Synthesis and Evaluation of a Silver Nanoparticle/Polyurethane Composite That Exhibits Antiviral Activity against SARS-CoV-2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterisation
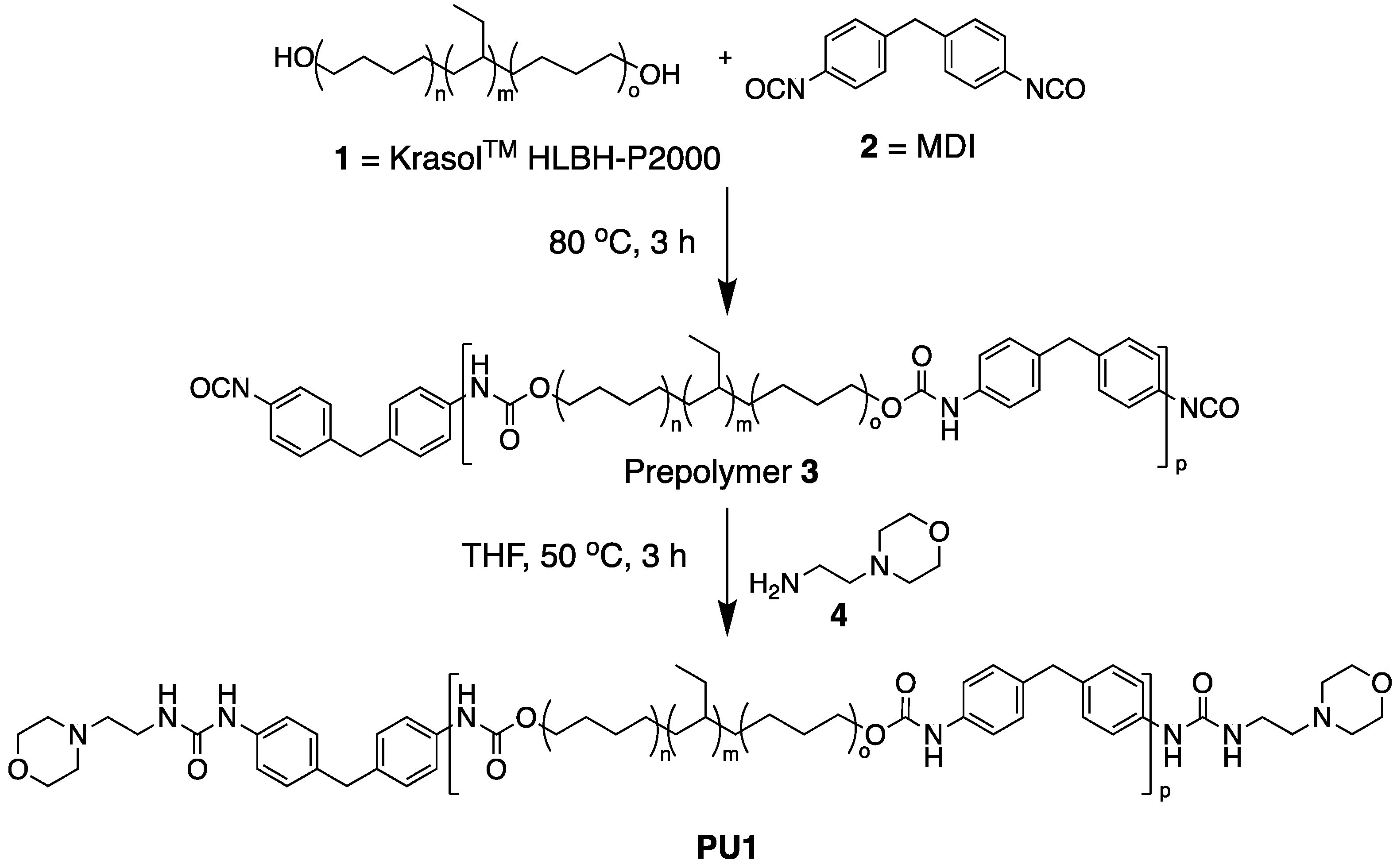
2.3. Synthesis of PU1
2.4. Film Casting and Composite Formation
2.5. Biological Testing
3. Results and Discussion
3.1. Polymer Design and Synthesis
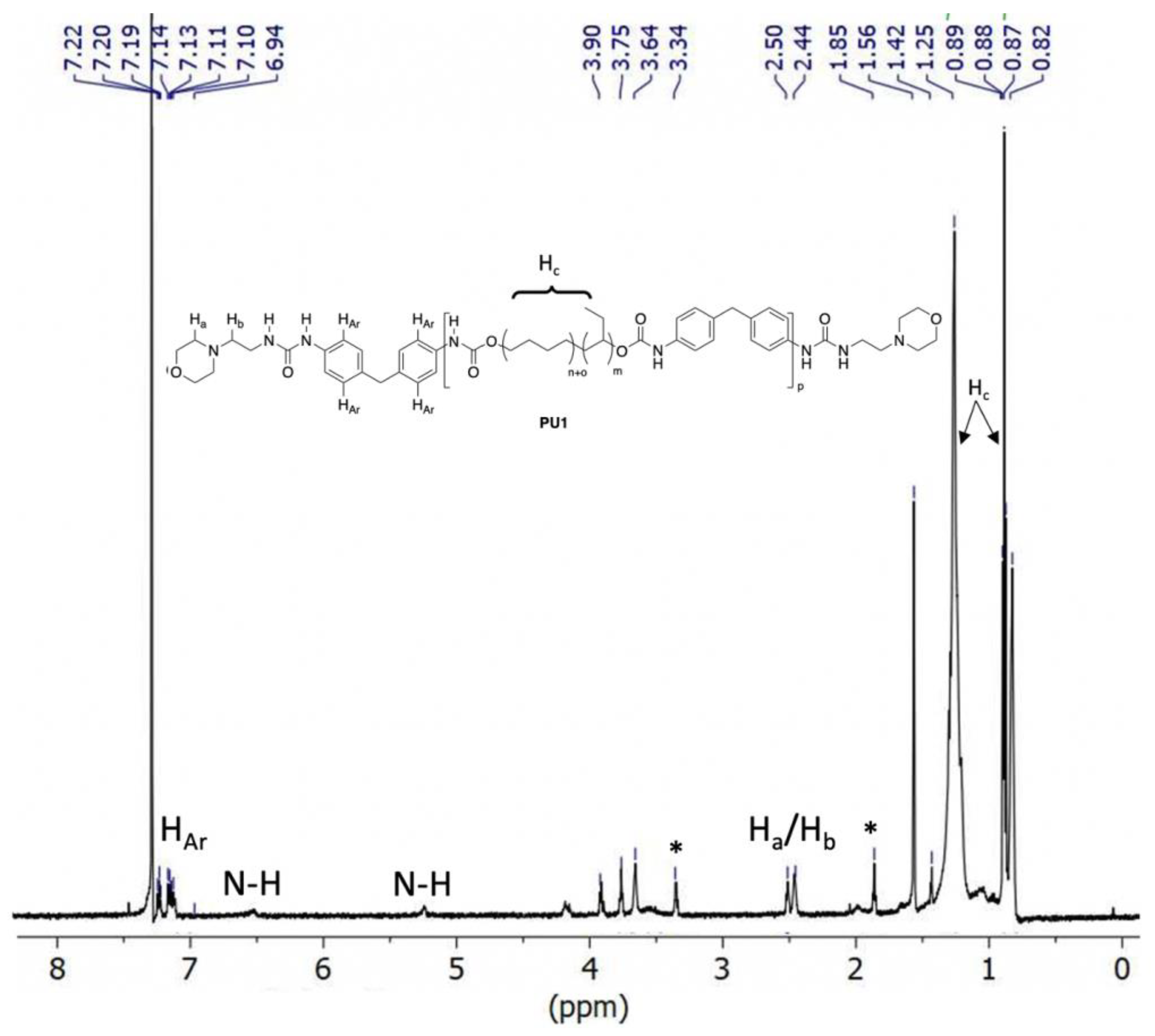
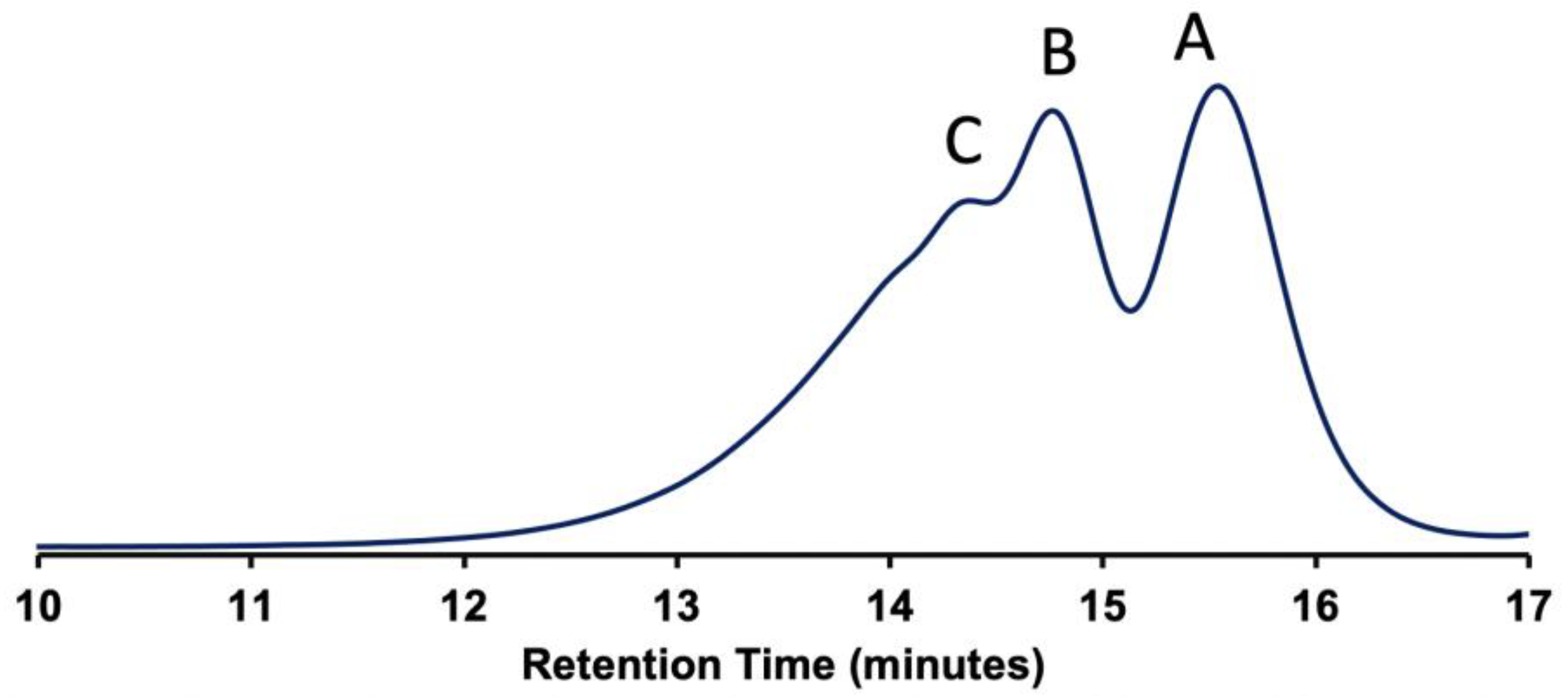
3.2. Polymer Characterisation
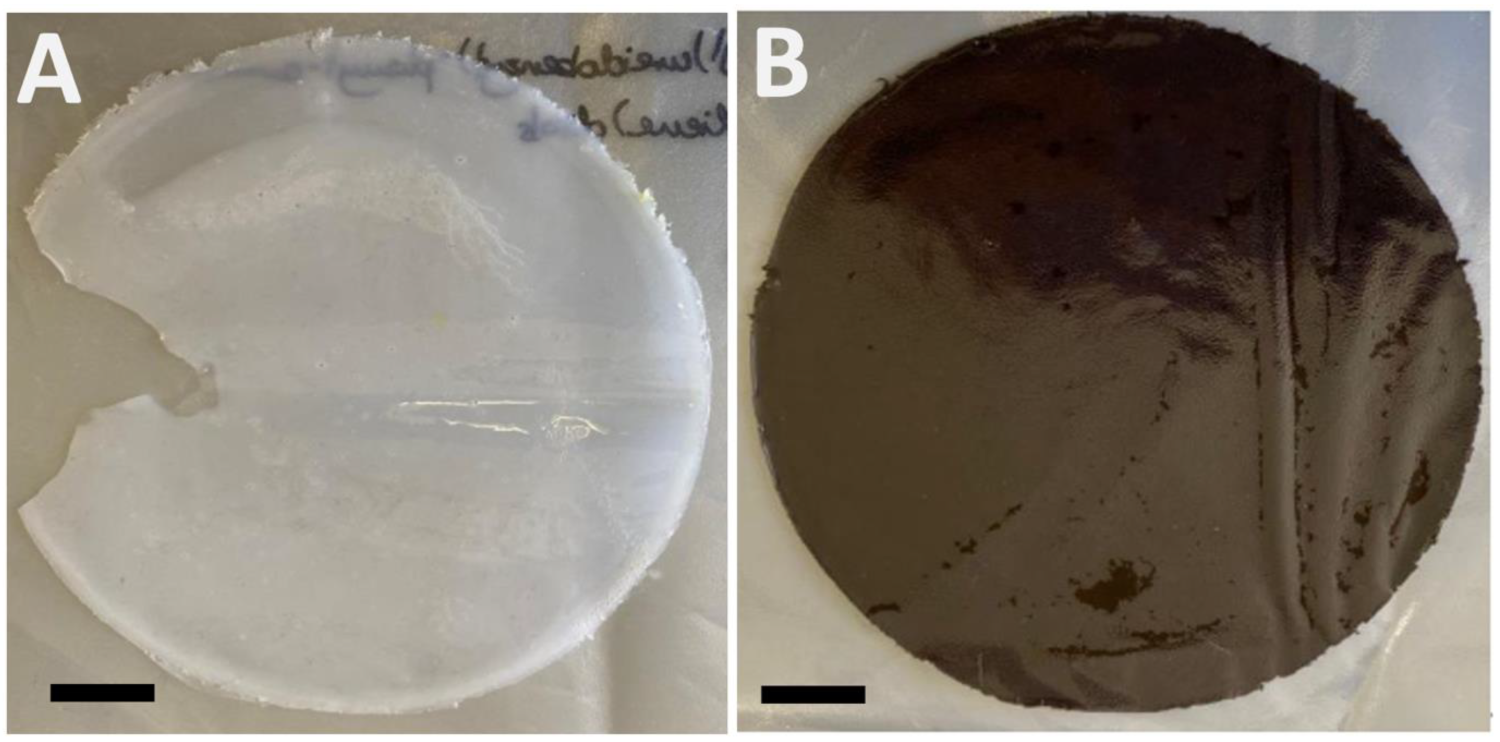
4. Composite Formation and Film Casting
4.1. Film Casting and IR Spectroscopic Analysis
4.2. Strength Testing
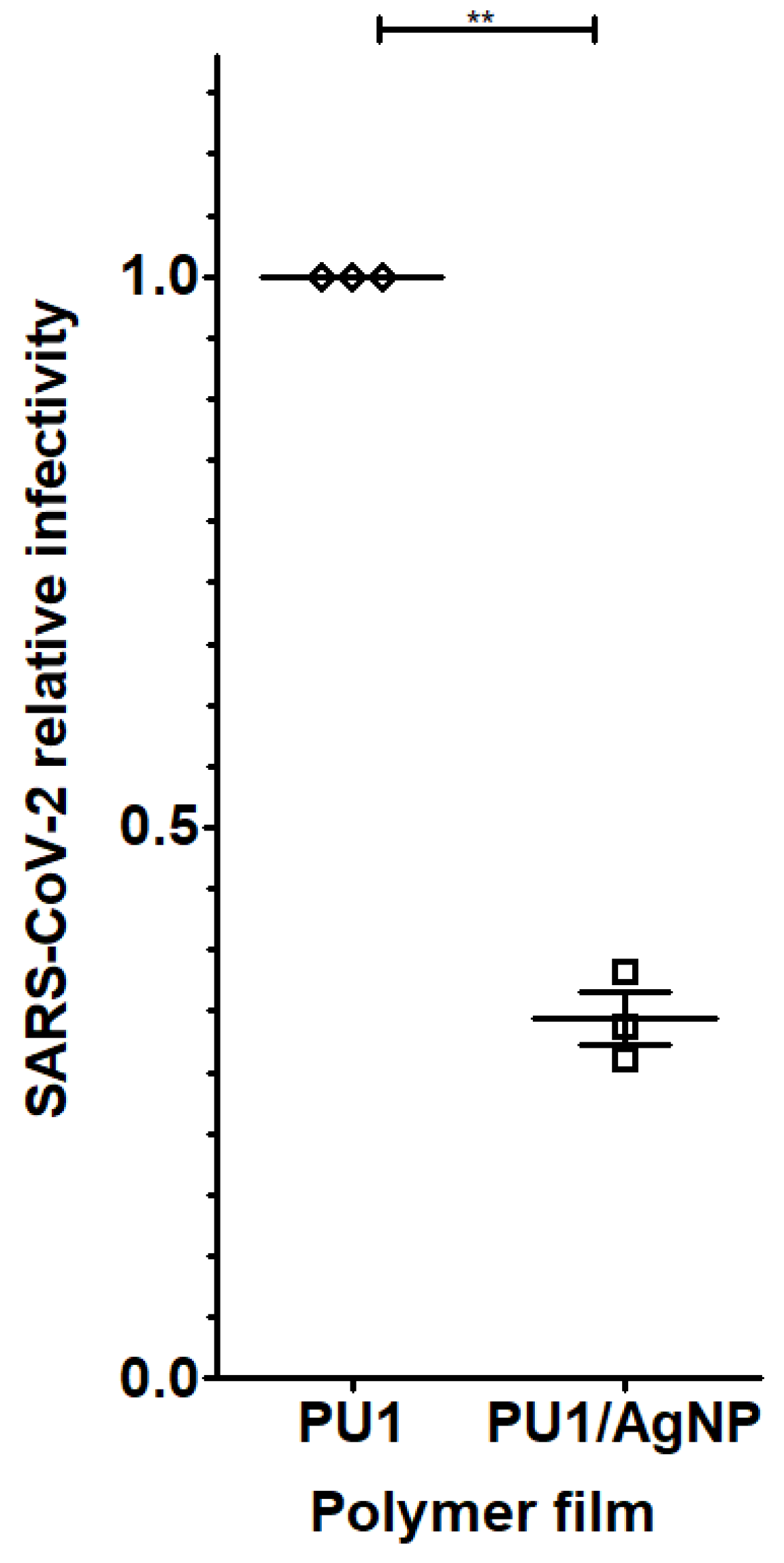
4.3. Biological Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (accessed on 28 June 2022).
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 28 June 2022).
- Meselson, M.N. Droplets and Aerosols in the Transmission of SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2063. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Morris, D.H.; Holbrook, M.G.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Chin, A.W.H.; Chu, J.T.S.; Perera, M.R.A.; Hui, K.P.Y.; Yen, H.-L.; Chan, M.C.W.; Peiris, M.; Poon, L.L.M. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef]
- Goldman, E. Exaggerated risk of transmission of COVID-19 by fomites. Lancet Infect. Dis. 2020, 20, 892–893. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Hermida-Merino, D.; Belal, M.; Greenland, B.W.; Woodward, P.; Slark, A.; Davis, F.; Mitchell, G.; Hamley, I.; Hayes, W. Electrospun supramolecular polymer fibres. Eur. Polym. J. 2012, 48, 1249–1255. [Google Scholar] [CrossRef]
- Bean, B.; Moore, B.M.; Sterner, B.; Peterson, L.R.; Gerding, D.N.; Balfour, H.H. Survival of influenza viruses on environmental surfaces. J. Infect. Dis. 1982, 146, 47–51. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Sharafeldin, T.A.; Goyal, S.M. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transbound. Emerg. Dis. 2021, 68, 296–312. [Google Scholar] [CrossRef]
- Haldar, J.; An, D.; de Cienfuegos, L.A.; Chen, J.; Klibanov, A.M. Polymeric coatings that inactivate both influenza virus and pathogenic bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 17667–17671. [Google Scholar] [CrossRef]
- Wang, Y.; Canady, T.D.; Zhou, Z.; Tang, Y.; Price, D.N.; Bear, D.G.; Chi, E.Y.; Schanze, K.S.; Whitten, D.G. Cationic phenylene ethynylene polymers and oligomers exhibit efficient antiviral activity. ACS Appl. Mater. Interfaces 2011, 3, 2209–2214. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, U.C.; Shrivastava, R. Interaction of viral proteins with metal ions: Role in maintaining the structure and functions of viruses. FEMS Immunol. Med. Microbiol. 2005, 43, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Lazarczyk, M.; Favre, M. Role of Zn2+ Ions in Host-Virus Interactions. J. Virol. 2008, 82, 11486–11494. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.; Tamura, H. Biomedical Applications of Chitin Nanomaterials: A Short Review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Vimbela, G.V.; Ngo, S.M.; Fraze, C.; Yang, L.; Stout, D.A. Antibacterial properties and toxicity from metallic nanomaterials. Int. J. Nanomed. 2017, 12, 3941–3965. [Google Scholar] [CrossRef]
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Berasain, M.A.; Gómez, M.; Ramírez, C.; Vidal, R.M. Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities. Antimicrob. Resist. Infect. Control. 2019, 8, 3. [Google Scholar] [CrossRef]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species—Bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Nedelcu, I.-A.; Ficai, A.; Sonmez, M.; Ficai, D.; Oprea, O.; Andronescu, E. Silver Based Materials for Biomedical Applications. Curr. Org. Chem. 2014, 18, 173–184. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Oprea, O.-C.; Ficai, A.; Ene, V.-L.; Vasile, B.-S.; Andronescu, E.; Holban, A.-M. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil—Innovative Packaging for Cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- Pica, A.; Guran, C.; Andronescu, E.; Oprea, O.; Ficai, D.; Ficai, A. Antimicrobial performances of some film forming materials based on silver nanoparticles. J. Optoelectron. Adv. Mater. 2012, 14, 863–868. [Google Scholar]
- Lin, N.; Verma, D.; Saini, N.; Arbi, R.; Munir, M.; Jovic, M.; Turak, A. Antiviral nanoparticles for sanitizing surfaces: A roadmap to self-sterilizing against COVID-19. Nano Today 2021, 40, 101267. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Salas, B.; Beltran-Partida, E.; Cheng, N.; Salvador-Carlos, J.; Valdez-Salas, E.A.; Curiel-Alvarez, M.; Ibarra-Wiley, R. Promotion of Surgical Masks Antimicrobial Activity by Disinfection and Impregnation with Disinfectant Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 2689–2701. [Google Scholar] [CrossRef] [PubMed]
- Vaiyapuri, R.; Greenland, B.W.; Colquhoun, H.M.; Elliott, J.M.; Hayes, W. Molecular recognition between functionalized gold nanoparticles and healable, supramolecular polymer blends—A route to property enhancement. Polym. Chem. 2013, 4, 4902–4909. [Google Scholar] [CrossRef]
- Vaiyapuri, R.; Greenland, B.W.; Colquhoun, H.M.; Elliott, J.M.; Hayes, W. Evolution of supramolecular healable composites: A minireview. Polym. Int. 2013, 63, 933–942. [Google Scholar] [CrossRef]
- Salimi, S.; Babra, T.S.; Dines, G.; Baskerville, S.W.; Hayes, W.; Greenland, B.W. Composite polyurethane adhesives that debond-on-demand by hysteresis heating in an oscillating magnetic field. Eur. Polym. J. 2019, 121, 109264. [Google Scholar] [CrossRef]
- Topuz, F.; Uyar, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020, 130, 108927. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R. Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef]
- Daima, H.K.; Selvakannan, P.R.; Kandjani, A.E.; Shukla, R.; Bhargava, S.K.; Bansal, V. Synergistic influence of polyoxometalate surface corona towards enhancing the antibacterial performance of tyrosine-capped Ag nanoparticles. Nanoscale 2014, 6, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Clement, J.L.; Jarrett, P.S. Antibacterial Silver. Met.-Based Drugs 1994, 1, 707103. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Coey, J.D.; O’Rourke, C.; Bamford, C.G.; Mills, A. Flexible, disposable photocatalytic plastic films for the destruction of viruses. J. Photochem. Photobiol. B Biol. 2022, 235, 112551. [Google Scholar] [CrossRef] [PubMed]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Szycher, M. Szycher’s Handbook of Polyurethanes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-4398-6313-8. [Google Scholar]
- Babra, T.S.; Wood, M.; Godleman, J.S.; Salimi, S.; Warriner, C.; Bazin, N.; Siviour, C.R.; Hamley, I.W.; Hayes, W.; Greenland, B.W. Fluoride-responsive debond on demand adhesives: Manipulating polymer crystallinity and hydrogen bonding to optimise adhesion strength at low bonding temperatures. Eur. Polym. J. 2019, 119, 260–271. [Google Scholar] [CrossRef]
- Woodward, P.J.; Hermida-Merino, D.; Greenland, B.W.; Hamley, I.; Light, Z.; Slark, A.T.; Hayes, W. Hydrogen Bonded Supramolecular Elastomers: Correlating Hydrogen Bonding Strength with Morphology and Rheology. Macromolecules 2010, 43, 2512–2517. [Google Scholar] [CrossRef]
- Hermida-Merino, D.; Feula, A.; Melia, K.; Slark, A.T.; Giannakopoulos, I.; Siviour, C.R.; Buckley, C.P.; Greenland, B.W.; Liu, D.; Gan, Y.; et al. A systematic study of the effect of the hard end-group composition on the microphase separation, thermal and mechanical properties of supramolecular polyurethanes. Polymer 2016, 107, 368–378. [Google Scholar] [CrossRef]
- Houton, K.A.; Wilson, A.J. Hydrogen-bonded supramolecular polyurethanes. Polym. Int. 2014, 64, 165–173. [Google Scholar] [CrossRef]
- O’Donnell, A.D.; Salimi, S.; Hart, L.R.; Babra, T.; Greenland, B.; Hayes, W. Applications of supramolecular polymer networks. React. Funct. Polym. 2022, 172, 105209. [Google Scholar] [CrossRef]
- Yan, X.Z.; Wang, F.; Zheng, B.; Huang, F. Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 2012, 41, 6042–6065. [Google Scholar] [CrossRef] [PubMed]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular Polymers. Chem. Rev. 2001, 101, 4071–4098. [Google Scholar] [CrossRef] [PubMed]
- De Greef, T.F.A.; Smulders, M.M.J.; Wolffs, M.; Schenning, A.P.H.J.; Sijbesma, R.P.; Meijer, E.W. Supramolecular Polymerization. Chem. Rev. 2009, 109, 5687–5754. [Google Scholar] [CrossRef] [PubMed]
- Feula, A.; Tang, X.; Giannakopoulos, I.; Chippindale, A.M.; Hamley, I.W.; Greco, F.; Buckley, C.P.; Siviour, C.R.; Hayes, W. An adhesive elastomeric supramolecular polyurethane healable at body temperature. Chem. Sci. 2016, 7, 4291–4300. [Google Scholar] [CrossRef]
- Babra, T.S.; Trivedi, A.; Warriner, C.N.; Bazin, N.; Castiglione, D.; Sivour, C.; Hayes, W.; Greenland, B.W. Fluoride Degradable and thermally responsible debondable polyurethane based adhesive. Polym. Chem. 2017, 8, 7207–7216. [Google Scholar] [CrossRef]
- Anancharoenwong, E.; Chueangchayaphan, W.; Rakkapao, N.; Marthosa, S.; Chaisrikhwun, B. Thermo-mechanical and antimicrobial properties of natural rubber-based polyurethane nanocomposites for biomedical applications. Polym. Bull. 2021, 78, 833–848. [Google Scholar] [CrossRef]
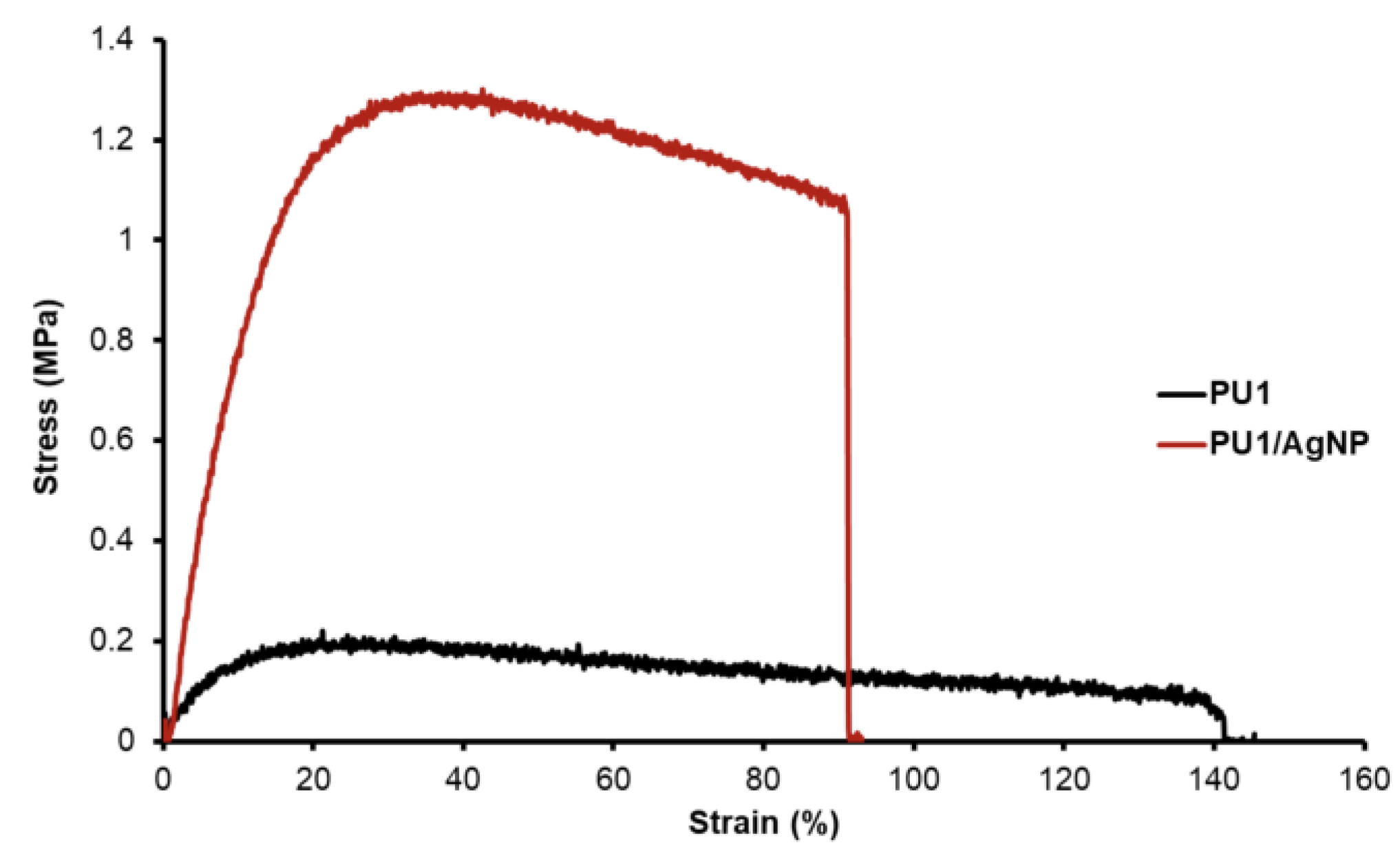
| Material | Ultimate Tensile Strength, MPa (s.d.) | Tensile Modulus, MPa (s.d.) | Modulus of Toughness, MJ m−3 (s.d.) | Elongation at Break % (s.d) |
|---|---|---|---|---|
| PU1 | 0.18 (0.02) | 2.37 (0.56) | 0.22 (0.04) | 243 (106) |
| PU1/AgNP | 1.20 (0.26) | 10.87 (3.81) | 0.52 (0.32) | 53 (27) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, W.T.; Babra, T.S.; Smith, J.H.D.; Bagley, M.C.; Spencer, J.; Wright, E.; Greenland, B.W. Synthesis and Evaluation of a Silver Nanoparticle/Polyurethane Composite That Exhibits Antiviral Activity against SARS-CoV-2. Polymers 2022, 14, 4172. https://doi.org/10.3390/polym14194172
Lam WT, Babra TS, Smith JHD, Bagley MC, Spencer J, Wright E, Greenland BW. Synthesis and Evaluation of a Silver Nanoparticle/Polyurethane Composite That Exhibits Antiviral Activity against SARS-CoV-2. Polymers. 2022; 14(19):4172. https://doi.org/10.3390/polym14194172
Chicago/Turabian StyleLam, Wing T., Tahkur S. Babra, Julian H. D. Smith, Mark C. Bagley, John Spencer, Edward Wright, and Barnaby W. Greenland. 2022. "Synthesis and Evaluation of a Silver Nanoparticle/Polyurethane Composite That Exhibits Antiviral Activity against SARS-CoV-2" Polymers 14, no. 19: 4172. https://doi.org/10.3390/polym14194172
APA StyleLam, W. T., Babra, T. S., Smith, J. H. D., Bagley, M. C., Spencer, J., Wright, E., & Greenland, B. W. (2022). Synthesis and Evaluation of a Silver Nanoparticle/Polyurethane Composite That Exhibits Antiviral Activity against SARS-CoV-2. Polymers, 14(19), 4172. https://doi.org/10.3390/polym14194172








