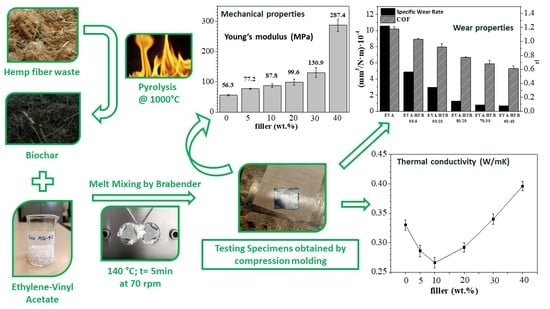
Ethylene-Vinyl Acetate (EVA) Containing Waste Hemp-Derived Biochar Fibers: Mechanical, Electrical, Thermal and Tribological Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Waste Hemp Fiber Pyrolysis and Preparation of Composites
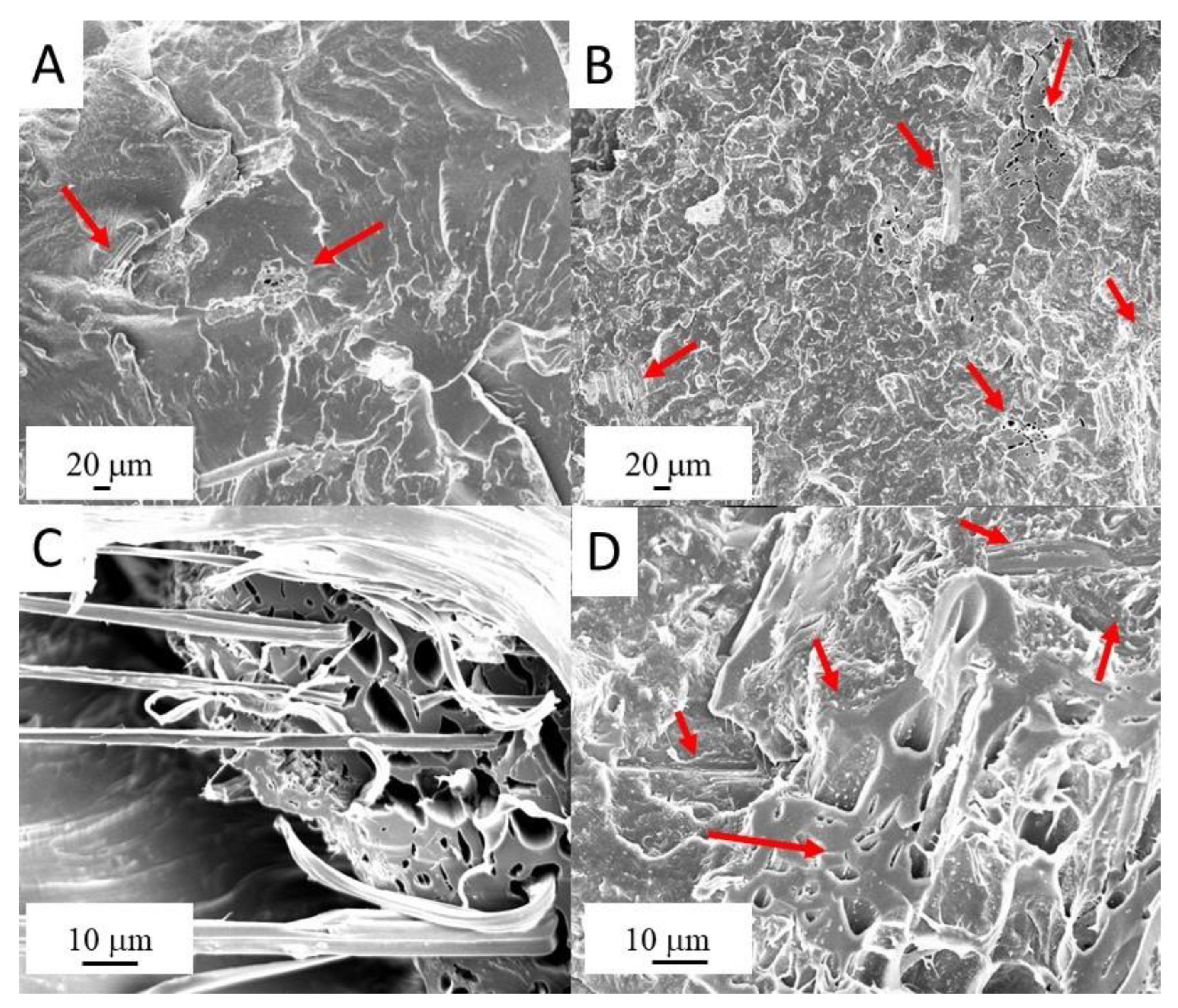
2.2.2. Scanning Electron Microscopy
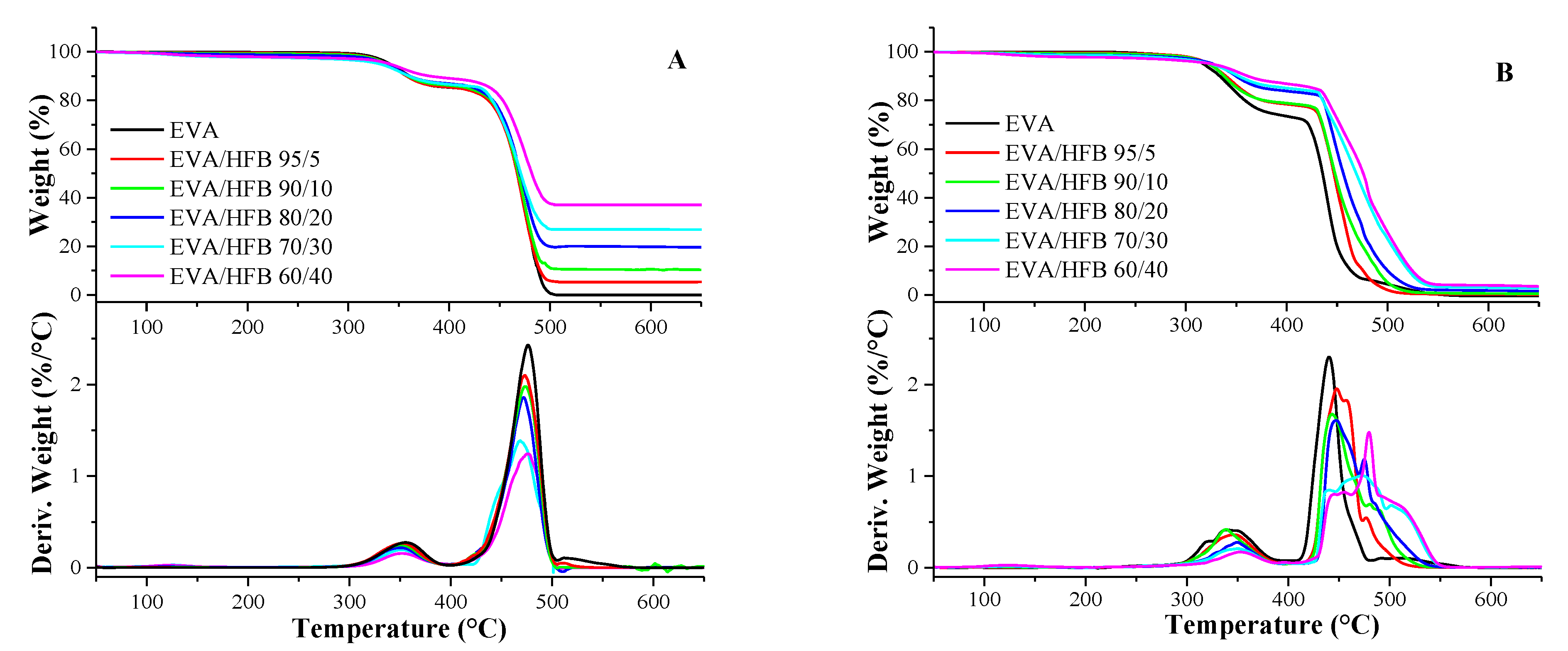
2.2.3. Thermal Analysis: Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
2.2.4. Thermal Conductivity
2.2.5. Electrical Conductivity
2.2.6. Tensile Tests
2.2.7. Tribological Tests
3. Results and Discussion
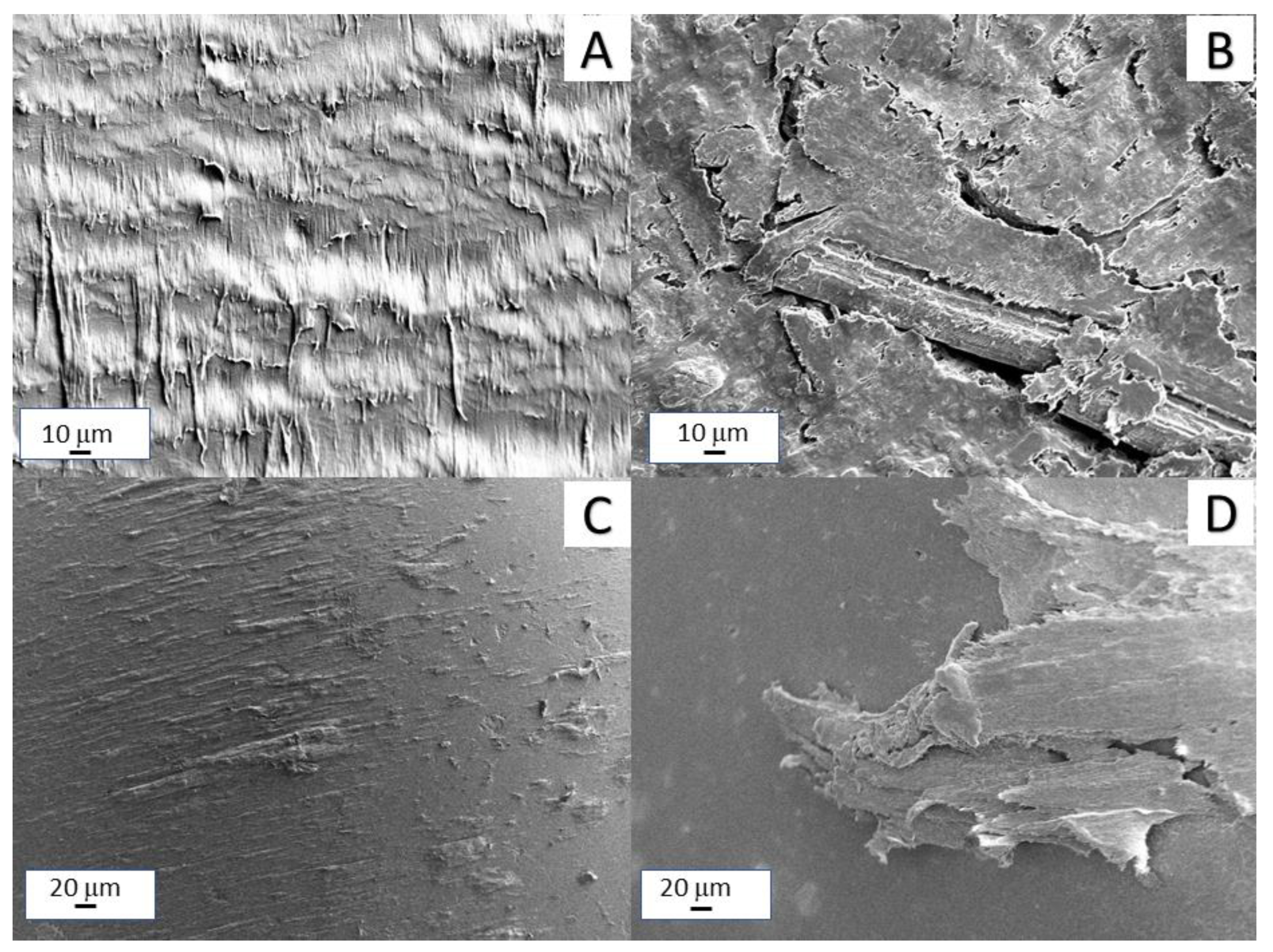
3.1. Morphological Analysis
3.2. Thermal Analyses
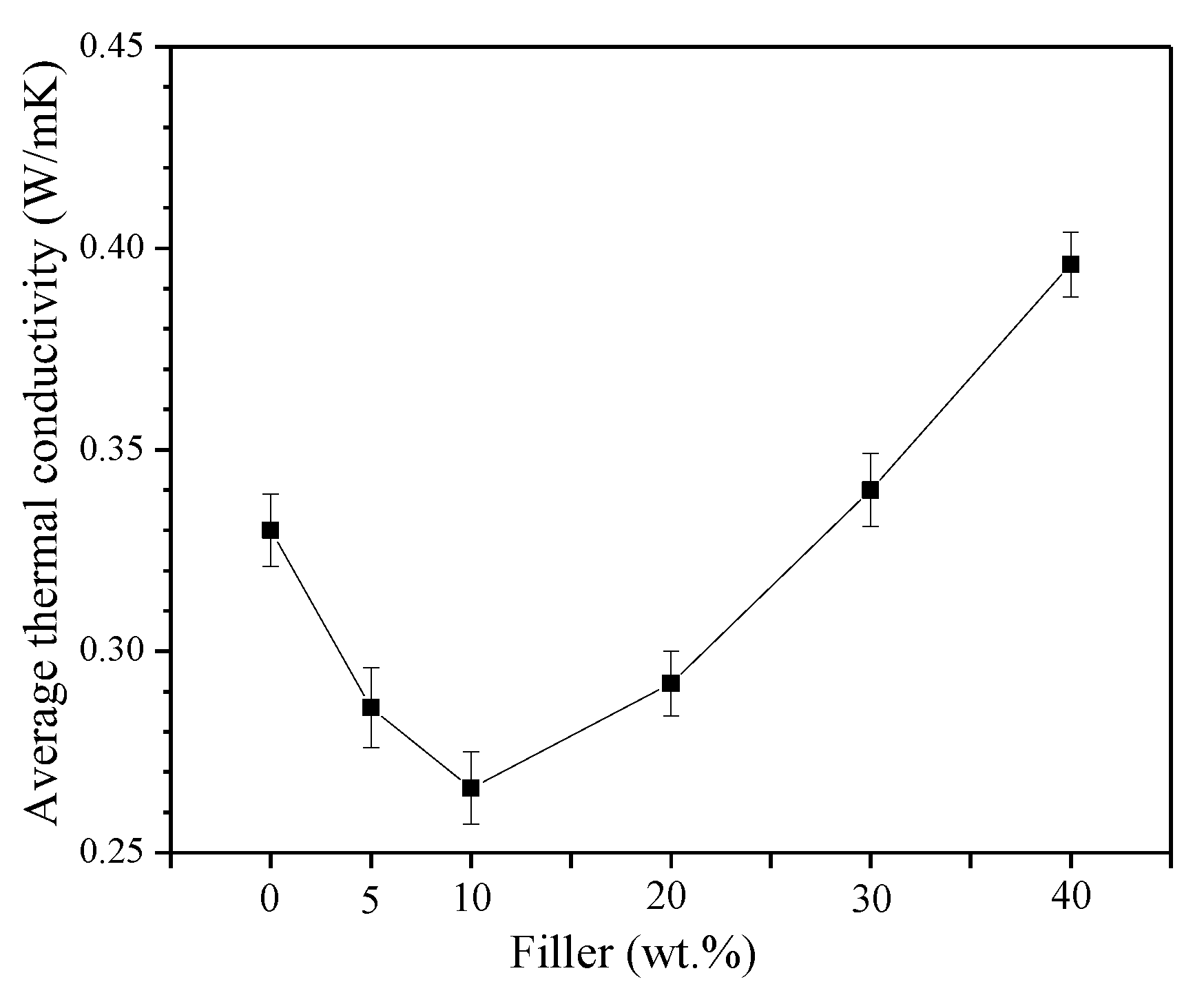
3.3. Thermal Conductivity Measurements
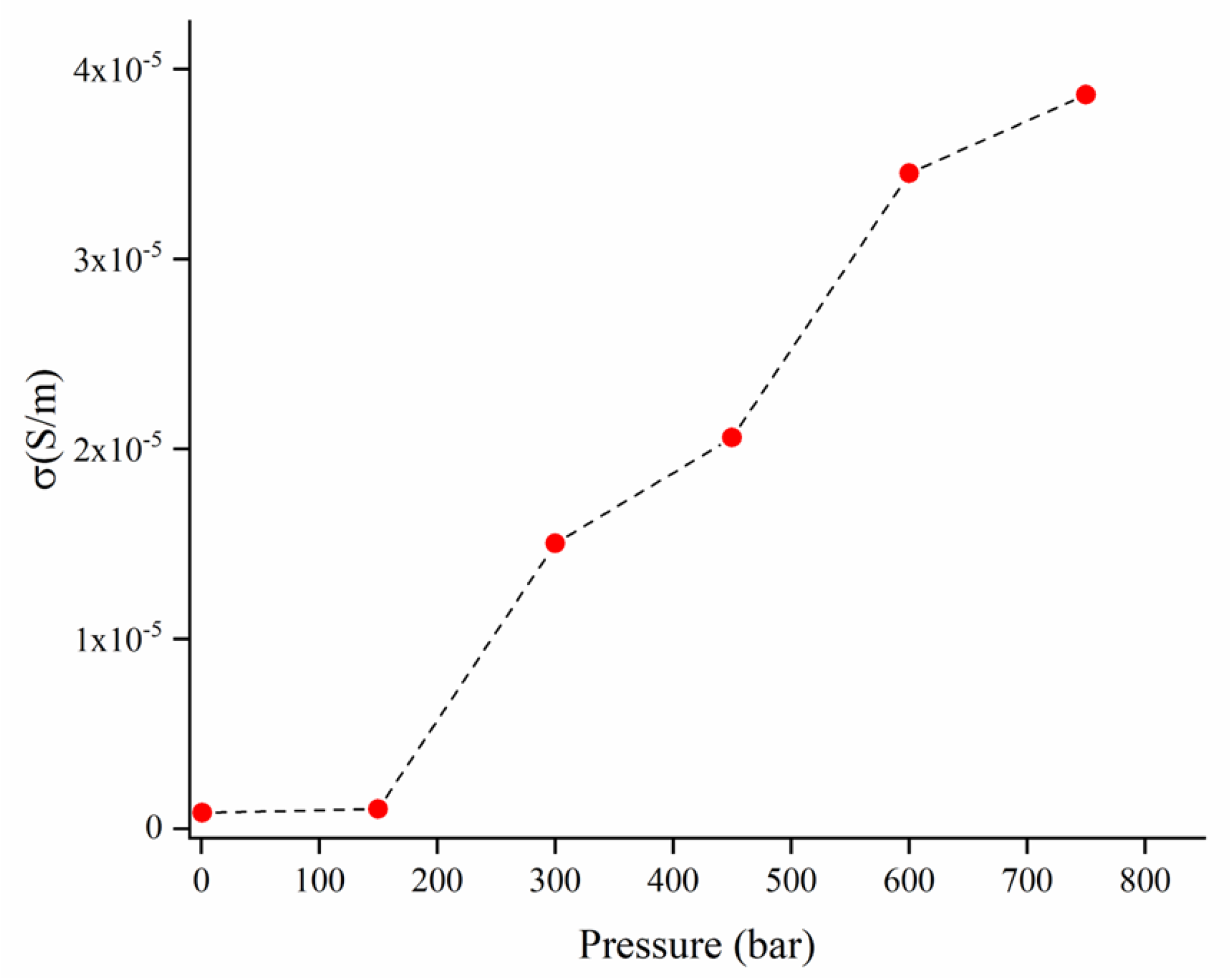
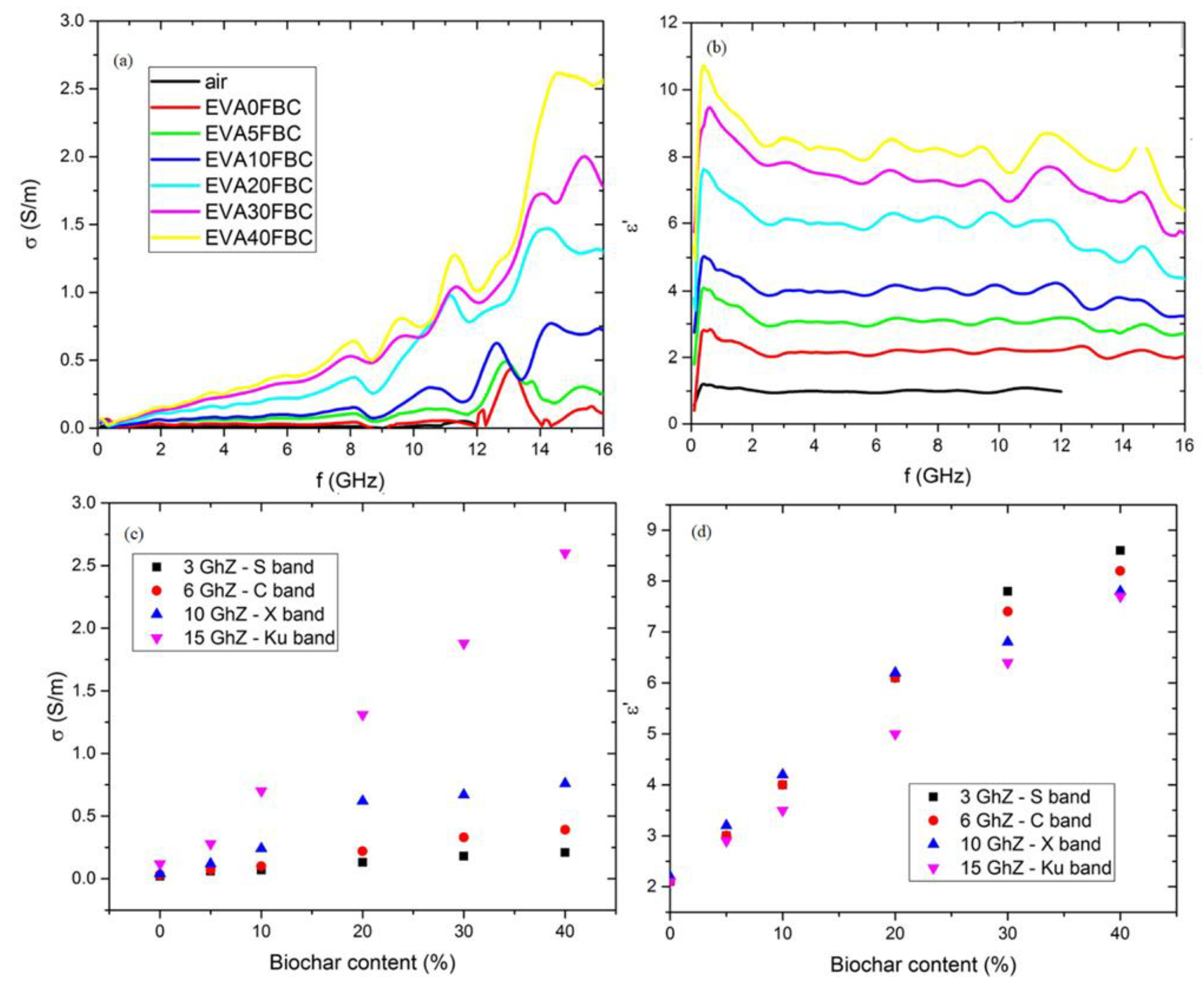
3.4. Electrical Conductivity Measurements
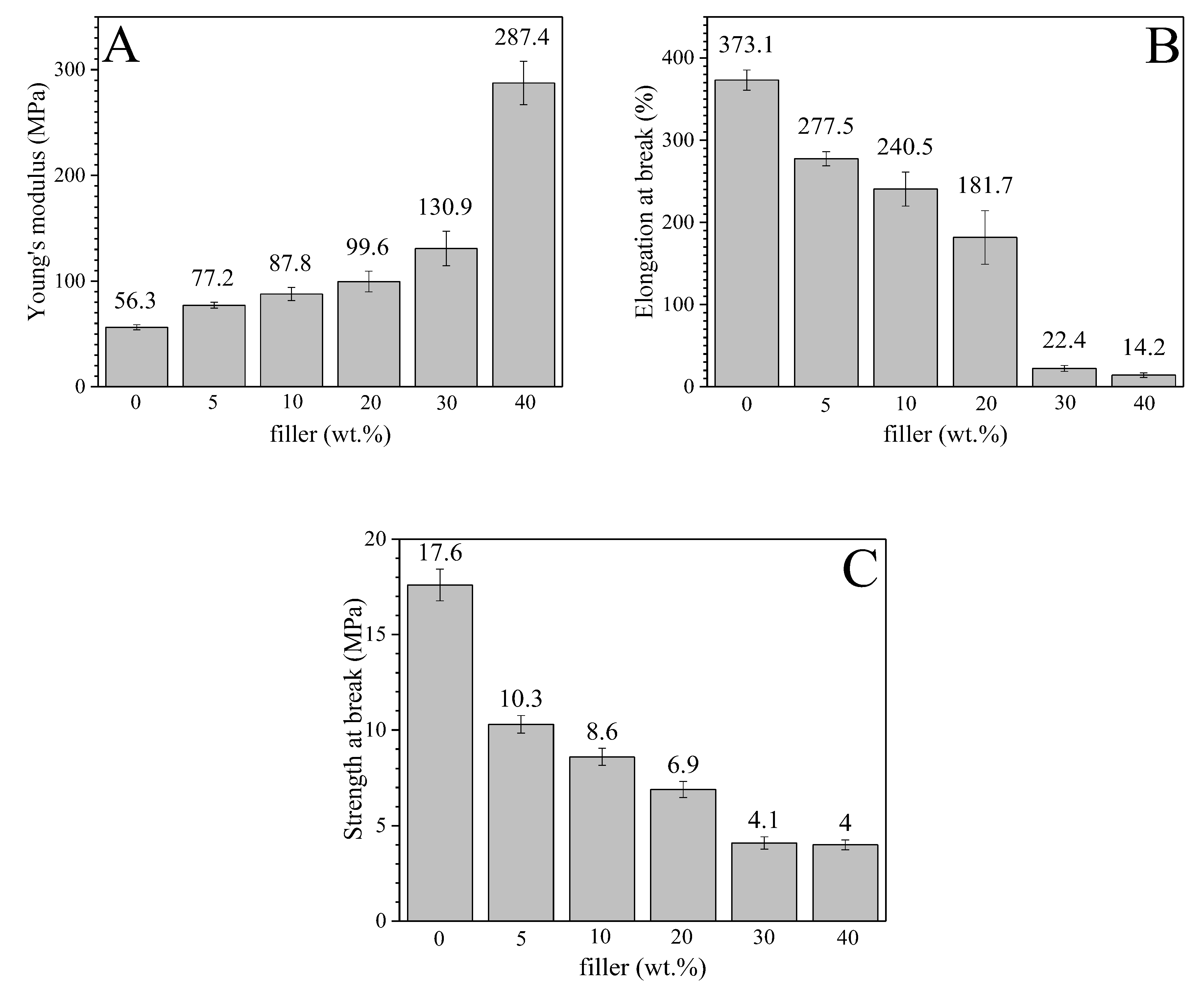
3.5. Mechanical Properties
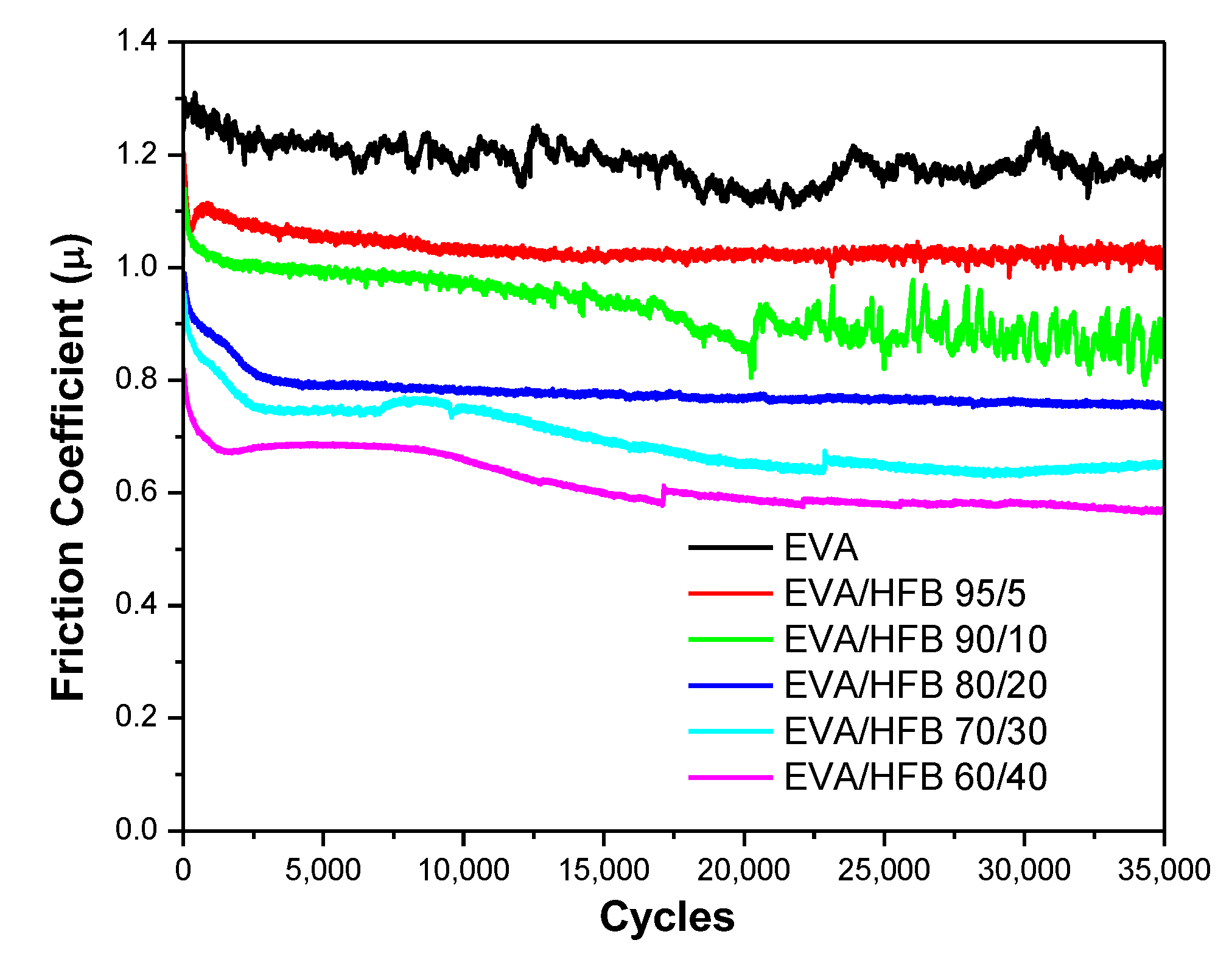
3.6. Tribological Tests
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venkatesh, G. Circular bio-economy—Paradigm for the future: Systematic review of scientific journal publications from 2015 to 2021. Circ. Econ. Sustain. 2021, 2, 231–279. [Google Scholar] [CrossRef]
- Das, O.; Bhattacharyya, D.; Sarmah, A.K. Sustainable eco–composites obtained from waste derived biochar: A consideration in performance properties, production costs, and environmental impact. J. Clean. Prod. 2016, 129, 159–168. [Google Scholar] [CrossRef]
- Espino, E.; Cakir, M.; Domenek, S.; Román-Gutiérrez, A.D.; Belgacem, N.; Bras, J. Isolation and characterization of cellulose nanocrystals from industrial by-products of Agave tequilana and barley. Ind. Crop. Prod. 2014, 62, 552–559. [Google Scholar] [CrossRef]
- Razzaq, H.A.; Gomez d’Ayala, G.; Santagata, G.; Bosco, F.; Mollea, C.; Larsen, N.; Duraccio, D. Bioactive films based on barley β-glucans and ZnO for wound healing applications. Carbohydr. Polym. 2021, 272, 118442. [Google Scholar] [CrossRef]
- Giubilini, A.; Sciancalepore, C.; Messori, M.; Bondioli, F. Valorization of oat hull fiber from agri-food industrial waste as filler for poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). J. Mater. Cycles Waste Manag. 2021, 23, 402–408. [Google Scholar] [CrossRef]
- Gargol, M.; Klepka, T.; Klapiszewski, Ł.; Podkościelna, B. Synthesis and thermo-mechanical study of epoxy resin-based composites with waste fibers of hemp as an eco-friendly filler. Polymers 2021, 13, 503. [Google Scholar] [CrossRef]
- Bartoli, M.; Arrigo, R.; Malucelli, G.; Tagliaferro, A.; Duraccio, D. Recent Advances in Biochar Polymer Composites. Polymers 2022, 14, 2506. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Emerging applications of biochar-based materials for energy storage and conversion. Energy Environ. Sci. 2019, 12, 1751–1779. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Sun, R. Application of biochar-based catalysts in biomass upgrading: A review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef]
- Yasim-Anuar, T.A.T.; Yee-Foong, L.N.; Lawal, A.A.; Farid, M.A.A.; Yusuf, M.Z.M.; Hassan, M.A.; Ariffin, H. Emerging application of biochar as a renewable and superior filler in polymer composites. RSC Adv. 2022, 12, 13938–13949. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, R.; Koubaa, A.; Braghiroli, F.; Migneault, S.; Wang, H.; Bradai, C. Effect of the pyro-gasification temperature of wood on the physical and mechanical properties of biochar-polymer biocomposites. Materials 2020, 13, 1327. [Google Scholar] [CrossRef] [PubMed]
- Poulose, A.M.; Elnour, A.Y.; Anis, A.; Shaikh, H.; Al-Zahrani, S.; George, J.; Al-Wabel, M.I.; Usman, A.R.; Ok, Y.S.; Tsang, D.C. Date palm biochar-polymer composites: An investigation of electrical, mechanical, thermal and rheological characteristics. Sci. Total Environ. 2018, 619, 311–318. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Misra, M.; Mohanty, A.K. Impact of interfacial adhesion on the microstructure and property variations of biocarbons reinforced nylon 6 biocomposites. Compos. Part A Appl. Sci. Manuf. 2017, 98, 32–44. [Google Scholar] [CrossRef]
- Das, O.; Bhattacharyya, D.; Hui, D.; Lau, K.-T. Mechanical and flammability characterisations of biochar/polypropylene biocomposites. Compos. Part B Eng. 2016, 106, 120–128. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Chen, C.; Wang, H.; Deng, Q.; Gong, M.; Li, D. Development of electrically conductive nano bamboo charcoal/ultra-high molecular weight polyethylene composites with a segregated network. Compos. Sci. Technol. 2016, 132, 31–37. [Google Scholar] [CrossRef]
- Giorcelli, M.; Bartoli, M. Development of coffee biochar filler for the production of electrical conductive reinforced plastic. Polymers 2019, 11, 1916. [Google Scholar] [CrossRef]
- Richard, S.; Selwin Rajadurai, J.; Manikandan, V. Effects of particle loading and particle size on tribological properties of biochar particulate reinforced polymer composites. J. Tribol. 2017, 139, 12202. [Google Scholar] [CrossRef]
- Bartoli, M.; Duraccio, D.; Faga, M.G.; Piatti, E.; Torsello, D.; Ghigo, G.; Malucelli, G. Mechanical, electrical, thermal and tribological behavior of epoxy resin composites reinforced with waste hemp-derived carbon fibers. J. Mater. Sci. 2022, 57, 14861–14876. [Google Scholar] [CrossRef]
- Çopuroğlu, M.; Şen, M. A comparative study of UV aging characteristics of poly(ethylene-co-vinyl acetate) and poly(ethylene-co-vinyl acetate)/carbon black mixture. Polym. Adv. Technol. 2005, 16, 61–66. [Google Scholar] [CrossRef]
- Takidis, G.; Bikiaris, D.; Papageorgiou, G.; Achilias, D.; Sideridou, I. Compatibility of low-density polyethylene/poly(ethylene-co-vinyl acetate) binary blends prepared by melt mixing. J. Appl. Polym. Sci. 2003, 90, 841–852. [Google Scholar] [CrossRef]
- Kuila, T.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Functionalized-graphene/ethylene vinyl acetate co-polymer composites for improved mechanical and thermal properties. Polym. Test. 2012, 31, 282–289. [Google Scholar] [CrossRef]
- Hou, Y.H.; Zhang, M.Q.; Rong, M.Z. Performance stabilization of conductive polymer composites. J. Appl. Polym. Sci. 2003, 89, 2438–2445. [Google Scholar] [CrossRef]
- Hou, Y.H.; Zhang, M.Q.; Rong, M.Z.; Yu, G.; Zeng, H.M. Improvement of conductive network quality in carbon black-filled polymer blends. J. Appl. Polym. Sci. 2002, 84, 2768–2775. [Google Scholar] [CrossRef]
- Jyoti, J.; Kumar, A.; Dhakate, S.; Singh, B.P. Dielectric and impedance properties of three dimension graphene oxide-carbon nanotube acrylonitrile butadiene styrene hybrid composites. Polym. Test. 2018, 68, 456–466. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Liang, J.Z. Electrical properties and morphology of carbon black-filled HDPE/EVA composites. J. Appl. Polym. Sci. 2010, 117, 1998–2002. [Google Scholar] [CrossRef]
- Azizi, S.; David, E.; Fréchette, M.F.; Nguyen-Tri, P.; Ouellet-Plamondon, C.M. Electrical and thermal conductivity of ethylene vinyl acetate composite with graphene and carbon black filler. Polym. Test. 2018, 72, 24–31. [Google Scholar] [CrossRef]
- Ratzsch, K.F.; Cecen, V.; Tölle, F.; Wartig, K.A.; Thomann, R.; Mülhaupt, R.; Friedrich, C. Rheology, electrical properties, and percolation of TRGO-filled EVA-copolymers. Macromol. Mater. Eng. 2014, 299, 1134–1144. [Google Scholar] [CrossRef]
- Yuan, N.; Ma, F.; Fan, Y.; Liu, Y.; Ding, J. High conductive ethylene vinyl acetate composites filled with reduced graphene oxide and polyaniline. Compos. Part A Appl. Sci. Manuf. 2012, 43, 2183–2188. [Google Scholar] [CrossRef]
- Das, N.C.; Khastgir, D.; Chaki, T.; Chakraborty, A. Electromagnetic interference shielding effectiveness of carbon black and carbon fibre filled EVA and NR based composites. Compos. Part A Appl. Sci. Manuf. 2000, 31, 1069–1081. [Google Scholar] [CrossRef]
- Lee, B.; Dai, G. Influence of interfacial modification on the thermal conductivity of polymer composites. J. Mater. Sci. 2009, 44, 4848–4855. [Google Scholar] [CrossRef]
- Wang, Z.-G.; Liu, W.; Liu, Y.-H.; Ren, Y.; Li, Y.-P.; Zhou, L.; Xu, J.-Z.; Lei, J.; Li, Z.-M. Highly thermal conductive, anisotropically heat-transferred, mechanically flexible composite film by assembly of boron nitride nanosheets for thermal management. Compos. Part B Eng. 2020, 180, 107569. [Google Scholar] [CrossRef]
- Allan, J.; Pinder, H.; Dehouche, Z. Enhancing the thermal conductivity of ethylene-vinyl acetate (EVA) in a photovoltaic thermal collector. AIP Adv. 2016, 6, 035011. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Guo, Y.; Lu, Y.; Huang, Y.; Xu, H.; Wu, D.; Sun, J. Optimal analysis for thermal conductivity variation of EVA/SCF composites prepared by spatial confining forced network assembly. Mater. Today Commun. 2020, 25, 101206. [Google Scholar] [CrossRef]
- Zec, J.; Tomić, N.Z.; Zrilić, M.; Lević, S.; Marinković, A.; Heinemann, R.J. Optimization of Al2O3 particle modification and UHMWPE fiber oxidation of EVA based hybrid composites: Compatibility, morphological and mechanical properties. Compos. Part B Eng. 2018, 153, 36–48. [Google Scholar] [CrossRef]
- Yao, D.; Yin, G.; Bi, Q.; Yin, X.; Wang, N.; Wang, D.-Y. Basalt fiber modified ethylene vinyl acetate/magnesium hydroxide composites with balanced flame retardancy and improved mechanical properties. Polymers 2020, 12, 2107. [Google Scholar] [CrossRef]
- Zimmermann, M.V.; Turella, T.C.; Santana, R.M.; Zattera, A.J. The influence of wood flour particle size and content on the rheological, physical, mechanical and morphological properties of EVA/wood cellular composites. Mater. Des. 2014, 57, 660–666. [Google Scholar] [CrossRef]
- Sonia, A.; Dasan, K.P.; Alex, R. Celluloses microfibres (CMF) reinforced poly (ethylene-co-vinyl acetate)(EVA) composites: Dynamic mechanical, gamma and thermal ageing studies. Chem. Eng. J. 2013, 228, 1214–1222. [Google Scholar] [CrossRef]
- Silviya, E.; Unnikrishnan, G.; Varghese, S.; Guthrie, J. Thermal and mechanical characterization of EVA/banana fiber-derived cellulose composites. J. Appl. Polym. Sci. 2012, 125, 786–792. [Google Scholar] [CrossRef]
- Ravi Kumar, B.N.; Suresha, B.; Sailaja, R.; Venkataramareddy, M. Experimental investigation on the abrasive wear behavior of nanoclay-filled EVA/LDPE composites. Polym. Compos. 2010, 31, 426–433. [Google Scholar]
- Ravi Kumar, B.N.; Venkataramareddy, M.; Suresha, B. Two-body abrasive wear behavior of nano-clay filled LDPE/EVA composites. J. Reinf. Plast. Compos. 2009, 28, 2999–3007. [Google Scholar] [CrossRef]
- Wang, Y. Fiber and textile waste utilization. Waste Biomass Valorization 2010, 1, 135–143. [Google Scholar] [CrossRef]
- Giraudo, F.; Delmastro, R. Macchina Sfibratrice Per Canapa Ad Uso Industriale. Italy’Patent 10TO2010A000554, 28 June 2010. [Google Scholar]
- Torsello, D.; Bartoli, M.; Giorcelli, M.; Rovere, M.; Arrigo, R.; Malucelli, G.; Tagliaferro, A.; Ghigo, G. High frequency electromagnetic shielding by biochar-based composites. Nanomaterials 2021, 11, 2383. [Google Scholar] [CrossRef] [PubMed]
- Ba, D.; Sabouroux, P. Epsimu, a toolkit for permittivity and permeability measurement in microwave domain at real time of all materials: Applications to solid and semisolid materials. Microw. Opt. Technol. Lett. 2010, 52, 2643–2648. [Google Scholar] [CrossRef]
- Baker-Jarvis, J.; Vanzura, E.J.; Kissick, W.A. Improved technique for determining complex permittivity with the transmission/reflection method. IEEE Trans. Microw. Theory Tech. 1990, 38, 1096–1103. [Google Scholar] [CrossRef]
- Kauzlarich, J.; Williams, J. Archard wear and component geometry. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2001, 215, 387–403. [Google Scholar] [CrossRef]
- Minale, M.; Carotenuto, C.; Paduano, L.P.; Grassia, L. Nonisothermal Crystallization Kinetics of an Ethylene-Vinyl-Acetate: I Calorimetry Versus Rheology. Polym. Eng. Sci. 2019, 59, 2557–2563. [Google Scholar] [CrossRef]
- Almeida, A.; Possemiers, S.; Boone, M.; De Beer, T.; Quinten, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. Eur. J. Pharm. Biopharm. 2011, 77, 297–305. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, J. Thermal conductivity of ethylene-vinyl acetate copolymers with different vinyl acetate contents dependent on temperature and crystallinity. Thermochim. Acta 2022, 708, 179141. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Zhang, J. Non-isothermal crystallization kinetics of partially miscible ethylene-vinyl acetate copolymer/low density polyethylene blends. eXPRESS Polym. Lett. 2010, 4, 141–152. [Google Scholar] [CrossRef]
- Marcilla, A.; Gómez-Siurana, A.; Menargues, S. TG/FTIR study of the thermal pyrolysis of EVA copolymers. J. Anal. Appl. Pyrolysis 2005, 74, 224–230. [Google Scholar] [CrossRef]
- Marcilla, A.; Gómez-Siurana, A.; Menargues, S. Qualitative study of the evolution of the composition of the gas evolved in the thermal and HY-catalytic oxidative degradation of EVA copolymers. Thermochim. Acta 2005, 438, 155–163. [Google Scholar] [CrossRef]
- Mohammed, Z.; Jeelani, S.; Rangari, V. Effective reinforcement of engineered sustainable biochar carbon for 3D printed polypropylene biocomposites. Compos. Part C Open Access 2022, 7, 100221. [Google Scholar] [CrossRef]
- Peterson, J.D.; Vyazovkin, S.; Wight, C.A. Kinetics of the thermal and thermo-oxidative degradation of polystyrene, polyethylene and poly (propylene). Macromol. Chem. Phys. 2001, 202, 775–784. [Google Scholar] [CrossRef]
- Alghyamah, A.A.; Elnour, A.Y.; Shaikh, H.; Haider, S.; Poulose, A.M.; Al-Zahrani, S.; Almasry, W.A.; Park, S.Y. Biochar/polypropylene composites: A study on the effect of pyrolysis temperature on crystallization kinetics, crystalline structure, and thermal stability. J. King Saud Univ. Sci. 2021, 33, 101409. [Google Scholar] [CrossRef]
- Noori, A.; Bartoli, M.; Frache, A.; Piatti, E.; Giorcelli, M.; Tagliaferro, A. Development of pressure-responsive polypropylene and biochar-based materials. Micromachines 2020, 11, 339. [Google Scholar] [CrossRef]
- Torsello, D.; Ghigo, G.; Giorcelli, M.; Bartoli, M.; Rovere, M.; Tagliaferro, A. Tuning the microwave electromagnetic properties of biochar-based composites by annealing. Carbon Trends 2021, 4, 100062. [Google Scholar] [CrossRef]
- Das, C.; Tamrakar, S.; Kiziltas, A.; Xie, X. Incorporation of biochar to improve mechanical, thermal and electrical properties of polymer composites. Polymers 2021, 13, 2663. [Google Scholar] [CrossRef]
- Sefadi, J.S.; Luyt, A. Morphology and properties of EVA/empty fruit bunch composites. J. Thermoplast. Compos. Mater. 2012, 25, 895–914. [Google Scholar] [CrossRef]
- Lin, H.Y.; Cheng, H.Z.; Lee, K.J.; Wang, C.F.; Liu, Y.C.; Wang, Y.W. Effect of Carbonaceous Components on Tribological Properties of Copper-Free NAO Friction Material. Materials 2020, 13, 1163. [Google Scholar] [CrossRef] [PubMed]
- Di Maro, M.; Duraccio, D.; Malucelli, G.; Faga, M.G. High density polyethylene composites containing alumina-toughened zirconia particles: Mechanical and tribological behavior. Compos. Part B Eng. 2021, 217, 108892. [Google Scholar] [CrossRef]
- Aranganathan, N.; Bijwe, J. Development of copper-free eco-friendly brake-friction material using novel ingredients. Wear 2016, 352–353, 79–91. [Google Scholar] [CrossRef]
- Manoharan, S.; Vijay, R.; Lenin Singaravelu, D.; Kchaou, M. Experimental investigation on the tribo-thermal properties of brake friction materials containing various forms of graphite: A comparative study. Arab. J. Sci. Eng. 2019, 44, 1459–1473. [Google Scholar] [CrossRef]
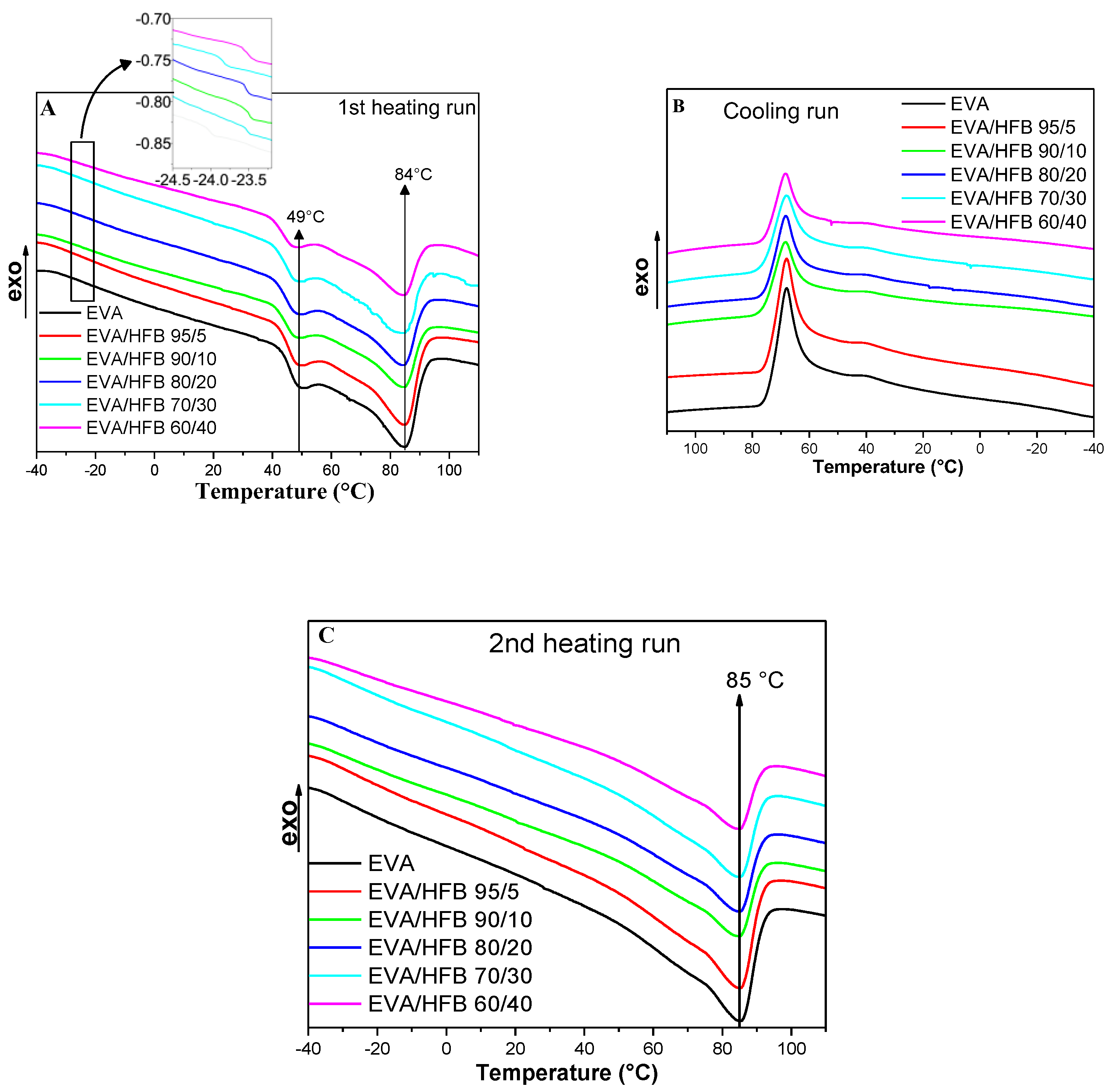
| Samples | Tg (°C) | Tm1,1 (°C) | Tm1,2 (°C) | Tc1 (°C) | Tc2 (°C) | Tm2 (°C) | ΔHm1 (J/g) | χc1 (%) |
|---|---|---|---|---|---|---|---|---|
| EVA | −24.0 | 50.1 | 84.5 | 68.1 | 39.7 | 85.4 | 60.5 | 22 |
| EVA/HFB 95/5 | −23.5 | 49.9 | 84.5 | 67.9 | 39.7 | 85.3 | 61.3 | 22 |
| EVA/HFB 90/10 | −23.5 | 48.8 | 83.9 | 68.3 | 39.9 | 85.0 | 66.5 | 24 |
| EVA/HFB 80/20 | −23.6 | 48.8 | 83.7 | 68.4 | 39.7 | 85.2 | 65.8 | 24 |
| EVA/HFB 70/30 | −23.8 | 48.4 | 83.3 | 68.2 | 39.6 | 84.9 | 64.1 | 23 |
| EVA/HFB 60/40 | −23.4 | 48.3 | 83.9 | 68.5 | 39.5 | 84.9 | 62.3 | 22 |
| N2 | Air | |||||||
|---|---|---|---|---|---|---|---|---|
| T10% (°C) | Tmax1 (°C) | Tmax2 (°C) | Residue @ 700 °C (%) | T10% (°C) | Tmax1 (°C) | Tmax2 (°) | Residue @ 700 °C (%) | |
| EVA | 361 | 356 | 477 | 0.5 | 334 | 342 | 440 | 0 |
| EVA/HFB 95/5 | 358 | 353 | 477 | 5.3 | 340 | 345 | 448 | 0.3 |
| EVA/HFB 90/10 | 359 | 351 | 475 | 10.0 | 338 | 339 | 443 | 0.7 |
| EVA/HFB 80/20 | 361 | 350 | 474 | 19.3 | 350 | 349 | 448 | 1.5 |
| EVA/HFB 70/30 | 361 | 351 | 468 | 26.9 | 352 | 350 | 473 | 2.4 |
| EVA/HFB 60/40 | 381 | 353 | 479 | 37.0 | 361 | 353 | 480 | 3.0 |
| Biomass Source | Matrix | Filler Loading (wt.%) | Conductivity (S/m) | Pressure (bar) | Ref. |
|---|---|---|---|---|---|
| Hemp | Epoxy resin | 10 | 6 | 750 | [20] |
| Hemp | EVA | 40 | 10−5 | 750 | This work |
| Coffee | Epoxy resin | 15 | 10−2 | 1500 | [18] |
| Tea | PP | 40 | 2 × 10−2 | 1500 | [58] |
| Sample | COF | Specific Wear Rate (mm3/N·m)·10−4 |
|---|---|---|
| EVA | 1.18 ± 0.03 | 10.6 |
| EVA/HFB 95/5 | 1.03 ± 0.01 | 4.86 |
| EVA/HFB 90/10 | 0.92 ± 0.05 | 2.95 |
| EVA/HFB 80/20 | 0.77 ± 0.01 | 1.27 |
| EVA/HFB 70/30 | 0.68 ± 0.05 | 0.79 |
| EVA/HFB 60/40 | 0.61 ± 0.04 | 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faga, M.G.; Duraccio, D.; Di Maro, M.; Pedraza, R.; Bartoli, M.; d’Ayala, G.G.; Torsello, D.; Ghigo, G.; Malucelli, G. Ethylene-Vinyl Acetate (EVA) Containing Waste Hemp-Derived Biochar Fibers: Mechanical, Electrical, Thermal and Tribological Behavior. Polymers 2022, 14, 4171. https://doi.org/10.3390/polym14194171
Faga MG, Duraccio D, Di Maro M, Pedraza R, Bartoli M, d’Ayala GG, Torsello D, Ghigo G, Malucelli G. Ethylene-Vinyl Acetate (EVA) Containing Waste Hemp-Derived Biochar Fibers: Mechanical, Electrical, Thermal and Tribological Behavior. Polymers. 2022; 14(19):4171. https://doi.org/10.3390/polym14194171
Chicago/Turabian StyleFaga, Maria Giulia, Donatella Duraccio, Mattia Di Maro, Riccardo Pedraza, Mattia Bartoli, Giovanna Gomez d’Ayala, Daniele Torsello, Gianluca Ghigo, and Giulio Malucelli. 2022. "Ethylene-Vinyl Acetate (EVA) Containing Waste Hemp-Derived Biochar Fibers: Mechanical, Electrical, Thermal and Tribological Behavior" Polymers 14, no. 19: 4171. https://doi.org/10.3390/polym14194171
APA StyleFaga, M. G., Duraccio, D., Di Maro, M., Pedraza, R., Bartoli, M., d’Ayala, G. G., Torsello, D., Ghigo, G., & Malucelli, G. (2022). Ethylene-Vinyl Acetate (EVA) Containing Waste Hemp-Derived Biochar Fibers: Mechanical, Electrical, Thermal and Tribological Behavior. Polymers, 14(19), 4171. https://doi.org/10.3390/polym14194171