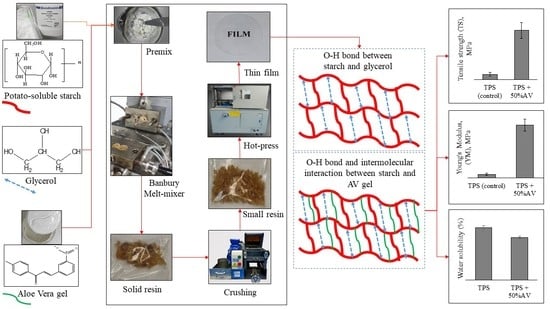
Production of Thermoplastic Starch-Aloe vera Gel Film with High Tensile Strength and Improved Water Solubility
Abstract
1. Introduction
2. Materials and Methods
2.1. Thermoplastic Starch Preparation
2.2. Film Preparation
2.3. Characterization of the Biodegradable Film
2.3.1. Mechanical Properties
2.3.2. Water Absorption and Water Solubility
2.3.3. Thermal Decomposition
2.3.4. Thermal Properties
2.3.5. Crystallinity
2.3.6. Visual Appearance and Thickness
2.3.7. Morphological Structure
2.3.8. Functional Compound
2.3.9. Statistical Analysis
3. Results
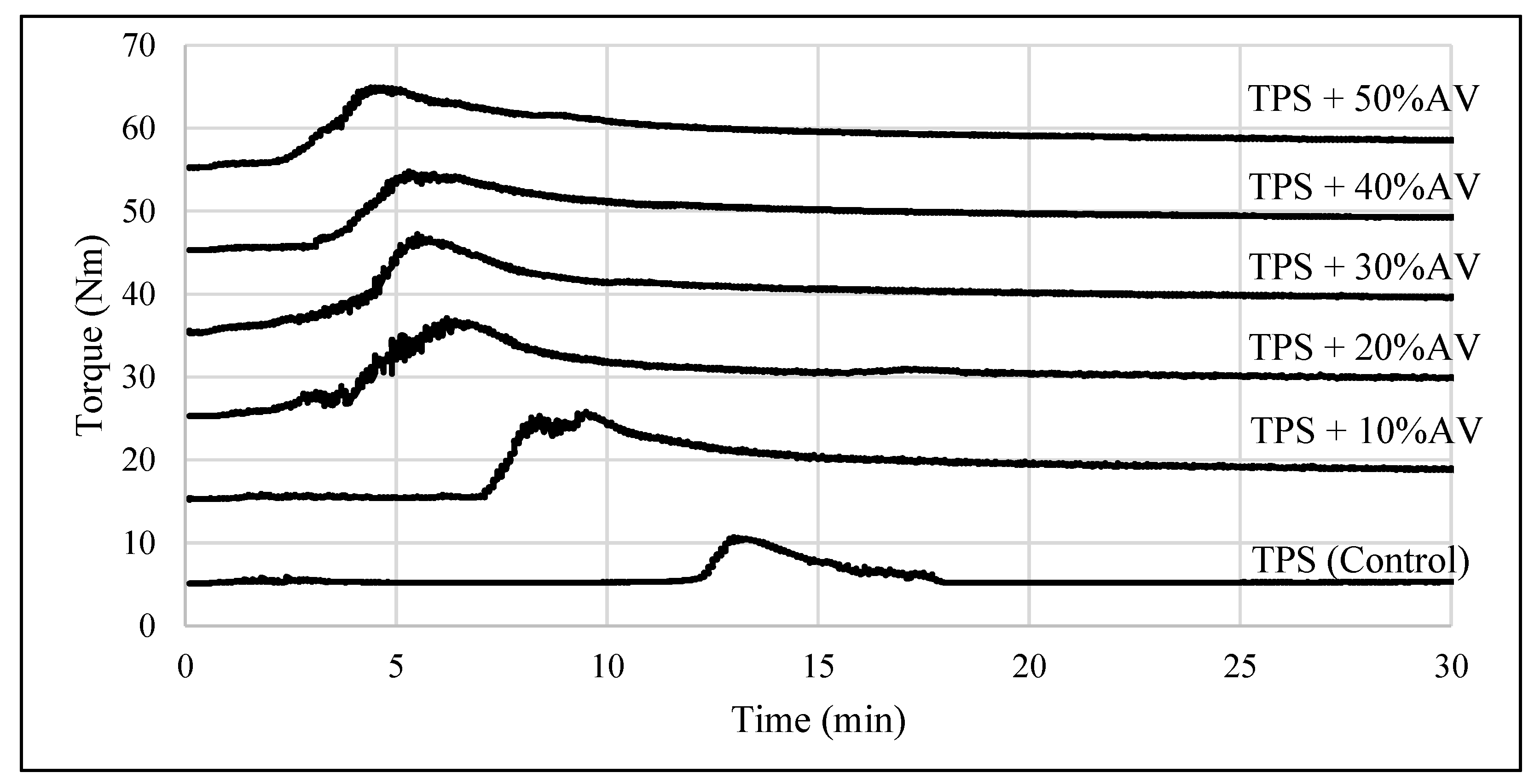
3.1. TPS Process Condition
3.2. Mechanical Properties
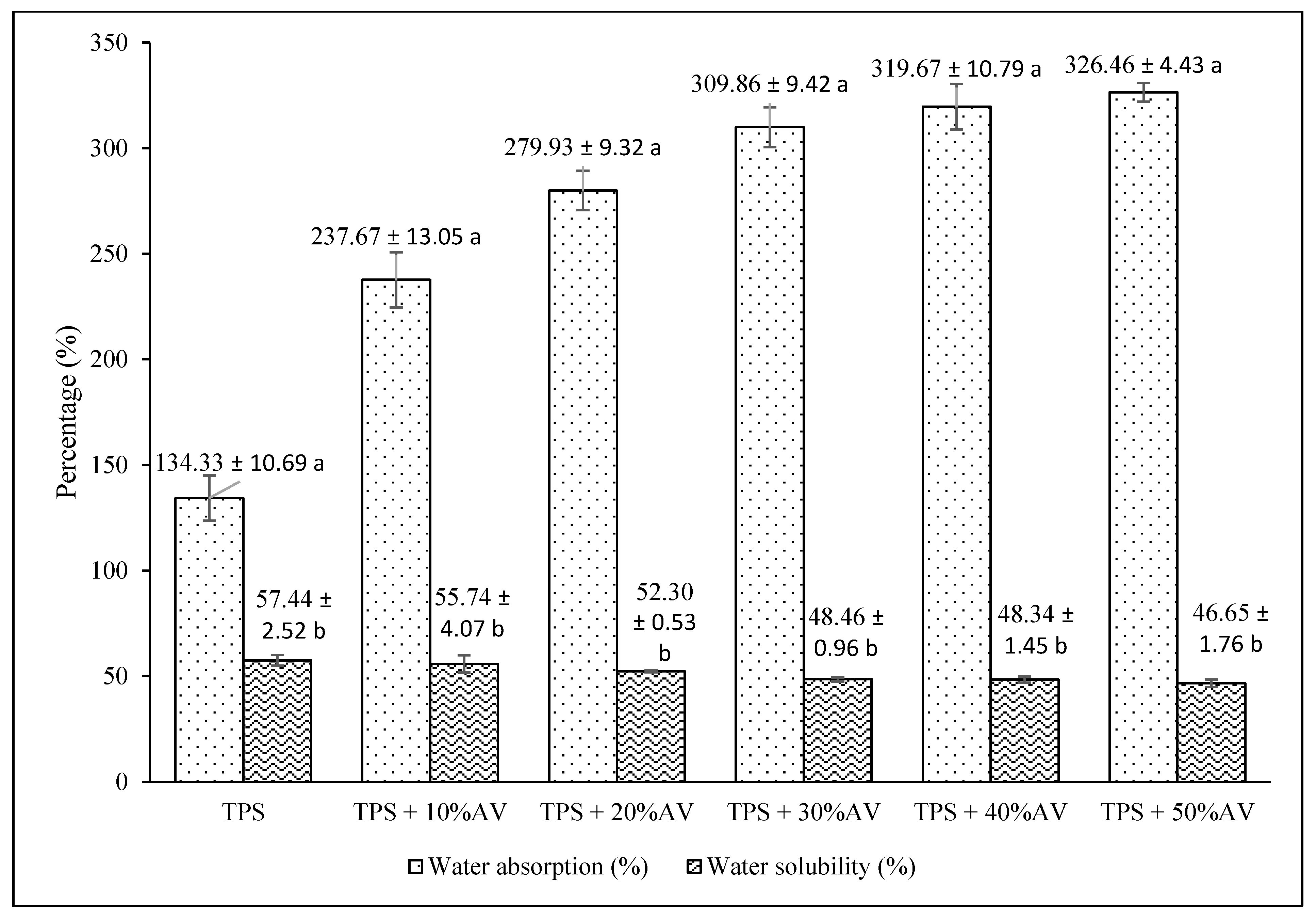
3.3. Water Absorption versus Water Solubility Percentage
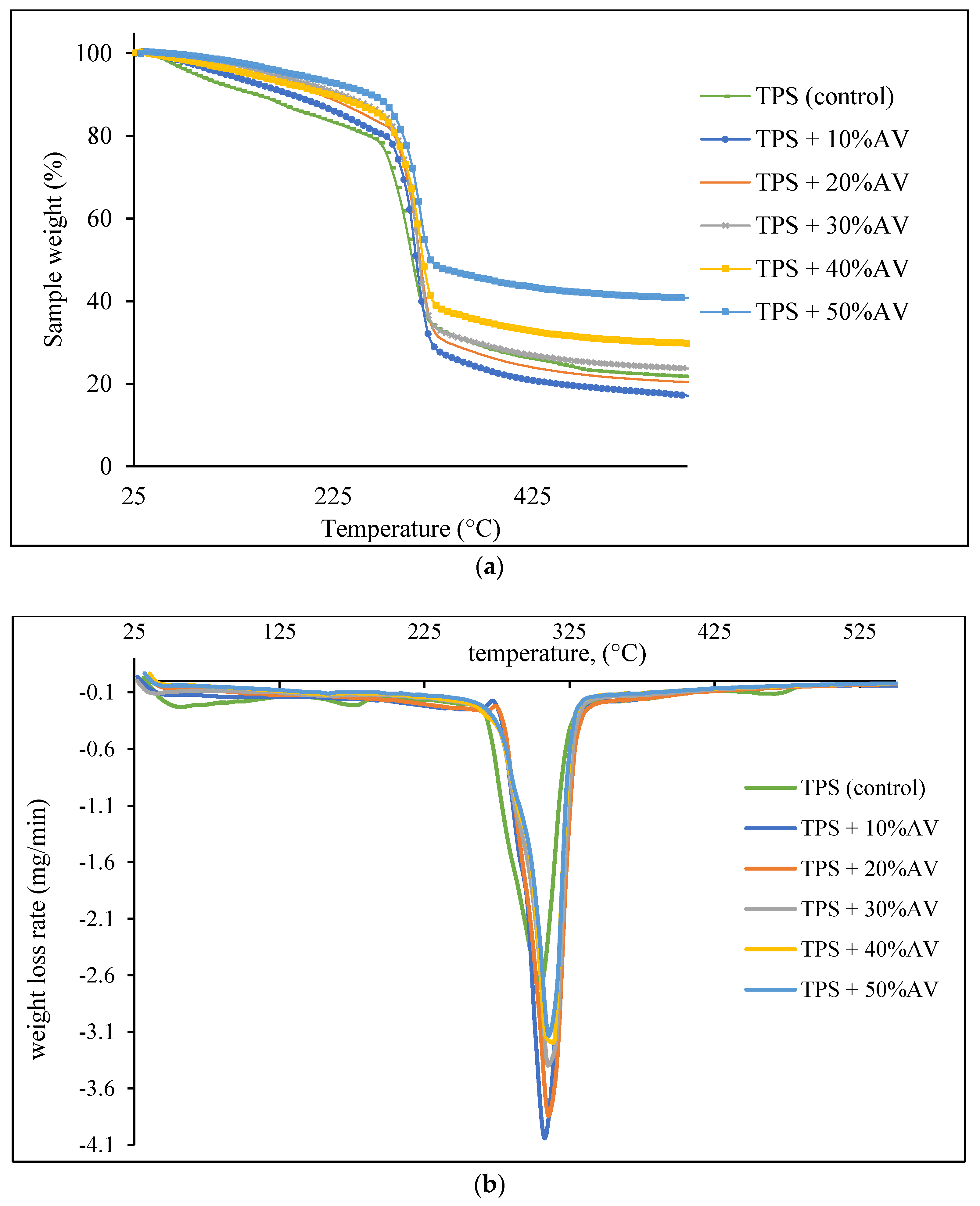
3.4. Thermal Degradation
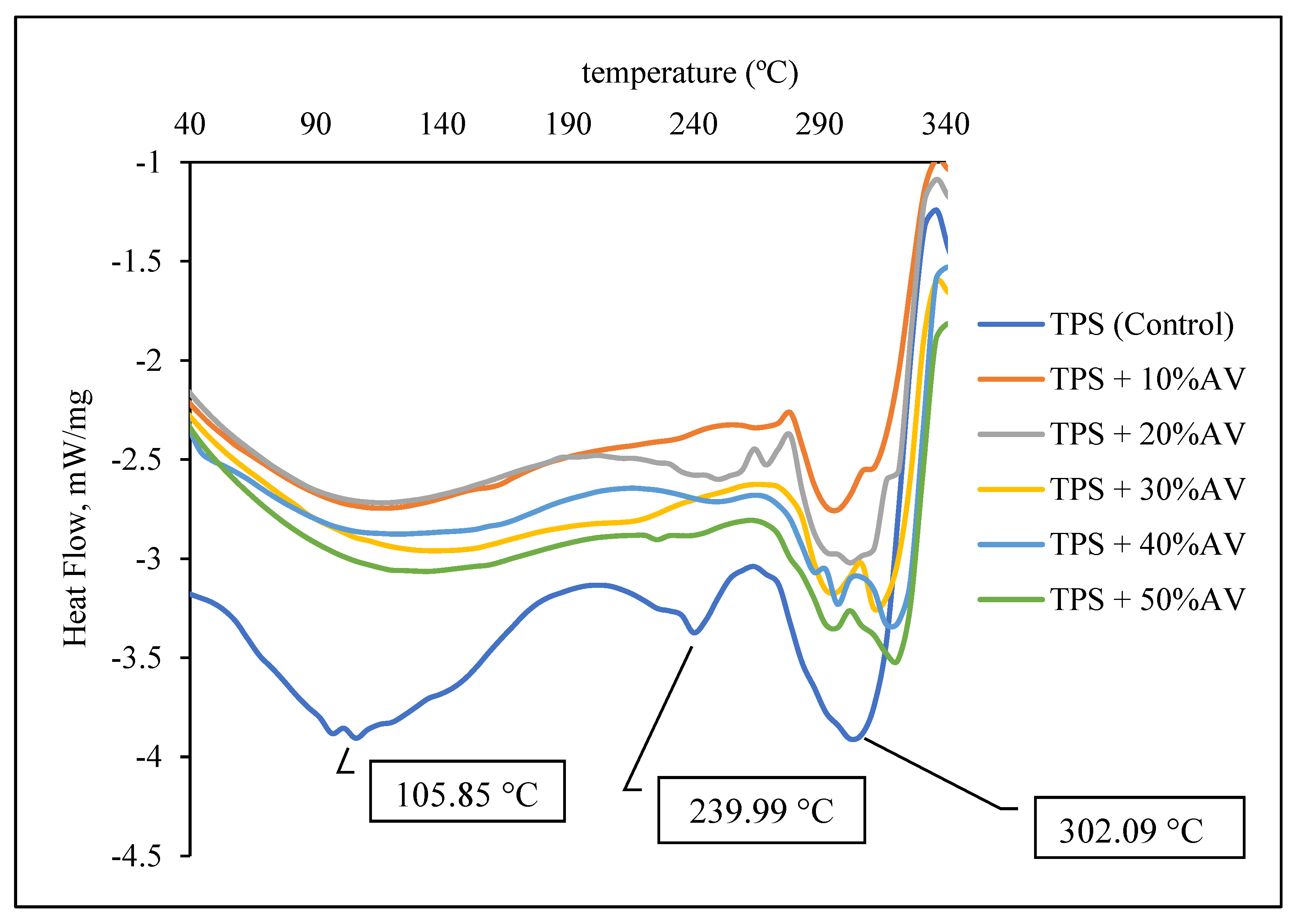
3.5. Thermal Properties
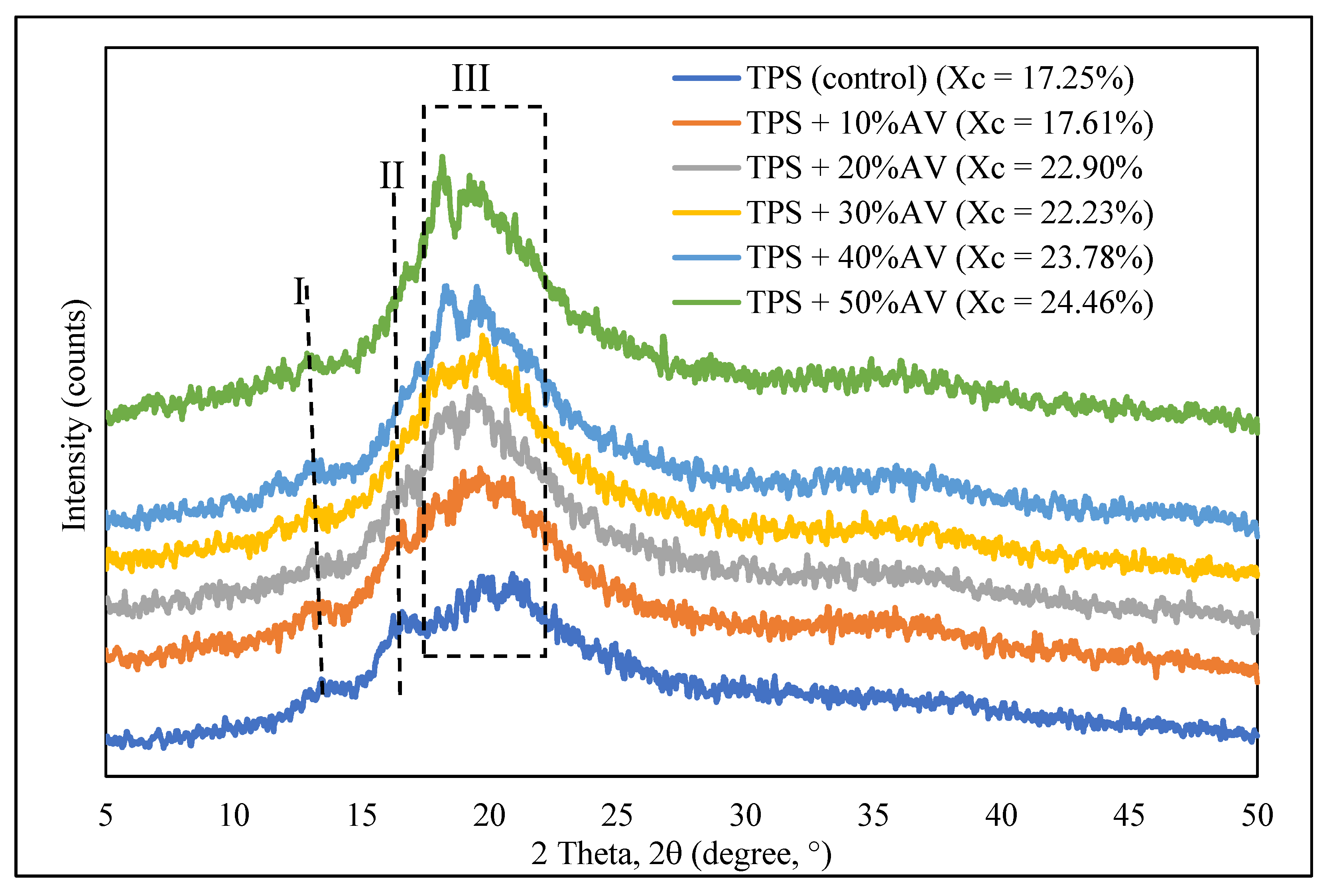
3.6. Crystallinity
3.7. Visual Appearance
3.8. Thickness
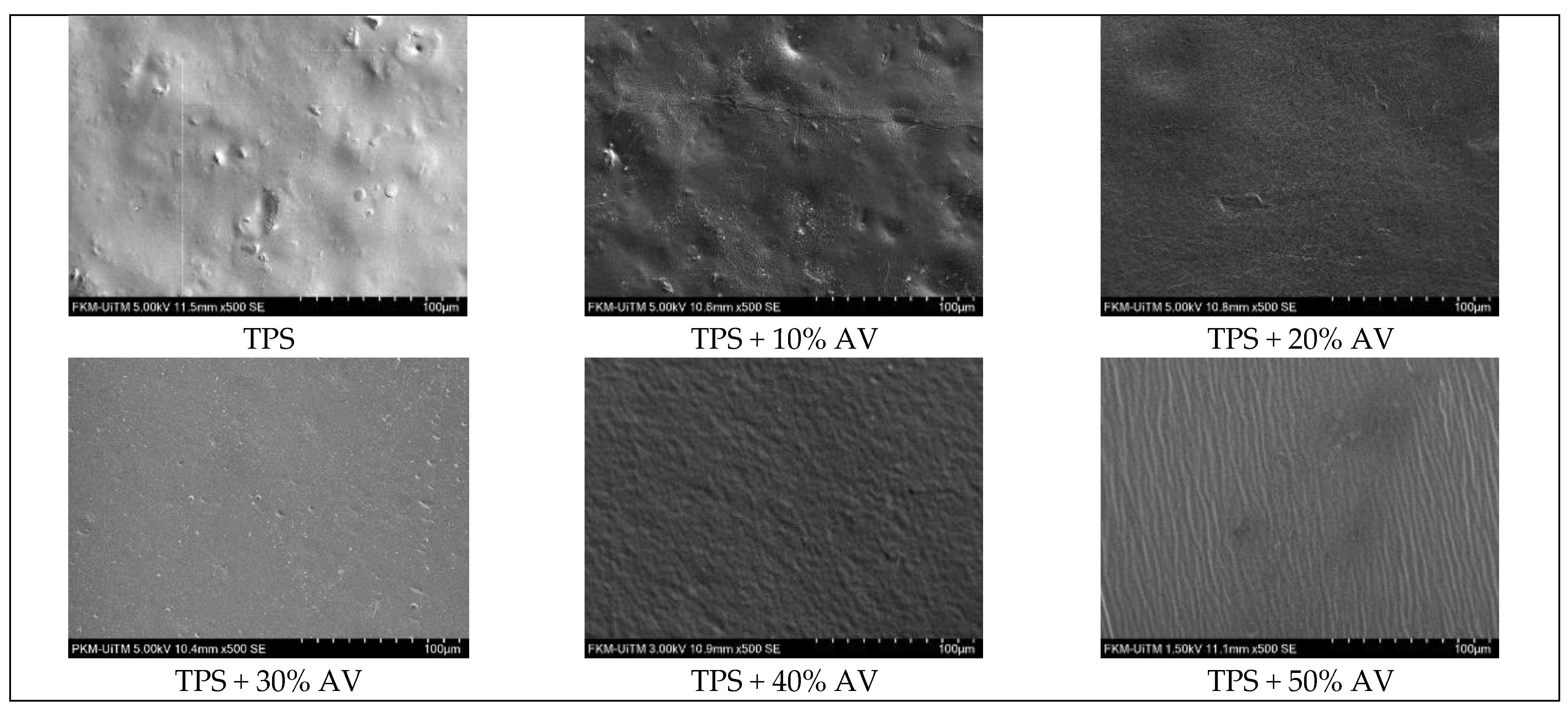
3.9. Surface Morphology
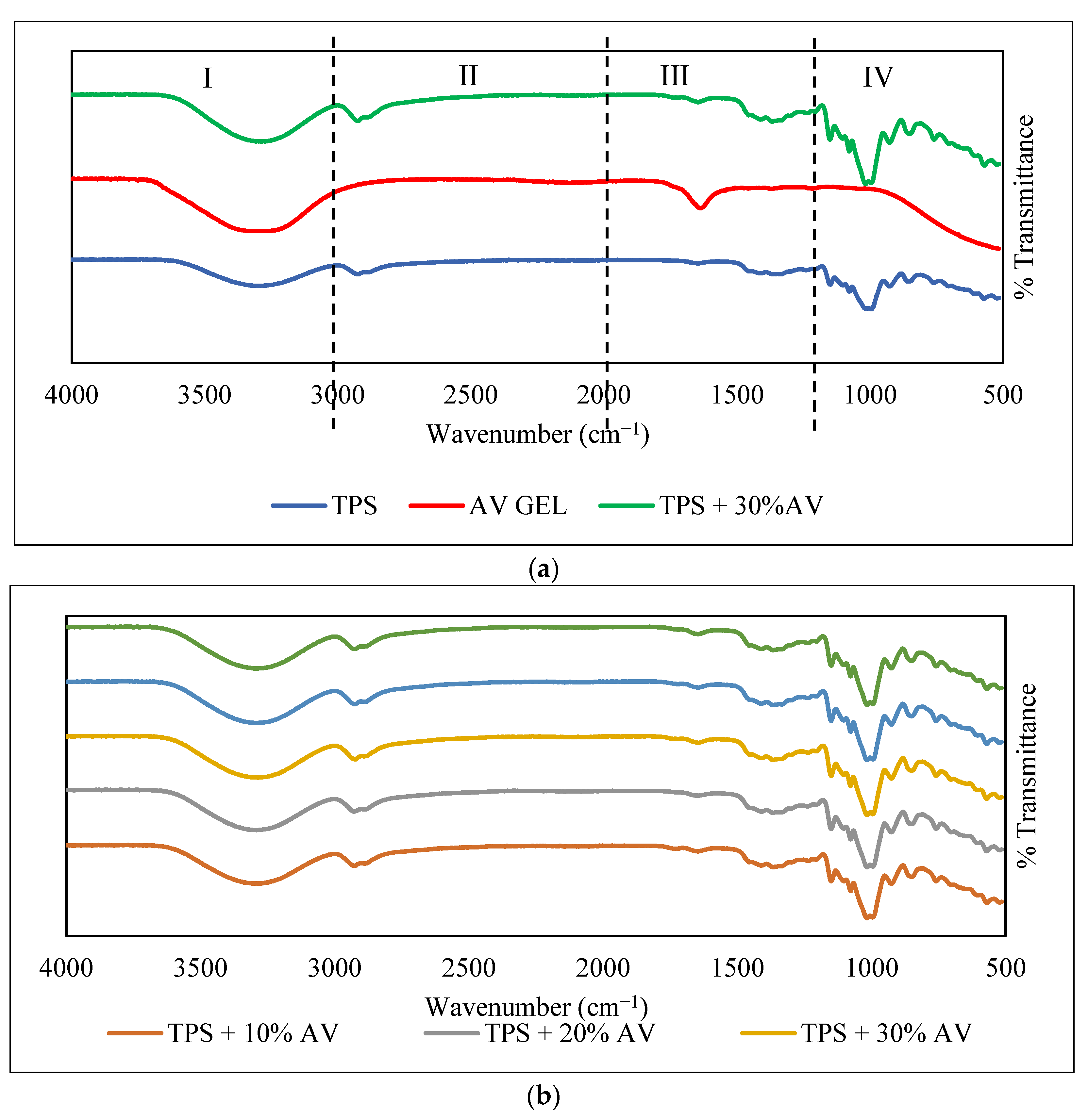
3.10. Functional Compound
3.11. Summary of Results and Data Comparison with Other Studies
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, D.; Banerjee, C.; Halder, G.; Dhawane, S.H. Elucidating Enhanced Biodegradability of Starch Blended Polyolefinic Sheet by Physico-Mechanical and Thermal Property Assessment. Mater. Today Proc. 2022, 49, 464–472. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Sultan, H.H.; Karam, H.J.; Al-Dhafeeri, A.T. Determination of Biodegradation Rate of Commercial Oxo-Biodegradable Polyethylene Film Products Using ASTM D 5988. J. Polym. Res. 2019, 26, 1–7. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Ashogbon, A.O.; Kumar, M. Recent Advances in Thermoplastic Starches for Food Packaging: A Review. Food Packag. Shelf Life 2021, 30, 100743. [Google Scholar] [CrossRef]
- Homthawornchoo, W.; Han, J.; Kaewprachu, P.; Romruen, O.; Rawdkuen, S. Green Tea Extract Enrichment: Mechanical and Physicochemical Properties Improvement of Rice Starch-Pectin Composite Film. Polymers 2022, 14, 2696. [Google Scholar] [CrossRef]
- Díaz-Cruz, C.A.; Caicedo, C.; Jiménez-Regalado, E.J.; de León, R.D.; López-González, R.; Aguirre-Loredo, R.Y. Barrier Properties of Corn Starch–Chitosan Biodegradable Films Reinforced with Cellulose Nanocrystals. Polymers 2022, 14, 2166. [Google Scholar] [CrossRef]
- Wang, C.; Lu, Y.; Li, Z.; An, X.; Gao, Z.; Tian, S. Preparation and Performance Characterization of a Composite Film Based on Corn Starch, κ-Carrageenan, and Ethanol Extract of Onion Skin. Polymers 2022, 14, 2986. [Google Scholar] [CrossRef]
- Charles, A.L.; Motsa, N.; Abdillah, A.A. A Comprehensive Characterization of Biodegradable Edible Films Based on Potato Peel Starch Plasticized with Glycerol. Polymers 2022, 14, 3462. [Google Scholar] [CrossRef] [PubMed]
- Todhanakasem, T.; Jaiprayat, C.; Sroysuwan, T.; Suksermsakul, S.; Suwapanich, R.; Maleenont, K.K.; Koombhongse, P.; Young, B.M. Active Thermoplastic Starch Film with Watermelon Rind Extract for Future Biodegradable Food Packaging. Polymers 2022, 14, 3232. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chen, L.; McClements, D.J.; Yang, T.; Zhang, Z.; Ren, F.; Miao, M.; Tian, Y.; Jin, Z. Starch-Based Biodegradable Packaging Materials: A Review of Their Preparation, Characterization and Diverse Applications in the Food Industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar] [CrossRef]
- Othman, S.H.; Majid, N.A.; Tawakkal, I.S.M.A.; Basha, R.K.; Nordin, N.; Shapi’i, R.A. Tapioca Starch Films Reinforced with Microcrystalline Cellulose for Potential Food Packaging Application. Food Sci. Technol. 2019, 39, 605–612. [Google Scholar] [CrossRef]
- Martins, P.C.; Latorres, J.M.; Martins, V.G. Impact of Starch Nanocrystals on the Physicochemical, Thermal and Structural Characteristics of Starch-Based Films. LWT–Food Sci. Technol. 2022, 156, 113041. [Google Scholar] [CrossRef]
- Méité, N.; Konan, L.K.; Tognonvi, M.T.; Oyetola, S. Effect of Metakaolin Content on Mechanical and Water Barrier Properties of Cassava Starch Films. S. Afr. J. Chem. Eng. 2022, 40, 186–194. [Google Scholar] [CrossRef]
- Shapii, R.A.; Othman, S.H.; Basha, R.K.; Naim, M.N. Mechanical, Thermal, and Barrier Properties of Starch Films Incorporated with Chitosan Nanoparticles. Nanotechnol. Rev. 2022, 11, 1464–1477. [Google Scholar] [CrossRef]
- Jeevahan, J.J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Joseph, G.B.; Mageshwaran, G.; Durairaj, R.B. Scaling up Difficulties and Commercial Aspects of Edible Films for Food Packaging: A Review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Khumkomgool, A.; Saneluksana, T.; Harnkarnsujarit, N. Active Meat Packaging from Thermoplastic Cassava Starch Containing Sappan and Cinnamon Herbal Extracts via LLDPE Blown-Film Extrusion. Food Packag. Shelf Life 2020, 26, 100557. [Google Scholar] [CrossRef]
- Altayan, M.M.; Al Darouich, T.; Karabet, F. On the Plasticization Process of Potato Starch: Preparation and Characterization. Food Biophys. 2017, 12, 397–403. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Geethamma, V.G.; Gopi, S.; Thomas, M.G.; Kunaver, M.; Huskić, M.; Kalarikkal, N.; Volova, T.; Rouxel, D.; Thomas, S. Thermal, Biodegradation and Theoretical Perspectives on Nanoscale Confinement in Starch/Cellulose Nanocomposite Modified via Green Crosslinker. Int. J. Biol. Macromol. 2019, 134, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Lauer, M.K.; Smith, R.C. Recent Advances in Starch-Based Films toward Food Packaging Applications: Physicochemical, Mechanical, and Functional Properties. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3031–3083. [Google Scholar] [CrossRef]
- Peidayesh, H.; Heydari, A.; Mosnáčková, K.; Chodák, I. In Situ Dual Crosslinking Strategy to Improve the Physico-Chemical Properties of Thermoplastic Starch. Carbohydr. Polym. 2021, 269, 118250. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Yu, H.; Zhang, J.; Cheng, F. Preparation and Characterization of Cross-Linked Starch Nanocrystals and Self-Reinforced Starch-Based Nanocomposite Films. Int. J. Biol. Macromol. 2021, 181, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Prachayawarakorn, J.; Suthichujit, A. Characterization and Properties of Biodegradable Thermoplastic Arrowroot Starch Crosslinked by Glutaraldehyde Processed by Compression Molding Technique. Curr. Appl. Sci. Technol. 2021, 22, 1–13. [Google Scholar] [CrossRef]
- Khan, B.; Khan Niazi, M.B.; Jahan, Z.; Farooq, W.; Naqvi, S.R.; Ali, M.; Ahmed, I.; Hussain, A. Effect of Ultra-Violet Cross-Linking on the Properties of Boric Acid and Glycerol Co-Plasticized Thermoplastic Starch Films. Food Packag. Shelf Life 2019, 19, 184–192. [Google Scholar] [CrossRef]
- Sukkaneewat, B.; Panrot, T.; Rojruthai, P.; Wongpreedee, T.; Prapruddivongs, C. Plasticizing Effects from Citric Acid/Palm Oil Combinations for Sorbitol-Crosslinked Starch Foams. Mater. Chem. Phys. 2022, 278, 125732. [Google Scholar] [CrossRef]
- Sornsumdaeng, K.; Seeharaj, P.; Prachayawarakorn, J. Property Improvement of Biodegradable Citric Acid-Crosslinked Rice Starch Films by Calcium Oxide. Int. J. Biol. Macromol. 2021, 193, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Mota, L.O.; Gimenez, F.I. Cellulose-Based Materials Crosslinked with Epichlorohydrin: A Mini Review. Rev. Virtual Quím. 2022, 1–12. [Google Scholar] [CrossRef]
- Chen, W.C.; Mohd Judah, S.N.M.S.; Ghazali, S.K.; Munthoub, D.I.; Alias, H.; Mohamad, Z.; Majid, R.A. The Effects of Citric Acid on Thermal and Mechanical Properties of Crosslinked Starch Film. Chem. Eng. Trans. 2021, 83, 199–204. [Google Scholar] [CrossRef]
- Yin, P.; Dong, X.; Zhou, W.; Zha, D.; Xu, J.; Guo, B.; Li, P. A Novel Method to Produce Sustainable Biocomposites Based on Thermoplastic Corn-Starch Reinforced by Polyvinyl Alcohol Fibers. RSC Adv. 2020, 10, 23632–23643. [Google Scholar] [CrossRef]
- Mallick, N.; Soni, A.B.; Pal, D. Improving the Mechanical, Water Vapor Permeability, Antimicrobial Properties of Corn-Starch/Ply Vinyl Alcoholfilm (PVA): Effect of Rice Husk Fiber (RH) & Alovera Gel(AV). IOP Conf. Ser. Mater. Sci. Eng. 2020, 798, 12002. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; Collazo-Bigliardi, S.; Roselló, J.; Santamarina, P.; Chiralt, A. Antifungal Starch-Based Edible Films Containing Aloe Vera. Food Hydrocoll. 2017, 72, 1–10. [Google Scholar] [CrossRef]
- Dagmara, B.; Katarzyna, J.; Krzysztof, B. Novel Starch/Chitosan/Aloe Vera Composites as Promising Biopackaging Materials. J. Polym. Environ. 2020, 28, 1021–1039. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Garcia, O.R.; Villa, C.C. The Influence of Aloe Vera Gel Incorporation on the Physicochemical and Mechanical Properties of Banana Starch-Chitosan Edible Films. J. Sci. Food Agric. 2018, 98, 4042–4049. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J.; González, G. Effect of Cross-Linking with Aloe Vera Gel on Surface and Physicochemical Properties of Edible Films Made from Plantain Flour. Food Biophys. 2017, 12, 11–22. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Álvarez, K. Physico-Chemical Properties and in Vitro Digestibility of Edible Films Made from Plantain Flour with Added Aloe Vera Gel. J. Funct. Foods 2016, 26, 750–762. [Google Scholar] [CrossRef]
- Suriati, L.; Utama, I.M.S.; Harsojuwono, B.A.; Gunam, I.B.W. Incorporating Additives for Stability of Aloe Gel Potentially as an Edible Coating. AIMS Agric. Food 2020, 5, 327–336. [Google Scholar] [CrossRef]
- Nieto-Suaza, L.; Acevedo-Guevara, L.; Sánchez, L.T.; Pinzón, M.I.; Villa, C.C. Characterization of Aloe Vera-Banana Starch Composite Films Reinforced with Curcumin-Loaded Starch Nanoparticles. Food Struct. 2019, 22, 100131. [Google Scholar] [CrossRef]
- Siti Fatma, A.K.; Jai, J.; Ku Halim, K.H.; Mohd Hariz, M.M.; Nadia, K.; Rabiatul Adawiyah, A.A. Full Factorial Design Analysis and Characterization of Polyethylene, Starch and Aloe Vera Gel Thin Film Formulation. Int. J. Adv. Sci. Eng. Inf. Technol. 2021, 11, 2139–2147. [Google Scholar] [CrossRef]
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-Based Films Enriched with Nanocellulose-Stabilized Pickering Emulsions Containing Different Essential Oils for Possible Applications in Food Packaging. Food Packag. Shelf Life 2021, 27, 100615. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Do, T.V.V.; Grillet, A.C.; Ha Thuc, H.; Ha Thuc, C.N. Biodegradability of Polymer Fi Lm Based on Low Density Polyethylene and Cassava Starch. Int. Biodeterior. Biodegrad. 2016, 115, 257–265. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Vu, T.T.; Grillet, A.C.; Ha Thuc, H.; Ha Thuc, C.N. Effect of Organoclay on Morphology and Properties of Linear Low Density Polyethylene and Vietnamese Cassava Starch Biobased Blend. Carbohydr. Polym. 2016, 136, 163–170. [Google Scholar] [CrossRef]
- Ramírez-Hernández, A.; Hernández-Mota, C.E.; Páramo-Calderón, D.E.; González-García, G.; Báez-García, E.; Rangel-Porras, G.; Vargas-Torres, A.; Aparicio-Saguilán, A. Thermal, Morphological and Structural Characterization of a Copolymer of Starch and Polyethylene. Carbohydr. Res. 2020, 488, 107907. [Google Scholar] [CrossRef]
- Panrong, T.; Karbowiak, T.; Harnkarnsujarit, N. Effects of Acetylated and Octenyl-Succinated Starch on Properties and Release of Green Tea Compounded Starch/LLDPE Blend Films. J. Food Eng. 2020, 284, 110057. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Makwana, S.H. Development of Active, Water-Resistant Carboxymethyl Cellulose-Poly Vinyl Alcohol-Aloe Vera Packaging Film. Carbohydr. Polym. 2020, 227, 115303. [Google Scholar] [CrossRef] [PubMed]
- Kormin, S.; Kormin, F.; Beg, M.D.H. Effect of Plasticizer on Physical and Mechanical Properties of Ldpe/Sago Starch Blend. J. Phys. Conf. Ser. 2019, 1150, 12032. [Google Scholar] [CrossRef]
- Paiva, D.; Pereira, A.M.; Pires, A.L.; Martins, J.; Carvalho, L.H.; Magalhães, F.D. Reinforcement of Thermoplastic Corn Starch with Crosslinked Starch/Chitosan Microparticles. Polymers 2018, 10, 985. [Google Scholar] [CrossRef]
- Bulatović, V.O.; Mandić, V.; Kučić Grgić, D.; Ivančić, A. Biodegradable Polymer Blends Based on Thermoplastic Starch. J. Polym. Environ. 2021, 29, 492–508. [Google Scholar] [CrossRef]
- Khoshgozaran-Abras, S.; Azizi, M.H.; Hamidy, Z.; Bagheripoor-Fallah, N. Mechanical, Physicochemical and Color Properties of Chitosan Based-Films as a Function of Aloe Vera Gel Incorporation. Carbohydr. Polym. 2012, 87, 2058–2062. [Google Scholar] [CrossRef]
- Boonsuk, P.; Sukolrat, A.; Bourkaew, S.; Kaewtatip, K.; Chantarak, S.; Kelarakis, A.; Chaibundit, C. Structure-Properties Relationships in Alkaline Treated Rice Husk Reinforced Thermoplastic Cassava Starch Biocomposites. Int. J. Biol. Macromol. 2021, 167, 130–140. [Google Scholar] [CrossRef]
- Hafila, K.Z.; Jumaidin, R.; Ilyas, R.A.; Selamat, M.Z.; Yusof, F.A.M. Effect of Palm Wax on the Mechanical, Thermal, and Moisture Absorption Properties of Thermoplastic Cassava Starch Composites. Int. J. Biol. Macromol. 2021, 190, 224–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic Starch Processing and Characteristics-A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353–1370. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.A.; Akram, M.E.; Al-Tawaha, A.R.M.S.; Laishevtcev, A.; Shariati, M.A. Characterization of Compisote Edible Films from Aloe Vera Gel, Beeswax and Chitosan. Potravin. Slovak J. Food Sci. 2019, 13, 854–862. [Google Scholar] [CrossRef][Green Version]
- Lopez, O.; Garcia, M.A.; Villar, M.A.; Gentili, A.; Rodriguez, M.S.; Albertengo, L. Thermo-Compression of Biodegradable Thermoplastic Corn Starch Films Containing Chitin and Chitosan. LWT–Food Sci. Technol. 2014, 57, 106–115. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Liu, J.; Dai, B.; Hu, S.; Hong, R.; Xie, H.; Li, Z.; Chen, Y.; Zeng, G. Preparation, Reinforcement and Properties of Thermoplastic Starch Film by Film Blowing. Food Hydrocoll. 2020, 108, 106006. [Google Scholar] [CrossRef]
- Vedove, T.M.A.R.D.; Maniglia, B.C.; Tadini, C.C. Production of Sustainable Smart Packaging Based on Cassava Starch and Anthocyanin by an Extrusion Process. J. Food Eng. 2021, 289, 110274. [Google Scholar] [CrossRef]
- Hazrol, M.D.; Sapuan, S.M.; Zainudin, E.S.; Zuhri, M.Y.M.; Abdul Wahab, N.I. Corn Starch (Zea mays) Biopolymer Plastic Reaction in Combination with Sorbitol and Glycerol. Polymers 2021, 13, 242. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C. Retrogradation and Antiplasticization of Thermoplastic Starch. In Thermoplastic Elastomers; IntechOpen: London, UK, 2012; pp. 117–134. [Google Scholar]
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the Integrity of Natural Biopolymer Films Used in Food Packaging by Crosslinking Approach: A Review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef]
- Nevoralová, M.; Koutný, M.; Ujčić, A.; Horák, P.; Kredatusová, J.; Šerá, J.; Růžek, L.; Růžková, M.; Krejčíková, S.; Šlouf, M.; et al. Controlled Biodegradability of Functionalized Thermoplastic Starch Based Materials. Polym. Degrad. Stab. 2019, 170, 108995. [Google Scholar] [CrossRef]
- Niu, X.; Ma, Q.; Li, S.; Wang, W.; Ma, Y.; Zhao, H.; Sun, J.; Wang, J. Preparation and Characterization of Biodegradable Composited Films Based on Potato Starch/Glycerol/Gelatin. J. Food Qual. 2021, 2021, 6633711. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Wang, Y.; Xiong, G.; Mei, X.; Wu, W.; Ding, A.; Li, X.; Qiao, Y.; Liao, L. Effects of Fatty Acid Chain Length on Properties of Potato Starch–Fatty Acid Complexes under Partially Gelatinization. Int. J. Food Prop. 2018, 21, 2121–2134. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Development of Thermoplastic Starch Blown Film by Incorporating Plasticized Chitosan. Carbohydr. Polym. 2015, 115, 575–581. [Google Scholar] [CrossRef]

| SAMPLE | TS ± SD | EAB ± SD | YM ± SD | Thick ± SD |
|---|---|---|---|---|
| TPS (control) | 1.03 ± 0.34 a | 11.26 ± 1.88 a | 51.92 ± 15.50 a | 0.2342 ± 0.03 a |
| TPS + 10% AV | 4.48 ± 1.68 b | 5.63 ± 1.87 b | 189.79 ± 106.49 b | 0.4357 ± 0.08 b |
| TPS + 20% AV | 6.71 ± 1.90 c | 1.62 ± 0.48 c | 443.04 ± 284.27 c | 0.4471 ± 0.08 c |
| TPS + 30% AV | 7.78 ± 1.67 d | 2.11 ± 0.99 d | 504.33 ± 174.98 d | 0.4484 ± 0.06 d |
| TPS + 40% AV | 8.96 ± 1.80 e | 1.83 ± 0.82 e | 687.67 ± 159.46 e | 0.5094 ± 0.07 e |
| TPS + 50% AV | 9.14 ± 1.47 f | 2.02 ± 0.99 f | 769.00 ± 88.07 f | 0.5127 ± 0.05 f |
| Characterization | TPS Film | TPS/10% AV–TPS/50% AV |
|---|---|---|
| Tensile strength, MPa | 1.03 ± 0.34 a | 4.48 ± 1.68 b–9.14 ± 1.47 f |
| Elongation at break, % | 11.26 ± 1.88 a | 5.63 ± 1.87 b–2.02 ± 0.99 f |
| Young’s modulus, MPa | 51.92 ± 15.50 a | 189.79 ± 106.49 b–769.00 ± 88.07 f |
| Water absorption, % | 134.33 ± 10.69 a | 237.67 ± 13.05 b–326.46 ± 4.43 f |
| Water solubility, % | 57.44 ± 2.52 a | 55.74 ± 4.07 b–46.65 ± 1.76 f |
| Maximum temperature, Tmax, °C | 304.87 | 307.75–312.29 |
| Melting temperature, Tm, °C | 306.89 | 307.01–320.93 |
| Crystallinity, % | 17.2458 | 17.6077–24.4592 |
| Thickness, mm | 0.2342 ± 0.03 a | 0.4357 ± 0.08 b–0.5127 ± 0.05 f |
| Physical appearance |  |  – – |
| Morphology |  |  – – |
| Functional group | 3288 cm−1 (broader and sharper) 3275 cm−1 (slighter) 2920 and 2871 cm−1 (shifted) 1647 cm−1 (sharpened) 1640 cm−1 (dampened) 1646 cm−1 (shifted) 1410 cm−1, 1366 cm−1, and 1244 cm−1 (stronger) | |
| Sources | Method | Formulation | TS (MPa) | EAB (%) | YM (MPa) | TGA (°C) | Tm (°C) | Crystallinity (%) | Thickness (mm) | Water Absorption (%) | Water Solubility (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| This paper | Melt-blend and hot-press technique | Potato starch + glycerol + AV gel: 10–50% | 9.14 | 2.11 | 769 | 304.87 increased to 307.75–312.29 | Peak shift from: 105 to 134, 306 to 320. Peak disappeared: 239.99 | 24.46 | 0.52 | 326.46 | 46.65 |
| [34] | Film-forming solution | Plantain flour + glycerol + AV gel: 0–6% | - | - | - | 100, 160–290, 330 | - | 9–16 | 0.017–0.042 | - | 55–57 |
| [33] | Film-forming solution | Plantain flour + glycerol + AV gel: 0–6% | 1.43–2.1 | 24–31 | 82–190 | - | - | - | - | - | - |
| [30] | Casting | Corn starch + glycerol + AV: starch: 1:3, 1:2, and 1:1 | - | - | - | - | - | 6.8–13.1 | 0.064–0.067 | - | - |
| [32] | Film-forming solution | Plantain starch + chitosan + sorbitol + AV gel: 200–1000% | 9.7–4.6 | 30.3–10.4 | - | - | - | - | 0.0735–0.1745 | - | 36.3–45.2 |
| [36] | Casting | Banana starch + glycerol + AV gel + different types of curcumin | 3.74–5.01 | 45.4–56.3 | - | 70, 201, 300 | - | No significant effect | 0.096–0.1404 | - | 11.8–32.9 |
| [31] | Film-forming solution | Potato starch + chitosan + AV gel: 10–50% | - | - | - | First stage: 78–95 Second stage: 247, 278, 303 | - | 25.63–33.71 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Karim, S.F.; Idris, J.; Jai, J.; Musa, M.; Ku Hamid, K.H. Production of Thermoplastic Starch-Aloe vera Gel Film with High Tensile Strength and Improved Water Solubility. Polymers 2022, 14, 4213. https://doi.org/10.3390/polym14194213
Abd Karim SF, Idris J, Jai J, Musa M, Ku Hamid KH. Production of Thermoplastic Starch-Aloe vera Gel Film with High Tensile Strength and Improved Water Solubility. Polymers. 2022; 14(19):4213. https://doi.org/10.3390/polym14194213
Chicago/Turabian StyleAbd Karim, Siti Fatma, Juferi Idris, Junaidah Jai, Mohibah Musa, and Ku Halim Ku Hamid. 2022. "Production of Thermoplastic Starch-Aloe vera Gel Film with High Tensile Strength and Improved Water Solubility" Polymers 14, no. 19: 4213. https://doi.org/10.3390/polym14194213
APA StyleAbd Karim, S. F., Idris, J., Jai, J., Musa, M., & Ku Hamid, K. H. (2022). Production of Thermoplastic Starch-Aloe vera Gel Film with High Tensile Strength and Improved Water Solubility. Polymers, 14(19), 4213. https://doi.org/10.3390/polym14194213