Fabrication of Low-Fouling Surfaces on Alkyne-Functionalized Poly-(p-xylylenes) Using Click Chemistry
Abstract
:1. Introduction
2. Materials and Methods
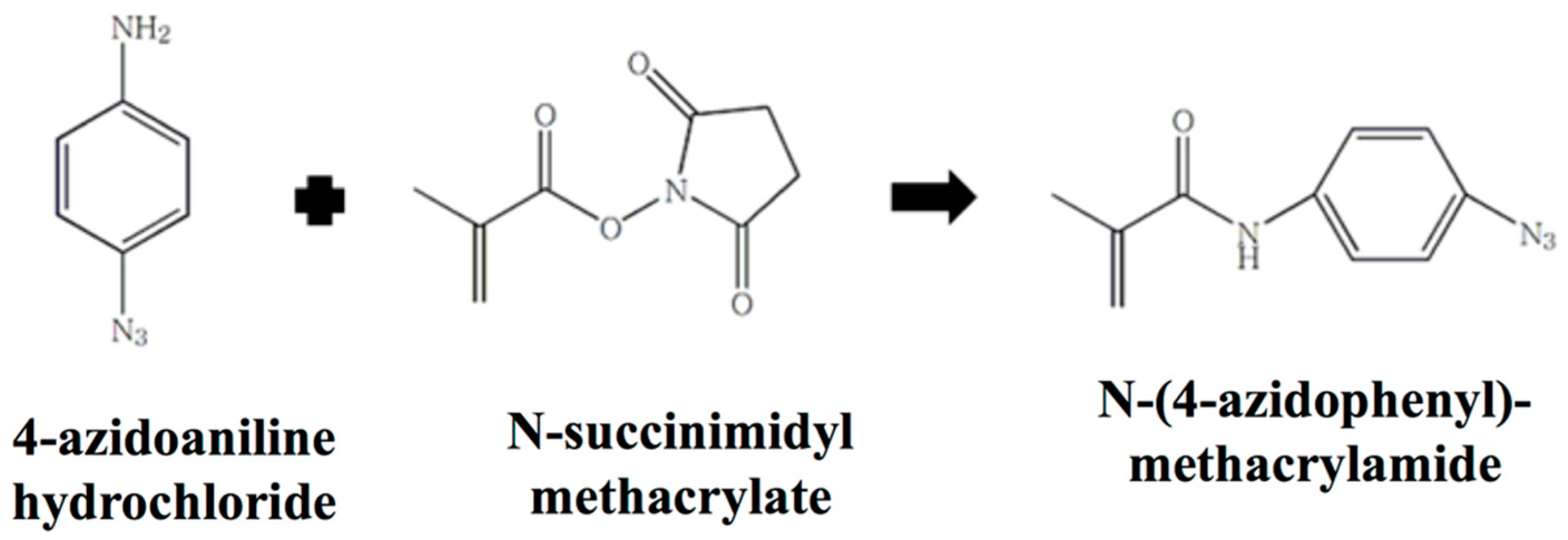
2.1. Synthesis and Characterization of N-(4-Azidophenyl) Methacrylamide (AzMA)
2.2. Synthesis and Characterization of Azide-Containing Sulfobetaine Polymers
2.3. Conjugation of Azide-Containing Polymers to Alkyne-PPX via Click Chemistry
2.4. Surface Analysis
2.5. The Adhesion of L929 Cells and Platelets
2.6. Determination of Protein Adsorption Using Quartz Crystal Microbalance (QCM)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Surfaces That Were Conjugated with Azide-Containing Sulfobetaine Polymers
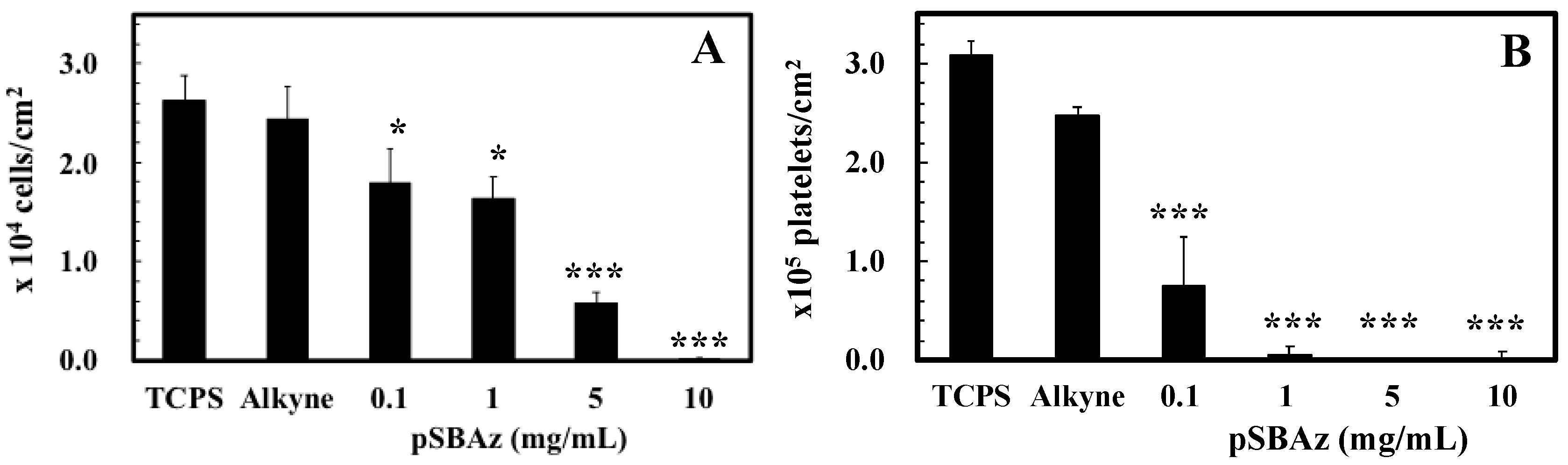
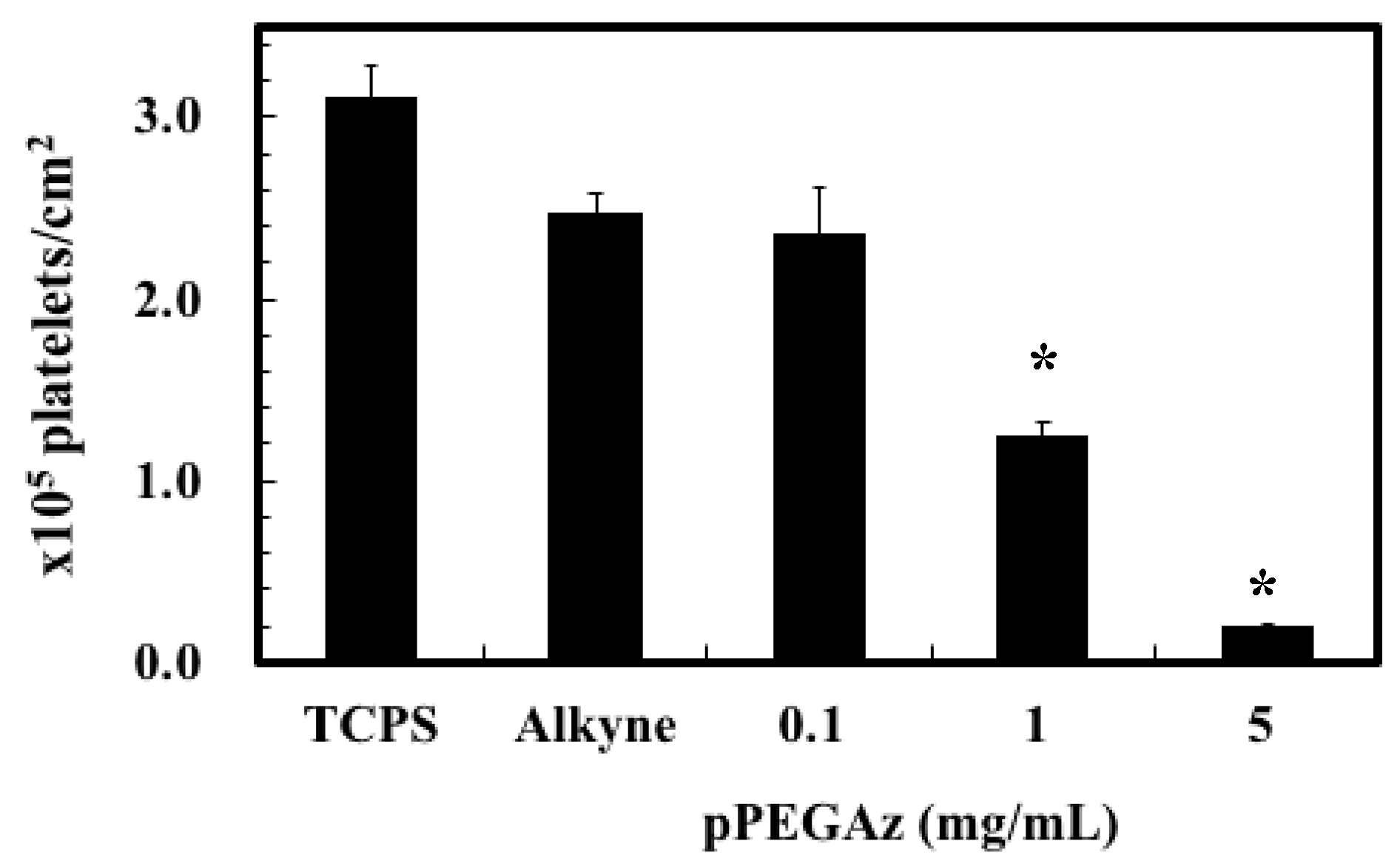
3.2. The Adhesion of L929 Cells and Platelets to pSBAz-Modified Surfaces
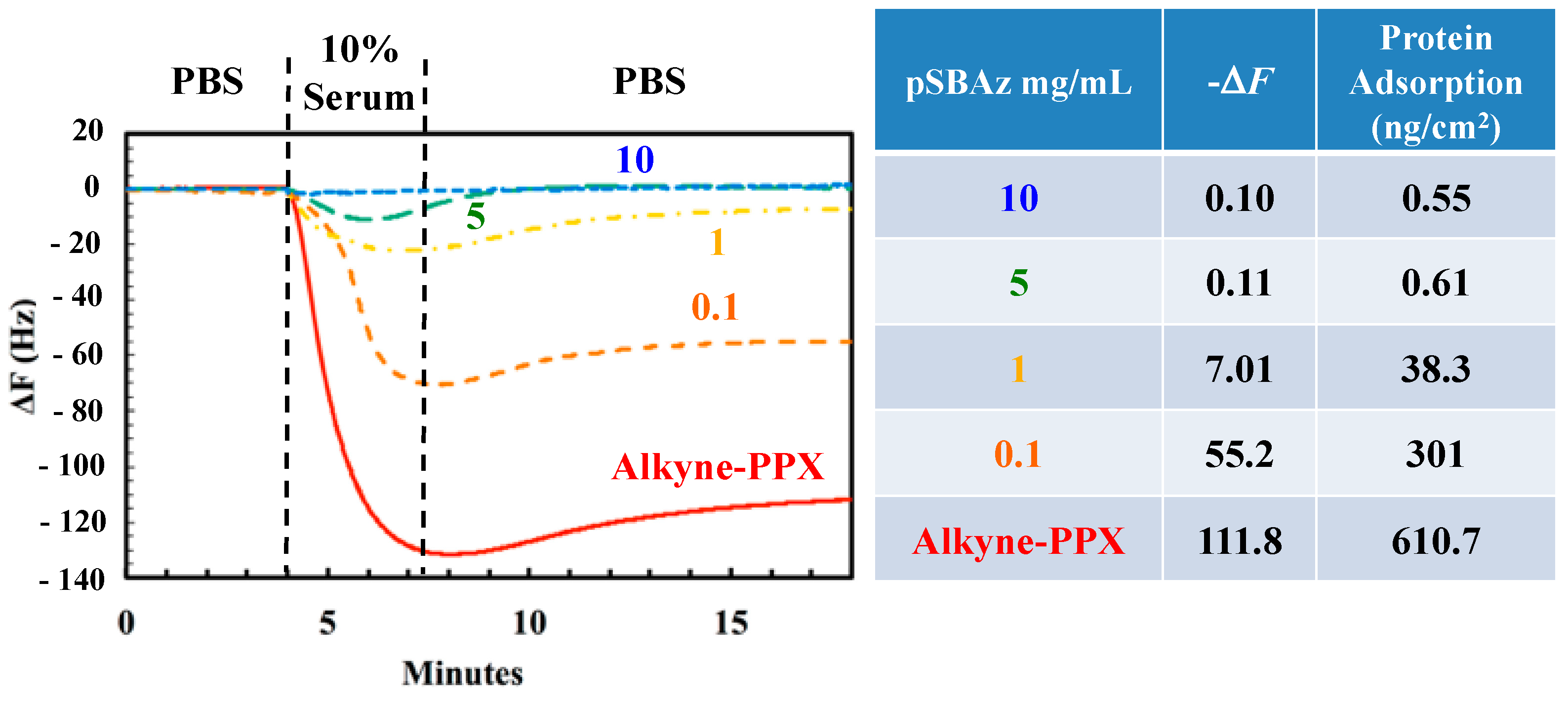
3.3. The Adsorption of Serum Proteins to pSBAz-Modified Surfaces
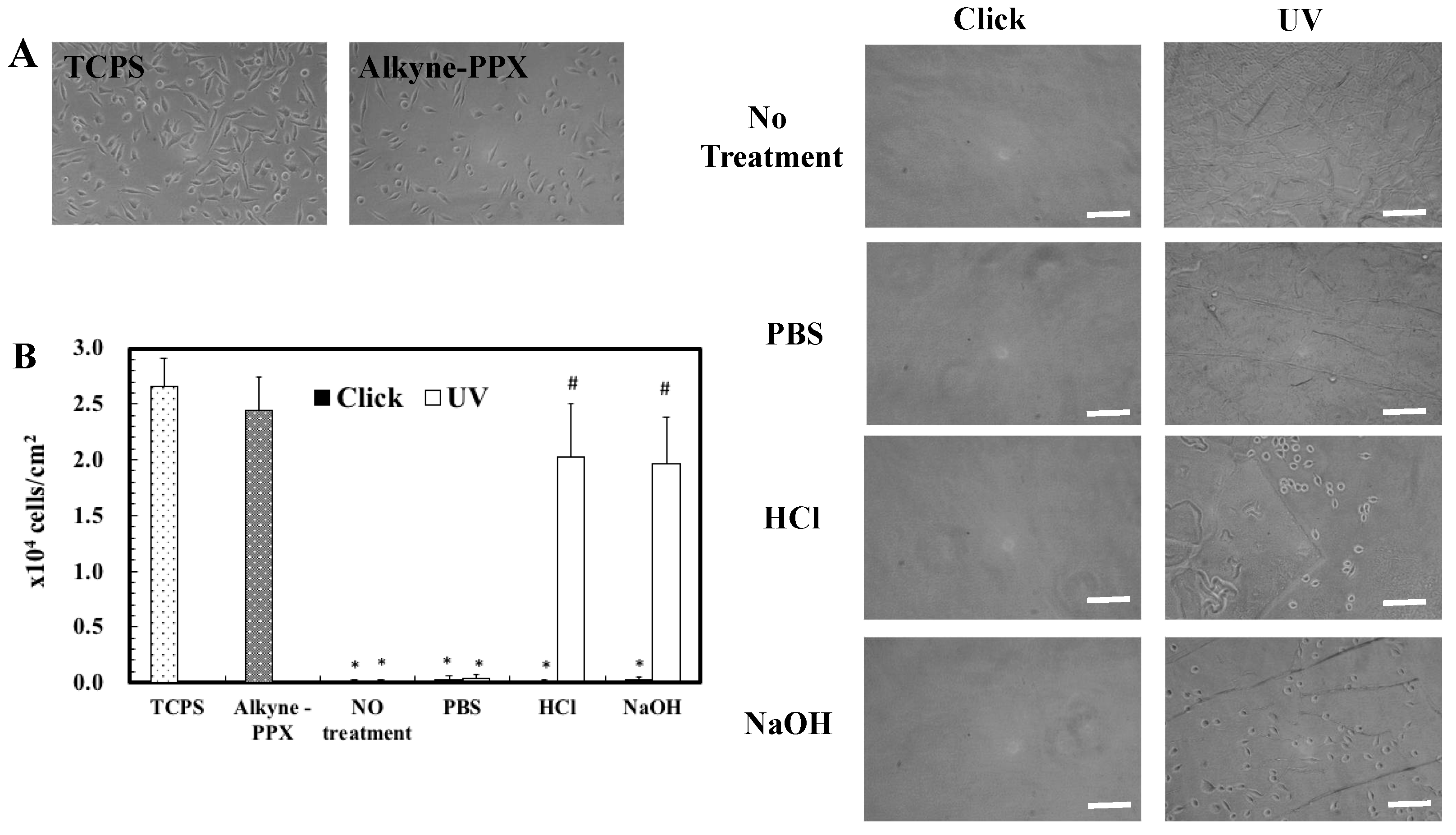
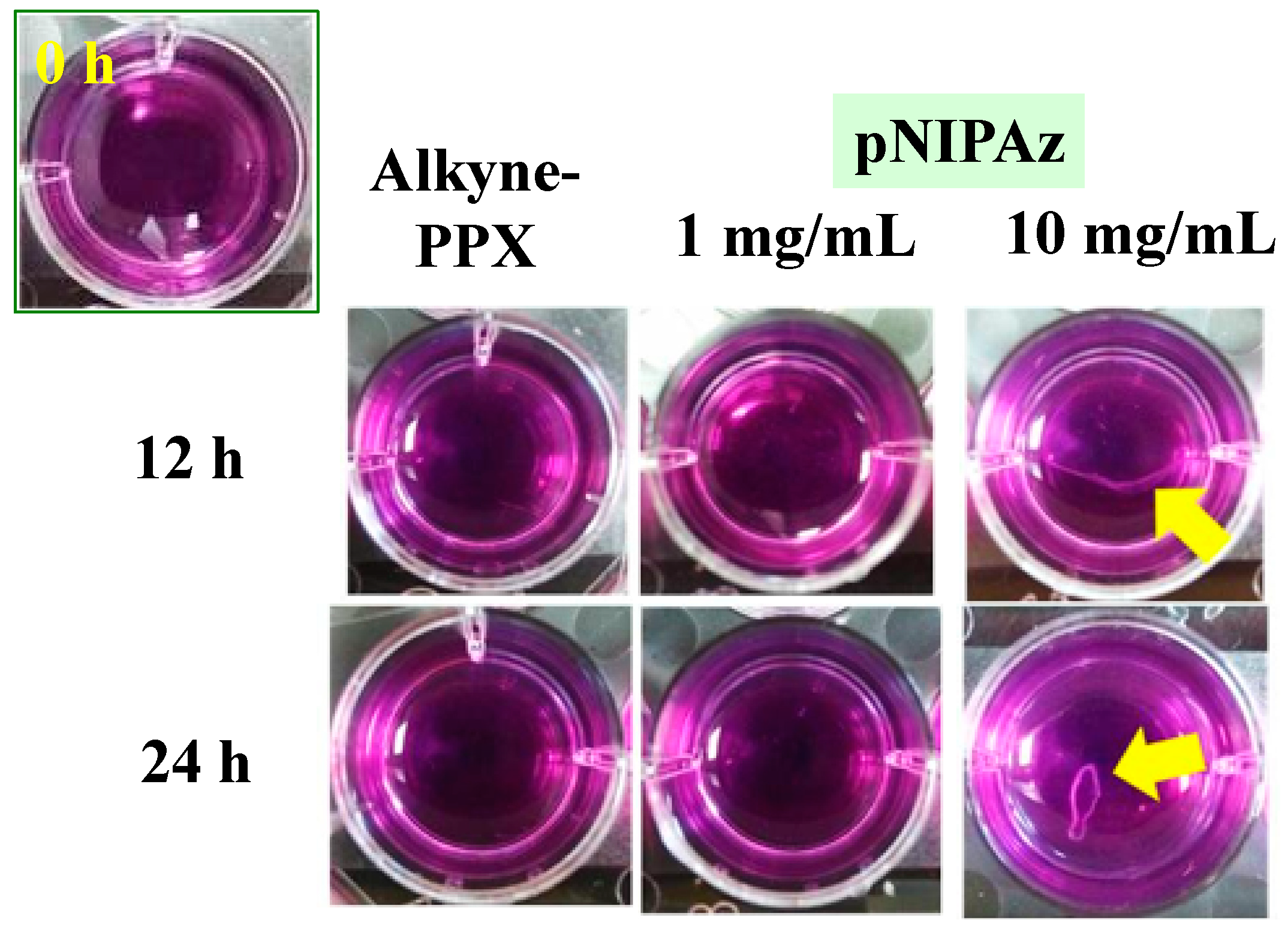
3.4. The Stability of pSBAz Deposition
3.5. Conjugation of Other Functional Polymers via Click Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where we have been and where we are going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef]
- Chang, Y.; Shih, Y.J.; Ko, C.Y.; Jhong, J.F.; Liu, Y.L.; Wei, T.C. Hemocompatibility of Poly(vinylidene fluoride) Membrane Grafted with Network-Like and Brush-Like Antifouling Layer Controlled via Plasma-Induced Surface PEGylation. Langmuir 2011, 27, 5445–5455. [Google Scholar] [CrossRef]
- Liu, Q.S.; Singh, A.; Lalani, R.; Liu, L.Y. Ultralow fouling polyacrylamide on gold surfaces via surface-initiated atom transfer radical polymerization. Biomacromolecules 2012, 13, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Kyomoto, M.; Shobuike, T.; Moro, T.; Yamane, S.; Takatori, Y.; Tanaka, S.; Miyamoto, H.; Ishihara, K. Prevention of bacterial adhesion and biofilm formation on a vitamin E-blended, cross-linked polyethylene surface with a poly(2-methacryloyloxyethyl phosphorylcholine) layer. Acta Biomater. 2015, 24, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.W.; Tsai, C.C.; Tsai, W.B.; Wang, M.J.; Kuo, W.H.; Wei, T.C.; Huang, S.T. Surface conjugation of zwitterionic polymers to inhibit cell adhesion and protein adsorption. Colloids Surf. B Biointerfaces 2013, 107, 152–159. [Google Scholar] [CrossRef]
- Kuo, W.H.; Wang, M.J.; Chien, H.W.; Wei, T.C.; Lee, C.; Tsai, W.B. Surface modification with poly(sulfobetaine methacrylate-co-acrylic acid) to reduce fibrinogen adsorption, platelet adhesion, and plasma coagulation. Biomacromolecules 2011, 12, 4348–4356. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.H.; Wang, M.J.; Chang, C.W.; Wei, T.C.; Lai, J.Y.; Tsai, W.B.; Lee, C. Improvement of hemocompatibility on materials by photoimmobilization of poly(ethylene glycol). J. Mater. Chem. 2012, 22, 9991–9999. [Google Scholar] [CrossRef]
- Chien, H.W.; Lin, H.Y.; Tsai, C.Y.; Chen, T.Y.; Chen, W.N. Superhydrophilic Coating with Antibacterial and Oil-Repellent Properties via NaIO4-Triggered Polydopamine/Sulfobetaine Methacrylate Polymerization. Polymers 2020, 12, 2008. [Google Scholar] [CrossRef]
- Yeh, S.L.; Wang, T.C.; Yusa, S.; Thissen, H.; Tsai, W.B. Conjugation of Polysulfobetaine via Poly(pyrogallol) Coatings for Improving the Antifouling Efficacy of Biomaterials. ACS Omega 2021, 6, 3517–3524. [Google Scholar] [CrossRef]
- Chen, P.R.; Wang, T.C.; Chen, S.T.; Chen, H.Y.; Tsai, W.B. Development of Antifouling Hyperbranched Polyglycerol Layers on Hydroxyl Poly-p-xylylene Coatings. Langmuir 2017, 33, 14657–14662. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Liao, S.C.; Higuchi, A.; Ruaan, R.C.; Chu, C.W.; Chen, W.Y. A highly stable nonbiofouling surface with well-packed grafted zwitterionic polysulfobetaine for plasma protein repulsion. Langmuir 2008, 24, 5453–5458. [Google Scholar] [CrossRef]
- Jiang, S.; Cao, Z. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef]
- Chien, H.W.; Cheng, P.H.; Chen, S.Y.; Yu, J.S.; Tsai, W.B. Low-fouling and functional poly(carboxybetaine) coating via a photo-crosslinking process. Biomater. Sci.-UK 2017, 5, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Fox, C.F. Chemical Cross-Linking in Biology. Annu. Rev. Biophys. Bioeng. 1979, 8, 165–193. [Google Scholar] [CrossRef] [PubMed]
- Cantini, M.; Sousa, M.; Moratal, D.; Mano, J.F.; Salmeron-Sanchez, M. Non-monotonic cell differentiation pattern on extreme wettability gradients. Biomater. Sci.-UK 2013, 1, 202–212. [Google Scholar] [CrossRef] [Green Version]
- Chien, H.W.; Chang, T.Y.; Tsai, W.B. Spatial control of cellular adhesion using photo-crosslinked micropatterned polyelectrolyte multilayer films. Biomaterials 2009, 30, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Edit 2002, 114, 2708–2711. [Google Scholar] [CrossRef]
- Tornoe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lahann, J. Designable biointerfaces using vapor-based reactive polymers. Langmuir 2011, 27, 34–48. [Google Scholar] [CrossRef]
- Chen, H.-Y.; McClelland, A.A.; Chen, Z.; Lahann, J. Solventless adhesive bonding using reactive polymer coatings. Anal. Chem. 2008, 80, 4119–4124. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lai, J.H.; Jiang, X.W.; Lahann, J. Substrate-selective chemical vapor deposition of reactive polymer coatings. Adv. Mater. 2008, 20, 3474–3480. [Google Scholar] [CrossRef] [Green Version]
- Bier, A.K.; Bognitzki, M.; Mogk, J.; Greiner, A. Synthesis, structure, and properties of alkyl-substituted PPXs by chemical vapor deposition for stent coatings. Macromolecules 2012, 45, 1151–1157. [Google Scholar] [CrossRef]
- Chen, L.; Tan, L.; Liu, S.; Bai, L.; Wang, Y. Surface modification by grafting of poly (SBMA-co-AEMA)-g-PDA coating and its application in CE. J. Biomater. Sci. Polym. Ed. 2014, 25, 766–785. [Google Scholar] [CrossRef]
- Nandivada, H.; Chen, H.Y.; Bondarenko, L.; Lahann, J. Reactive polymer coatings that “click”. Angew. Chem. Int. Edit 2006, 45, 3360–3363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Chen, S.F.; Zhang, Z.; Jiang, S.Y. Highly protein-resistant coatings from well-defined diblock copolymers containing sulfobetaines. Langmuir 2006, 22, 2222–2226. [Google Scholar] [CrossRef]
- Ito, Y.; Hasuda, H.; Sakuragi, M.; Tsuzuki, S. Surface modification of plastic, glass and titanium by photoimmobilization of polyethylene glycol for antibiofouling. Acta Biomater. 2007, 3, 1024–1032. [Google Scholar] [CrossRef]
- Tsai, W.B.; Shi, Q.; Grunkemeier, J.M.; McFarland, C.; Horbett, T.A. Platelet adhesion to radiofrequency glow-discharge-deposited fluorocarbon polymers preadsorbed with selectively depleted plasmas show the primary role of fibrinogen. J. Biomater. Sci. Polym. Ed. 2004, 15, 817–840. [Google Scholar] [CrossRef]
- Chien, H.W.; Wu, S.P.; Kuo, W.H.; Wang, M.J.; Lee, C.; Lai, J.Y.; Tsai, W.B. Modulation of hemocompatibility of polysulfone by polyelectrolyte multilayer films. Colloids Surf. B Biointerfaces 2010, 77, 270–278. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von schwingquarzen zur wagung dunner schichten und zur mikrowagung. Z. Für Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Fu, S.W.; Chien, H.W.; Tsai, W.B. Fabrication of poly(N-isopropylacrylamide) films containing submicrometer grooves for constructing aligned cell sheets. Langmuir 2013, 29, 14351–14355. [Google Scholar] [CrossRef] [PubMed]
| pSBAz | Atomic Percentage | WCA | |||
|---|---|---|---|---|---|
| C | O | N | S | ||
| Alkyne-PPX | 97.74 | 2.25 | - | - | 78.4° ± 2.4° * |
| 0.1 mg/mL | 96.00 | 2.35 | 1.23 | 0.42 | 44.4° ± 3.2° * |
| 1 mg/mL | 95.36 | 2.29 | 1.68 | 0.67 | 71.4° ± 4.5° * |
| 5 mg/mL | 95.74 | 1.89 | 1.58 | 0.83 | 25.1° ± 2.5° * |
| 10 mg/mL | 95.54 | 2.21 | 1.48 | 0.77 | 26.7° ± 1.8° * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-J.; Chen, H.-Y.; Tsai, W.-B. Fabrication of Low-Fouling Surfaces on Alkyne-Functionalized Poly-(p-xylylenes) Using Click Chemistry. Polymers 2022, 14, 225. https://doi.org/10.3390/polym14020225
Chen P-J, Chen H-Y, Tsai W-B. Fabrication of Low-Fouling Surfaces on Alkyne-Functionalized Poly-(p-xylylenes) Using Click Chemistry. Polymers. 2022; 14(2):225. https://doi.org/10.3390/polym14020225
Chicago/Turabian StyleChen, Pei-Ju, Hsien-Yeh Chen, and Wei-Bor Tsai. 2022. "Fabrication of Low-Fouling Surfaces on Alkyne-Functionalized Poly-(p-xylylenes) Using Click Chemistry" Polymers 14, no. 2: 225. https://doi.org/10.3390/polym14020225
APA StyleChen, P.-J., Chen, H.-Y., & Tsai, W.-B. (2022). Fabrication of Low-Fouling Surfaces on Alkyne-Functionalized Poly-(p-xylylenes) Using Click Chemistry. Polymers, 14(2), 225. https://doi.org/10.3390/polym14020225







