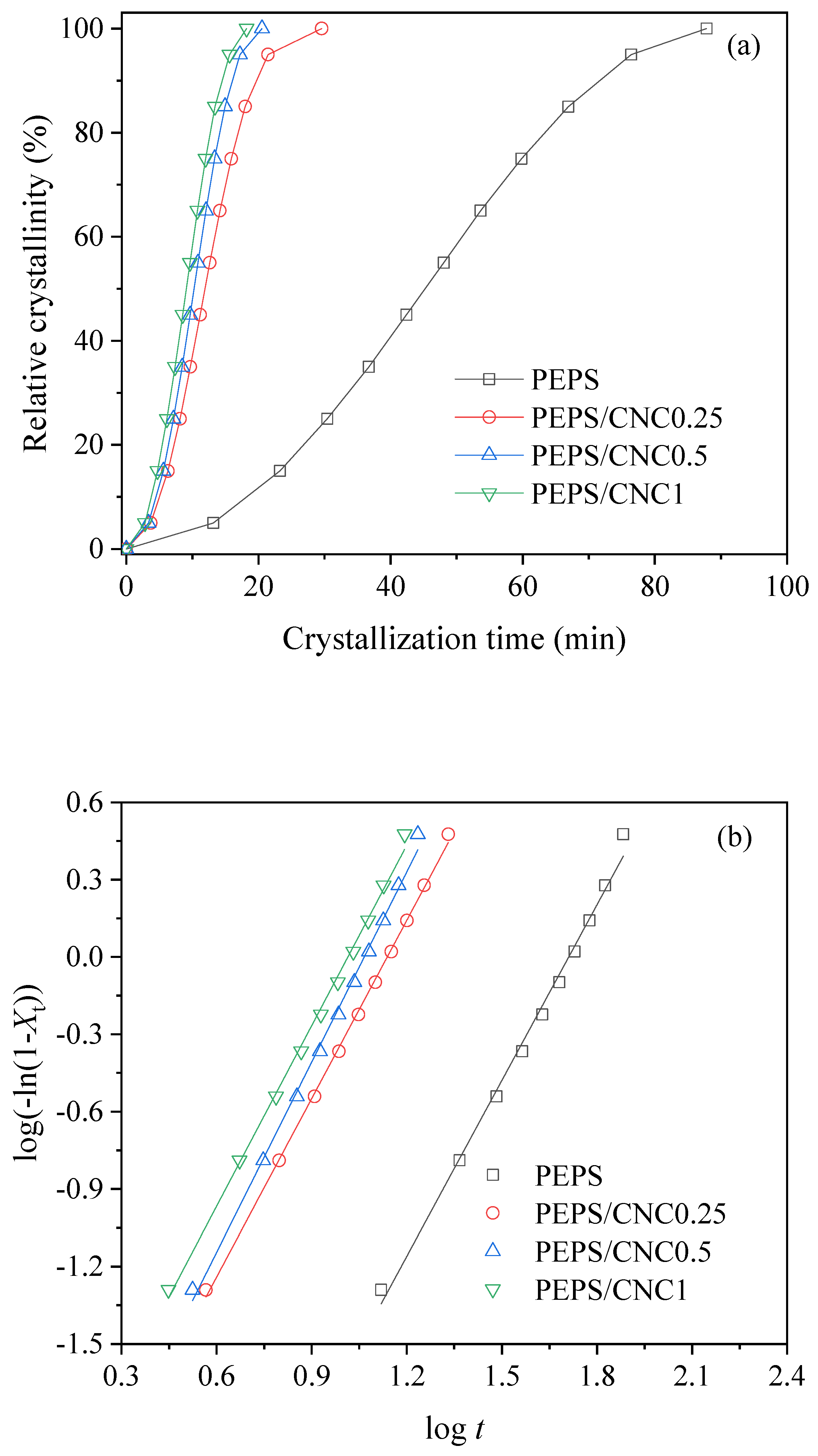
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rhim, J.; Park, H.; Ha, C. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Reddy, M.; Vivekanandhan, S.; Misra, M.; Bhatia, S. Mohanty, Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Tadahisa, I. Biodegradable and biobased polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Mohanty, A.; Vivekanandhan, S.; Pin, J.; Misra, M. Composites from renewable and sustainable resources: Challenges and innovations. Science 2018, 362, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.S.; Makhatha, M.E. Thermal properties of poly(ethylene succinate) nanocomposite. Polymer 2009, 50, 4635–4643. [Google Scholar]
- Wang, H.; Qiu, Z. Crystallization kinetics and morphology of biodegradable poly(L-lactic acid)/graphene oxide nanocomposites: Influences of graphene oxide loading and crystallization temperature. Thermochim. Acta 2012, 527, 40–46. [Google Scholar] [CrossRef]
- Vasileiou, A.A.; Papageorgiou, G.A.; Kontopoulou, M.; Docoslis, A.; Bikiaris, D. Covalently bonded poly (ethylene succinate)/SiO2 nanocomposites prepared by in situ polymerization. Polymer 2013, 54, 1018–1032. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Terzopoulou, Z.; Achilias, D.S.; Bikiaris, D.N.; Kapnisti, M.; Gournis, D. Biodegradable poly(ethylene succinate) nanocomposites. Effect of filler type on thermal behaviour and crystallization kinetics. Polymer 2013, 54, 4604–4616. [Google Scholar] [CrossRef]
- Jing, X.; Qiu, Z. Influence of thermally reduced graphene low-loadings on the crystallization behavior and morphology of biodegradable poly(ethylene succinate). Ind. Eng. Chem. Res. 2014, 53, 498–504. [Google Scholar] [CrossRef]
- Asadi, V.; Jafari, S.H.; Khonakdar, A.H.; Häuβler, L.; Wagenknecht, U. Incorporation of inorganic fullerene-like WS2 into poly(ethylene succinate) to prepare novel biodegradable nanocomposites: A study on isothermal and dynamic crystallization. RSC Adv. 2016, 6, 4925–4935. [Google Scholar] [CrossRef]
- Teng, S.; Qiu, Z. Effect of different POSS structures on the crystallization behavior and dynamic mechanical properties of biodegradable poly(ethylene succinate). Polymer 2019, 163, 68–73. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, Z. Effect of methyl as the simplest C−H side group on the significant variation of physical properties of biodegradable poly(ethylene succinate). Polym. Test. 2020, 90, 106755. [Google Scholar] [CrossRef]
- Lu, H.; Lu, S.; Chen, M.M. Characterization, crystallization kinetics, and melting behavior of poly(ethylene succinate) copolyester containing 7 mol% butylene succinate. J. Appl. Polym. Sci. 2009, 113, 876–886. [Google Scholar] [CrossRef]
- Chen, M.; Chang, W.; Lu, H. Characterization, crystallization kinetics and melting behavior of poly(ethylene succinate) copolyester containing 5 mol% trimethylene succinate. Polymer 2007, 48, 5408–5416. [Google Scholar] [CrossRef]
- Li, X.; Qiu, Z. Crystallization kinetics, morphology, and mechanical properties of novel poly(ethylene succinate-co-octamethylene succinate). Polym. Test. 2015, 48, 125–132. [Google Scholar] [CrossRef]
- Li, X.; Qiu, Z. Synthesis and properties of novel poly(ethylene succinate-co-decamethylene succinate) copolymers. RSC Adv. 2015, 5, 103713–103721. [Google Scholar] [CrossRef]
- Wu, H.; Qiu, Z. Synthesis, crystallization kinetics and morphology of novel poly(ethylene succinate-co-ethylene adipate) copolymer. CrystEngComm 2012, 14, 3586–3595. [Google Scholar] [CrossRef]
- Qiu, S.; Su, Z.; Qiu, Z. Crystallization kinetics, morphology and mechanical properties of novel biodegradable poly(ethylene succinate-co-ethylene suberate) copolyesters. Ind. Eng. Chem. Res. 2016, 55, 10286–10293. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, K.; Su, Z.; Qiu, Z. Thermal behavior, mechanical and rheological properties, and hydrolytic degradation of novel branched biodegradable poly(ethylene succinate) copolymers. Polym. Test. 2018, 66, 64–69. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, Z. Miscibility and crystallization behavior of novel branched poly(ethylene succinate)/poly(vinyl phenol) blends, Chinese. J. Polym. Sci. 2019, 37, 1169–1175. [Google Scholar]
- Zhang, K.; Qiu, Z. Effect of cyanuric acid as an efficient nucleating agent on the crystallization of novel biodegradable branched poly(ethylene succinate). Macromol 2021, 1, 112–120. [Google Scholar] [CrossRef]
- Zhang, K.; Jiang, Z.; Qiu, Z. Effect of different lengths of side groups on the thermal, crystallization and mechanical properties of novel biodegradable poly(ethylene succinate) copolymers. Polym. Degrad. Stabil. 2021, 187, 109542. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.; Dufresne, A.; Pinheiro, I.; Souza, D.; Gouveia, R.; Mei, L.; Lona, L. How do cellulose nanocrystals affect the overall properties of biodegradable polymer nanocomposites: A comprehensive review. Eur. Polym. J. 2018, 108, 274–285. [Google Scholar] [CrossRef]
- Younas, M.; Noreen, A.; Sharif, A.; Majeed, A.; Hassan, A.; Tabasum, S.; Mohammadi, K.; Zia, K.M. A review on versatile applications of blends and composites of CNC with natural and synthetic polymers with mathematical modeling. Int. J. Biol. Macromol. 2019, 124, 591–626. [Google Scholar] [CrossRef]
- Calvino, C.; Macke, N.; Kato, R.; Rowan, S. Development, processing and applications of bio-sourced cellulose nanocrystal composites. Prog. Polym. Sci. 2020, 103, 101221. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Yu, Y.; Xiao, L. Effect of loadings of nanocellulose on the significantly improved crystallization and mechanical properties of biodegradable poly(ε-caprolactone). Int. J. Biol. Macromol. 2020, 147, 34–45. [Google Scholar] [CrossRef]
- Kamal, M.; Khoshkava, V. Effect of cellulose nanocrystals (CNC) on rheological and mechanical properties and crystallization behavior of PLA/CNC nanocomposites. Carbohyd. Polym. 2015, 123, 105–114. [Google Scholar] [CrossRef]
- Xu, C.; Lv, Q.; Wu, D.; Wang, Z. Polylactide/cellulose nanocrystal composites: A comparative study on cold and melt crystallization. Cellulose 2017, 24, 2163–2175. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Z. Significantly enhanced crystallization of poly(L-lactide) by the synergistic effect of poly(diethylene glycol adipate) and cellulose nanocrystals in their fully biodegradable ternary composite. Ind. Eng. Chem. Res. 2019, 58, 15526–15532. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Q.; Wang, M.; Zeng, J. Morphology, crystallization and rheological behavior in poly(butylene succinate)/cellulose nanocrystal nanocomposites fabricated by solution coagulation. Carbohyd. Polym. 2017, 164, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Clarke, A.; Vasileiou, A.; Kontopoulou, M. Crystalline nanocellulose/thermoplastic polyester composites prepared by in situ polymerization. Polym. Eng. Sci. 2019, 59, 989–995. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.; Qiu, Z. Thermal and rheological properties of fully biodegradable poly(ethylene succinate)/cellulose nanocrystals composites. Compos. Commun. 2021, 23, 100571. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.; Qiu, Z. Isothermal melt crystallization kinetics study of cellulose nanocrystals nucleated biodegradable poly(ethylene succinate). Polymer 2021, 227, 123869. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Z. Nonisothermal melt crystallization study of poly(ethylene succinate)/cellulose nanocrystals composites. J. Polym. Environ. 2021. [Google Scholar] [CrossRef]
- Pan, S.; Qiu, Z. Fully biodegradable poly(hexamethylene succinate)/cellulose nanocrystals composites with enhanced crystallization rate and mechanical property. Polymers 2021, 13, 3667. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Z. Effect of low loadings of cellulose nanocrystals on the significantly enhanced crystallization of biodegradable poly(butylene succinate-co-butylene adipate). Carbohyd. Polym. 2019, 205, 211–216. [Google Scholar] [CrossRef]
- Li, J.; Qiu, Z. Influence of two different nanofillers on the crystallization behavior and dynamic mechanical properties of biodegradable poly(ethylene adipate). J. Polym. Environ. 2019, 27, 2674–2681. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, phase change, and microstructure kinetics of phase change III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Teng, S.; Jiang, Z.; Qiu, Z. Crystallization behavior and dynamic mechanical properties of poly(ε-caprolactone)/octaisobutyl-polyhedral oligomeric silsesquioxanes composites prepared via different methods. Chin. J. Polym. Sci. 2020, 38, 158–163. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, K.; Jiang, Z.; Qiu, Z. Synthesis and characterization of novel poly(butylene succinate)-b-poly(diethylene glycol terephthalate) multiblock copolyesters with high melting point and significantly improved mechanical property. Polymer 2021, 232, 124151. [Google Scholar] [CrossRef]
- Yang, F.; Qiu, Z. Preparation, crystallization and properties of biodegradable poly(butylene adipate-co-terephthalate)/organo-modified montmorillonite nanocomposites. J. Appl. Polym. Sci. 2011, 119, 1426–1434. [Google Scholar] [CrossRef]
- Xu, C.; Qiu, Z. Crystallization behavior and thermal property of biodegradable poly(3-hydroxybutyrate)/multi-walled carbon nanotubes nanocomposite. Polym. Adv. Technol. 2011, 22, 538–544. [Google Scholar] [CrossRef]
- Ueda, A.S.; Chatani, Y.; Tadokoro, H. Structure studies of polyesters. IV. Molecular and crystal structure of poly(ethylene succinate) and poly(ethylene oxalate). Polym. J. 1971, 2, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Wu, S. Polymer Interface and Adhesion; Marcel Dekker: New York, NY, USA, 1982. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, J.; Yu, J. Surface acetylation of cellulose nanocrystal and its reinforcing function in poly(lactic acid). Carbohyd. Polym. 2011, 83, 1834–1842. [Google Scholar] [CrossRef]
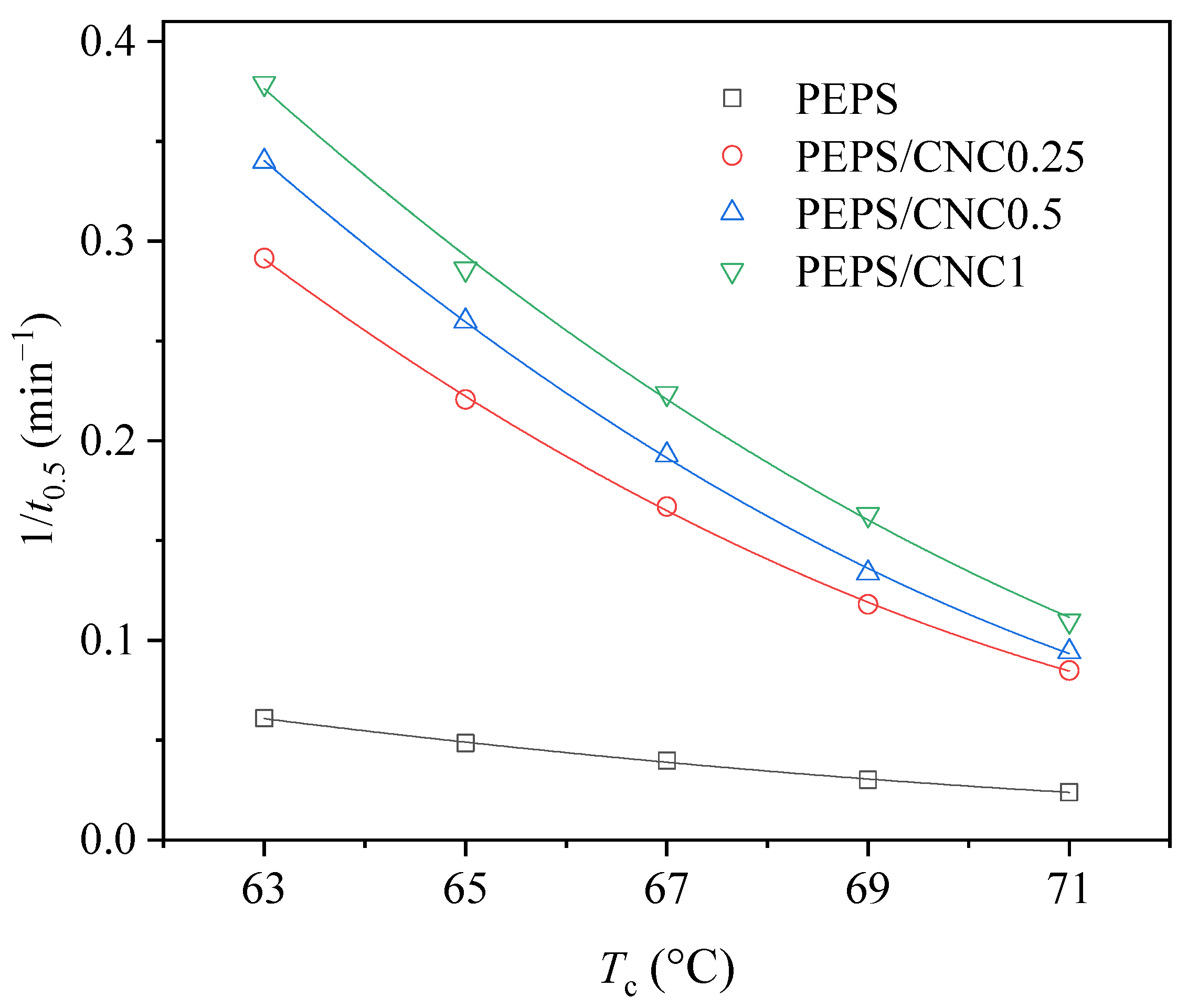
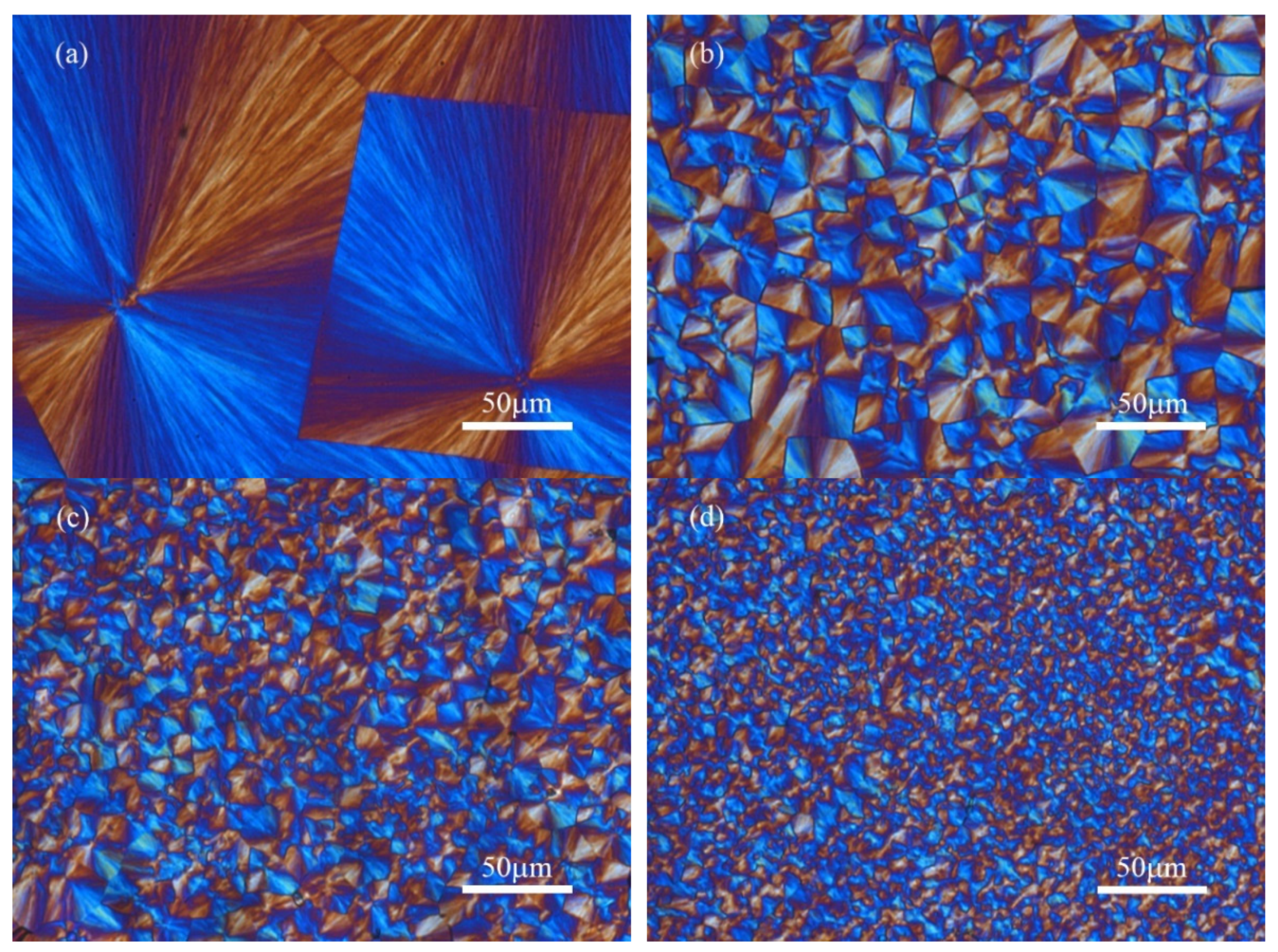
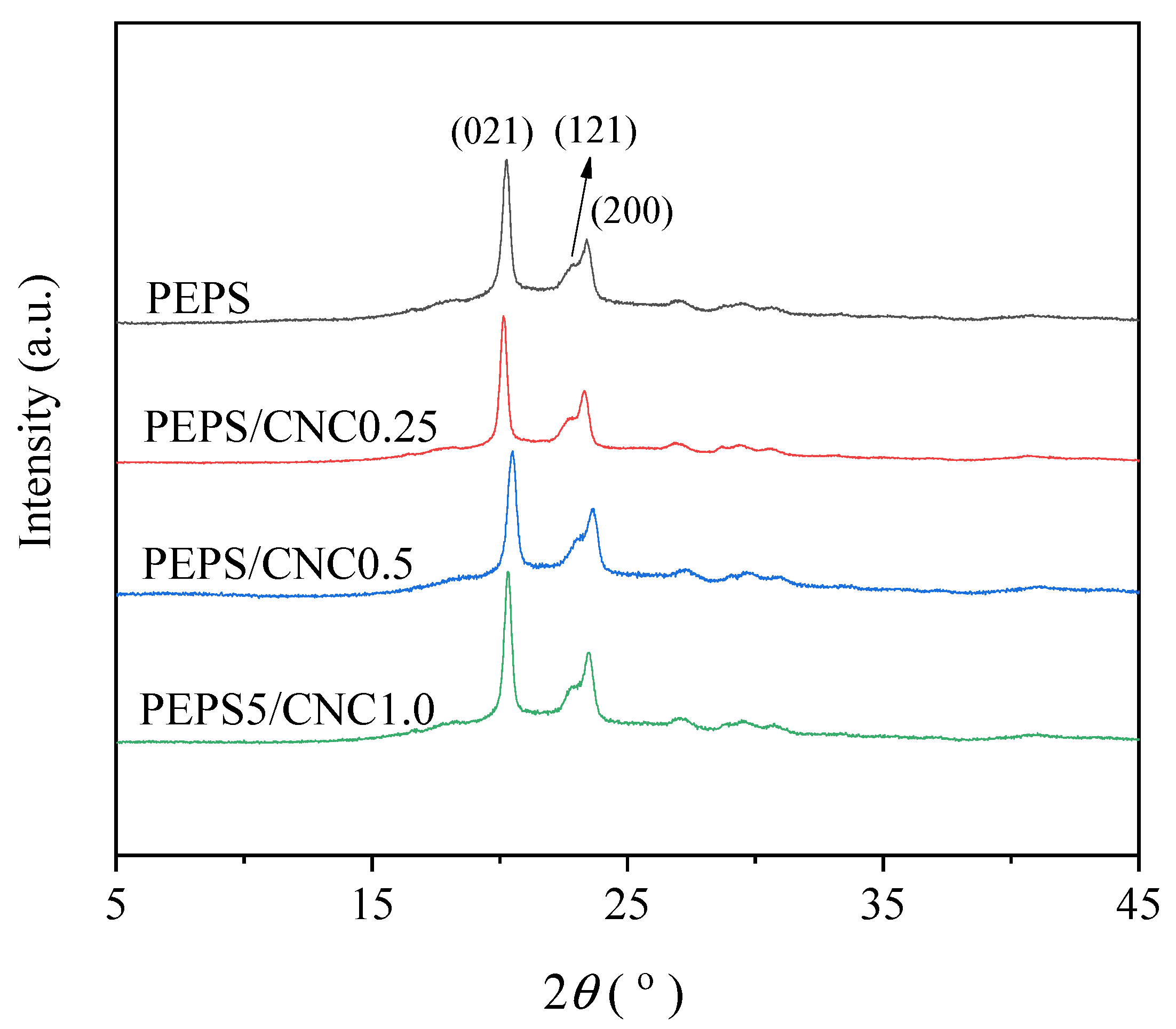
| Samples | Tc (°C) | n | k (min−n) | t0.5 (min) | t1/2 (min) |
|---|---|---|---|---|---|
| PEPS | 63 | 2.1 | 1.94 × 10−3 | 16.4 | 16.0 |
| 65 | 2.2 | 8.90 × 10−4 | 20.6 | 20.0 | |
| 67 | 2.2 | 5.71 × 10−4 | 25.2 | 26.7 | |
| 69 | 2.2 | 3.10 × 10−4 | 33.3 | 32.1 | |
| 71 | 2.3 | 1.30 × 10−4 | 41.8 | 45.1 | |
| PEPS/CNC0.25 | 63 | 2.6 | 2.81 × 10−2 | 3.4 | 3.5 |
| 65 | 2.5 | 2.30 × 10−2 | 3.9 | 4.2 | |
| 67 | 2.5 | 7.90 × 10−3 | 6.0 | 6.2 | |
| 69 | 2.4 | 6.37 × 10−3 | 7.1 | 8.4 | |
| 71 | 2.3 | 2.38 × 10−3 | 11.8 | 11.8 | |
| PEPS/CNC0.5 | 63 | 2.6 | 4.19 × 10−2 | 2.9 | 2.9 |
| 65 | 2.7 | 1.82 × 10−2 | 3.8 | 3.9 | |
| 67 | 2.6 | 8.43 × 10−3 | 5.5 | 5.3 | |
| 69 | 2.4 | 7.30 × 10−3 | 6.7 | 7.1 | |
| 71 | 2.4 | 2.39 × 10−3 | 10.6 | 10.2 | |
| PEPS/CNC1 | 63 | 2.8 | 3.72 × 10−2 | 2.8 | 2.7 |
| 65 | 2.6 | 2.68 × 10−2 | 3.5 | 3.5 | |
| 67 | 2.4 | 1.90 × 10−2 | 4.5 | 4.6 | |
| 69 | 2.3 | 1.52 × 10−2 | 5.3 | 5.9 | |
| 71 | 2.3 | 4.30 × 10−3 | 9.1 | 9.0 |
| Samples | γ (mN/m) | γd (mN/m) | γp (mN/m) |
|---|---|---|---|
| PEPS | 31.1 | 14.5 | 16.7 |
| CNC | 60.7 | 39.4 | 21.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.; Jiang, Z.; Qiu, Z. Significantly Enhanced Crystallization of Poly(ethylene succinate-co-1,2-propylene succinate) by Cellulose Nanocrystals as an Efficient Nucleating Agent. Polymers 2022, 14, 224. https://doi.org/10.3390/polym14020224
Pan S, Jiang Z, Qiu Z. Significantly Enhanced Crystallization of Poly(ethylene succinate-co-1,2-propylene succinate) by Cellulose Nanocrystals as an Efficient Nucleating Agent. Polymers. 2022; 14(2):224. https://doi.org/10.3390/polym14020224
Chicago/Turabian StylePan, Siyu, Zhiguo Jiang, and Zhaobin Qiu. 2022. "Significantly Enhanced Crystallization of Poly(ethylene succinate-co-1,2-propylene succinate) by Cellulose Nanocrystals as an Efficient Nucleating Agent" Polymers 14, no. 2: 224. https://doi.org/10.3390/polym14020224
APA StylePan, S., Jiang, Z., & Qiu, Z. (2022). Significantly Enhanced Crystallization of Poly(ethylene succinate-co-1,2-propylene succinate) by Cellulose Nanocrystals as an Efficient Nucleating Agent. Polymers, 14(2), 224. https://doi.org/10.3390/polym14020224







