Sol-Gel Coatings with Azofoska Fertilizer Deposited onto Pea Seeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. SiO2 Sol Synthesis
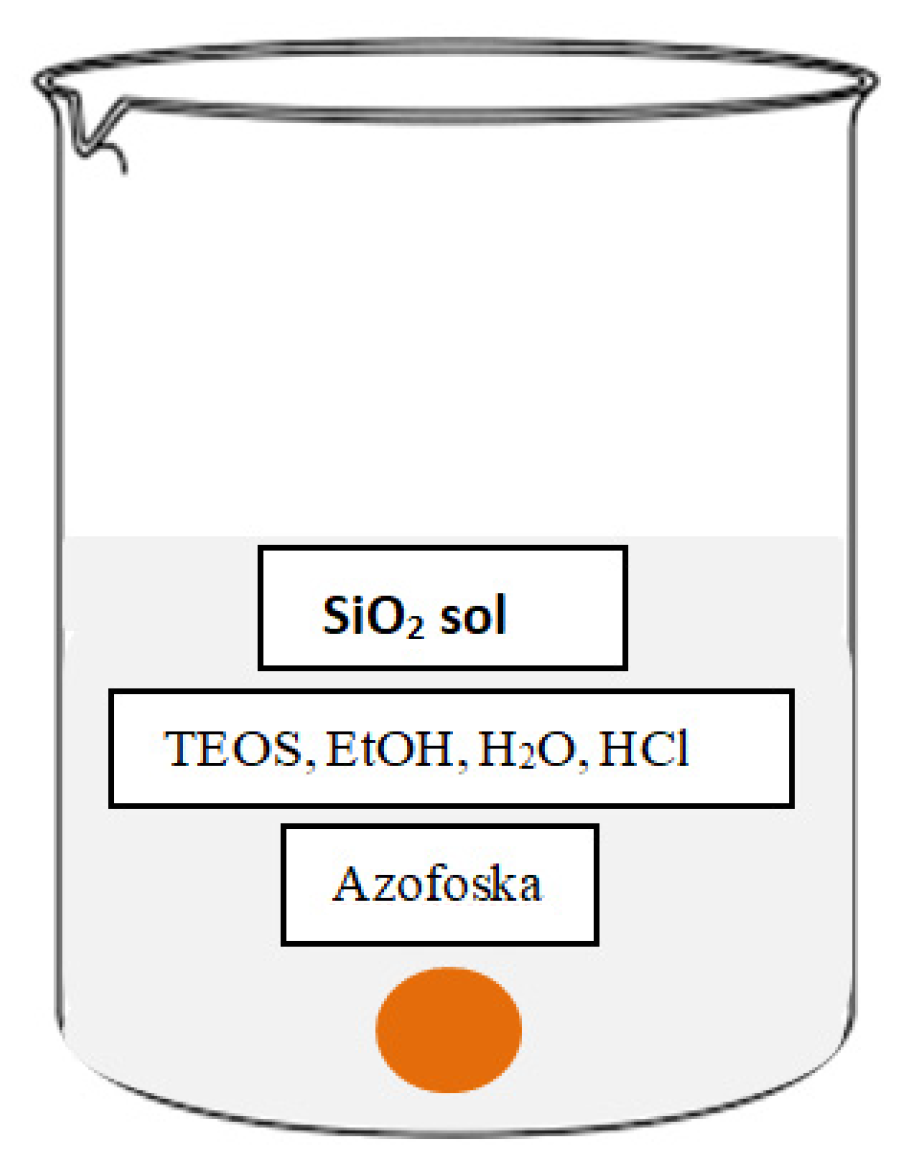
2.2.1. Preparation and Deposition of Pure SiO2 Sol
2.2.2. SiO2 Sol Dilution
2.3. Seed Germination Test
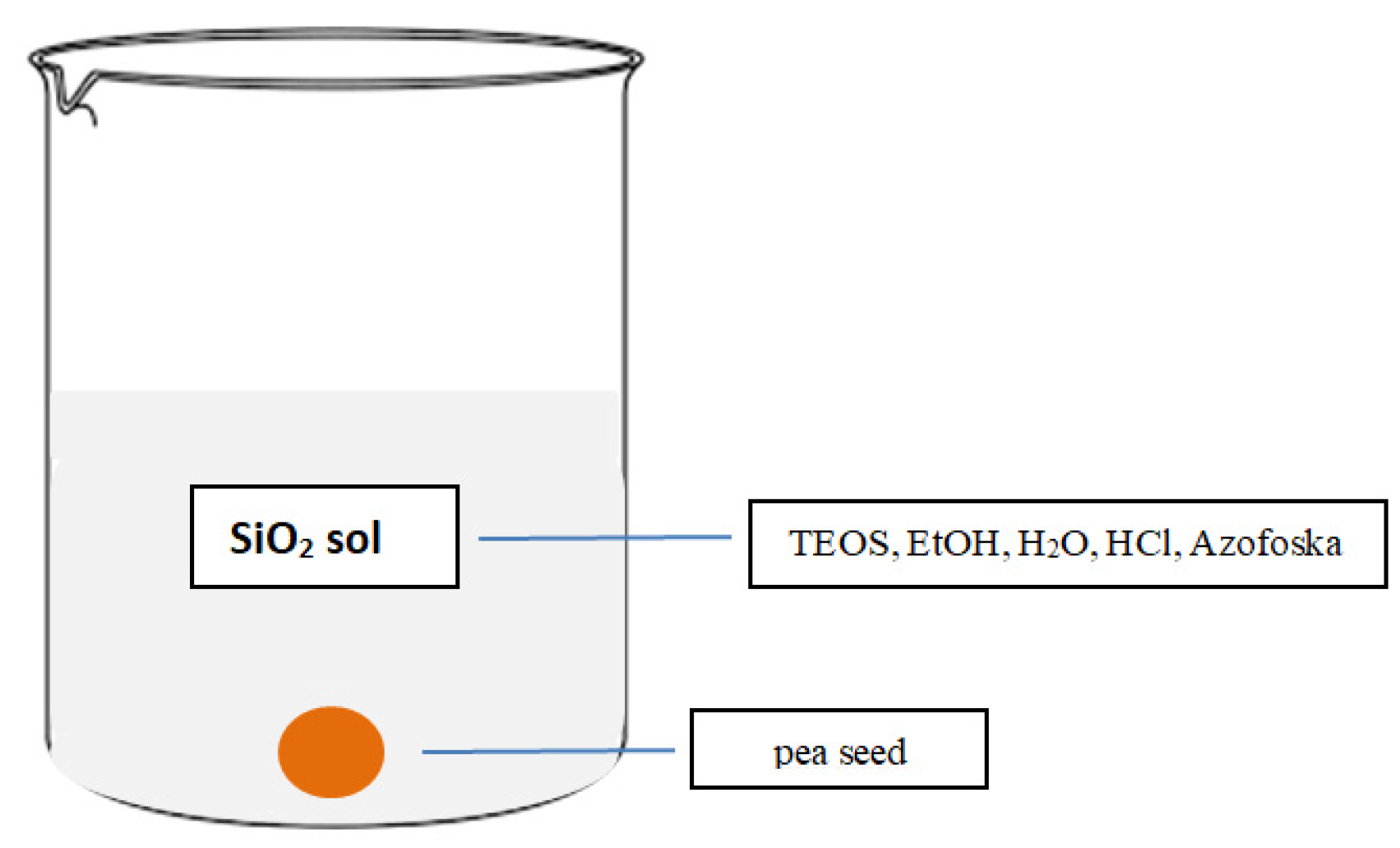
2.3.1. Preparation and Deposition of SiO2 Sol with Fertilizer
- (1)
- seeds covered with pure SiO2 sol;
- (2)
- seeds covered with SiO2-Azofoska sol and post-immersed in EtOH (thinner coating);
- (3)
- seeds covered with SiO2-Azofoska sol, not immersed in EtOH (thicker coating);
- (4)
- seeds without coatings as control.
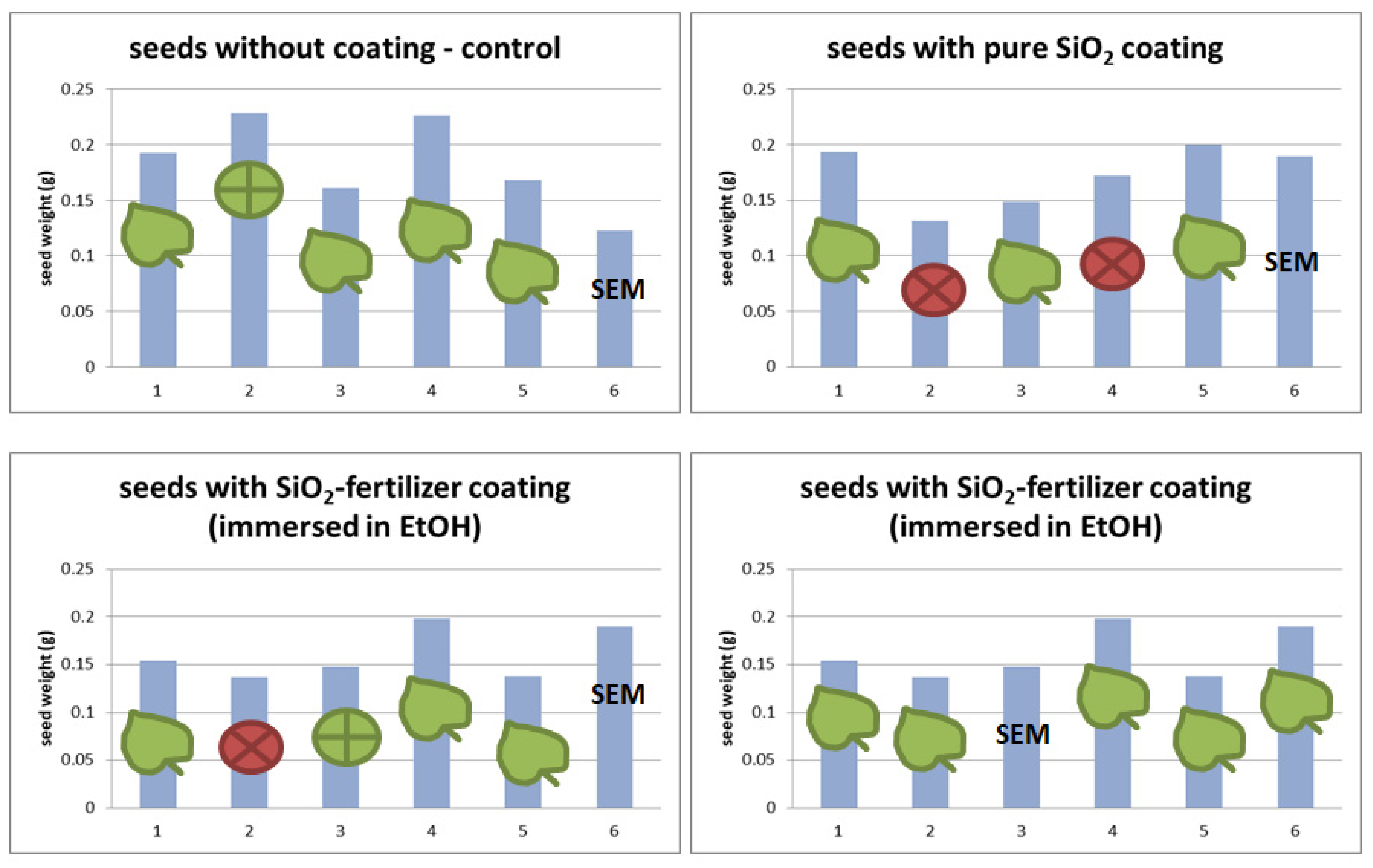
2.3.2. Seed Weight Changes over Time
2.3.3. Germination Test
2.4. Pea Seeds
2.5. Methods
3. Results
3.1. SiO2 Coatings Deposited in Different Conditions
3.2. Seed Germination Test
- (1)
- seeds covered with pure SiO2 sol;
- (2)
- seeds covered with SiO2-fertilizer sol—immersed in EtOH (thinner coating);
- (3)
- seeds covered with SiO2-fertilizer sol—not immersed in EtOH (thicker coating);
- (4)
- seeds without coating as control.
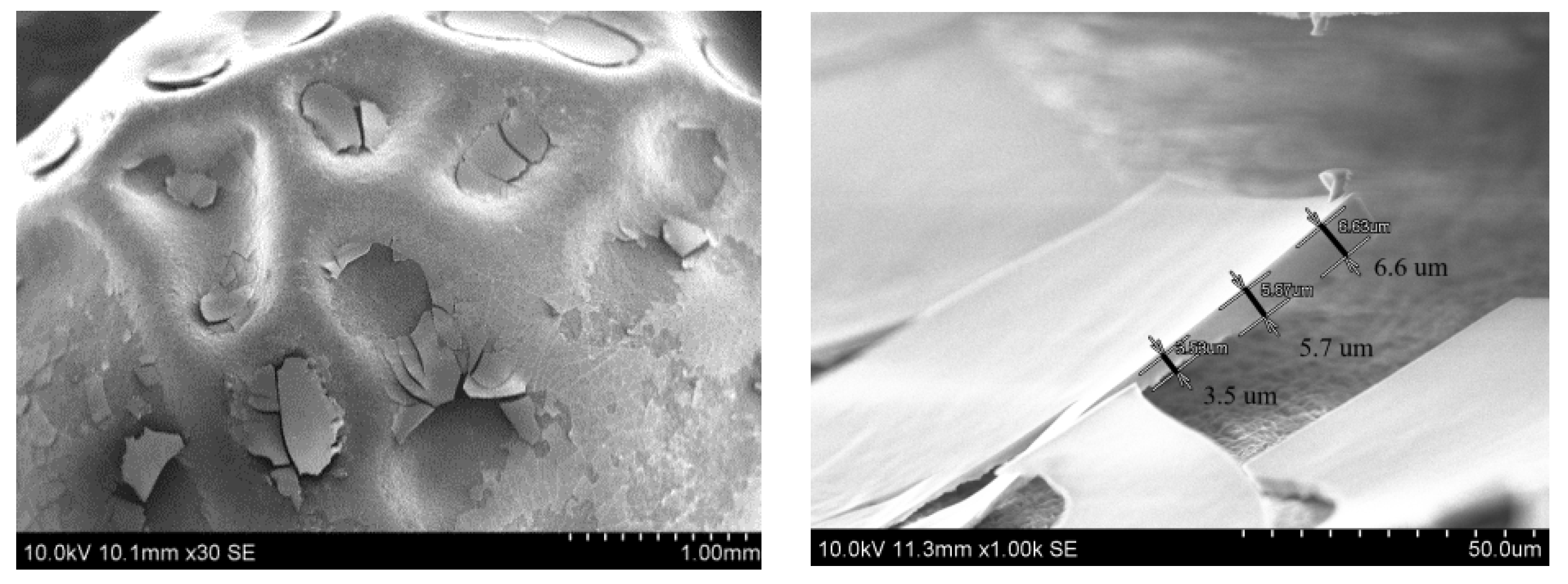
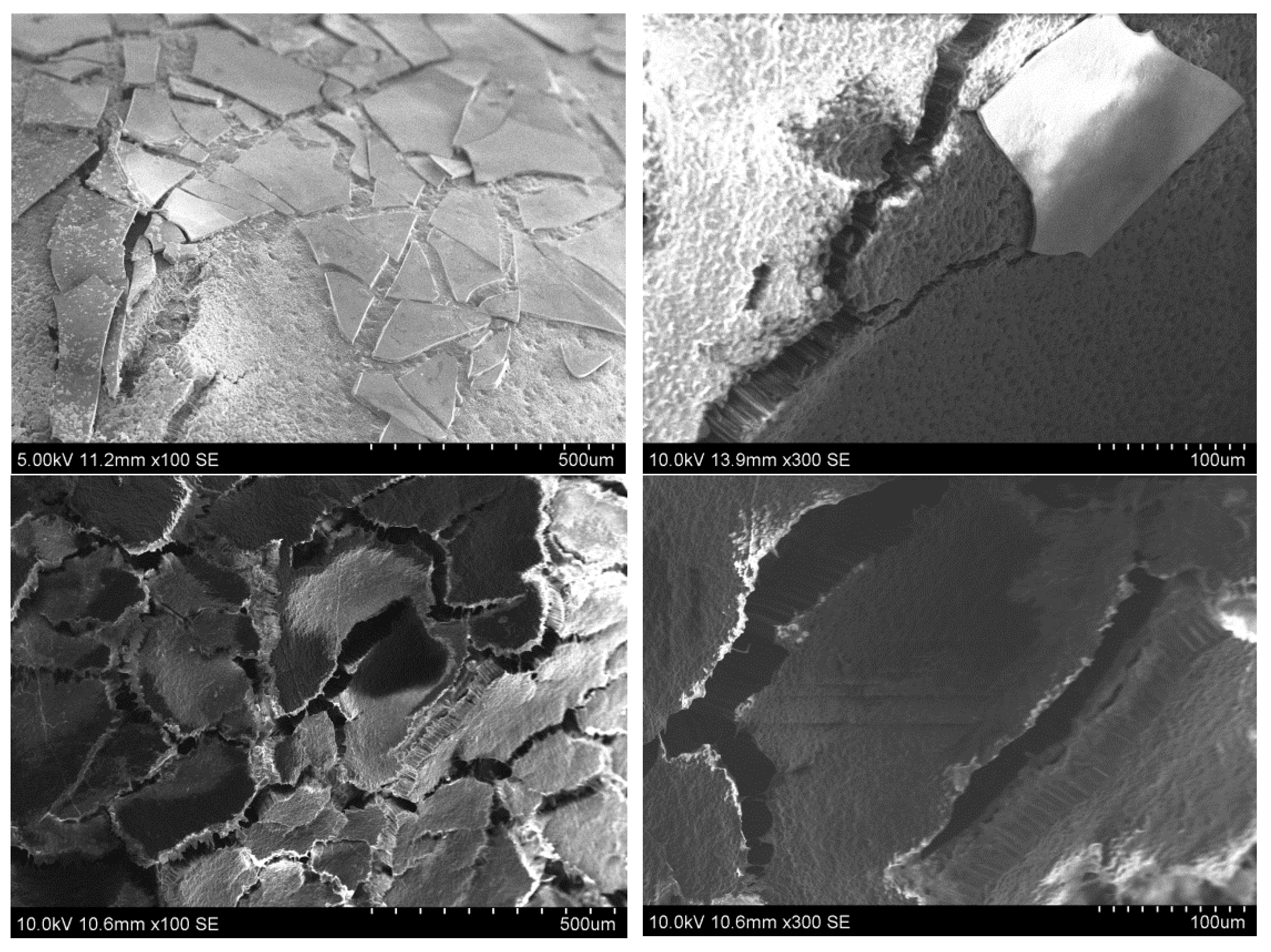
3.2.1. SEM Test of the Seeds Not Subjected to a Germination Test
3.2.2. Seeds after Germination
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Pirzada, T.; de Farias, B.V.; Mathew, R.; Guenther, R.H.; Byrd, M.V.; Sit, T.L.; Pal, L.; Opperman, C.H.; Khan, S.A. Recent advances in biodegradable matrices for active ingredient release in crop protection: Towards attaining sustainability in agriculture. Curr. Opin. Colloid Interface Sci. 2020, 48, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed Coating: Science or Marketing Spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef]
- Pedrini, S.; Balestrazzi, A.; Madsen, M.D.; Bhalsing, K.; Hardegree, S.P.; Dixon, K.W.; Kildisheva, O.A. Seed enhancement: Getting seeds restoration-ready. Restor. Ecol. 2020, 28, S266–S275. [Google Scholar] [CrossRef]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern Seed Technology: Seed Coating Delivery Systems for Enhancing Seed and Crop Performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Ruiz-de-la-Cruz, G.; Aguirre-Mancilla, C.L.; Godínez-Garrido, N.A.; Osornio-Flores, N.M.; Torres-Castillo, J.A. Chitosan mixed with beneficial fungal Condidia or fungicide for bean (Phaseolus vulgaris L.) seed coating. Interciencia 2017, 42, 307–312. [Google Scholar]
- Ayesha, M.S.; Suryanarayanan, T.S.; Nataraja, K.N.; Prasad, R.P.; Shaanker, R.U. Seed Treatment With Systemic Fungicides: Time for Review. Front. Plant Sci. 2021, 12, 654512. [Google Scholar] [CrossRef]
- Sharma, K.K.; Singh, U.S.; Sharma, P.; Ashish Kumar, A.; Sharma, L. Seed treatments for sustainable agriculture—A review. J. Appl. Nat. Sci. 2015, 7, 521–539. [Google Scholar] [CrossRef]
- Avelar, S.A.G.; de Sousa, F.V.; Fiss, G.; Baudet, L.; Peske, S.T. The use of film coating on the performance of treated corn seed. Rev. Bras. Sementes 2012, 34, 186–192. [Google Scholar] [CrossRef]
- Klarod, K.; Anoma Dongsansuk, A.; Piepho, H.-P.; Siri, B. Seed coating with plant nutrients enhances germination and seedling growth, and promotes total dehydrogenase activity during seed germination in tomato (Lycopersicon esculentum). Seed Sci. Technol. 2021, 49, 107–124. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef] [PubMed]
- Vercelheze, A.E.S.; Marim, B.M.; Oliveira, A.L.M.; Mali, S. Development of biodegradable coatings for maize seeds and their application for Azospirillum brasilense immobilization. Appl. Microbiol. Biotechnol. 2019, 103, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Seed coating with beneficial microorganisms for precision agriculture. Biotechnol. Adv. 2019, 37, 107423. [Google Scholar] [CrossRef]
- Zvinavashe, A.T.; Lim, E.; Sun, H.; Marelli, B. A bioinspired approach to engineer seed microenvironment to boost germination and mitigate soil salinity. Proc. Natl. Acad. Sci. USA 2019, 116, 25555–25561. [Google Scholar] [CrossRef]
- Gorim, L.; Asch, F. Effects of Composition and Share of Seed Coatings on the Mobilization Efficiency of Cereal Seeds during Germination. J. Agron. Crop Sci. 2012, 198, 81–91. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. Applications of Absorbent Polymers for Sustainable Plant Protection and Crop Yield. Sustainability 2021, 13, 3253. [Google Scholar] [CrossRef]
- Pathak, V.; Ambrose, R.P.K. Starch-based biodegradable hydrogel as seed coating for corn to improve early growth under water shortage. J. Appl. Polym. Sci. 2019, 137, 48523. [Google Scholar] [CrossRef]
- Huang, Q.; Qian, X.; Jiang, T.; Zheng, X. Effect of chitosan and guar gum based composite edible coating on quality of mushroom (Lentinus edodes) during postharvest storage. Sci. Hortic. 2019, 253, 382–389. [Google Scholar] [CrossRef]
- Adak, T.; Kumar, J.; Shakil, N.A.; Pandey, S. Role of nano-range amphiphilic polymers in seed quality enhancement of soybean and imidacloprid retention capacity on seed coatings. J. Sci. Food Agric. 2016, 96, 4351–4357. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.C.; de Araújo Rufino, C.; Dörr, C.S.; Barros, A.C.S.A.; Peske, S.T. Performance of lowland rice seeds coated with dolomitic limestone and aluminum silicate. Rev. Bras. Sementes 2012, 34, 202–211. [Google Scholar] [CrossRef][Green Version]
- Javed, T.; Afzal, I.; Mauro, R.P. Seed Coating in Direct Seeded Rice: An Innovative and Sustainable Approach to Enhance Grain Yield and Weed Management under Submerged Conditions. Sustainability 2021, 13, 2190. [Google Scholar] [CrossRef]
- Mattos, B.D.; Tardy, B.L.; Pezhman, M.; Kämäräinen, T.; Linder, M.; Schreiner, W.H.; Magalhães, W.L.E.; Rojas, O.J. Controlled biocide release from hierarchically-structured biogenic silica: Surface chemistry to tune release rate and responsiveness. Sci. Rep. 2018, 8, 5555. [Google Scholar] [CrossRef] [PubMed]
- Kaziema, A.E.; Gaoa, Y.; Zhanga, Y.; Qina, X.; Xiaoa, Y.; Zhanga, Y.; Youa, H.; Lia, J.; He, S. α-Amylase triggered carriers based on cyclodextrin anchored hollow mesoporous silica for enhancing insecticidal activity of avermectin against Plutella xylostella. J. Hazard. Mater. 2018, 359, 213–221. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef]
- Shilova, O.A.; Khamova, T.V.; Panova, G.G.; Anikina, L.M.; Artem’eva, A.M.; Kornyukhin, D.L. Using the Sol–Gel Technology for the Treatment of Barley Seeds. Glass Phys. Chem. 2018, 44, 26–32. [Google Scholar] [CrossRef]
- Neethirajan, S.; Gordon, R.; Wang, L. Potential of silica bodies (phytoliths) for nanotechnology. Trends Biotechnol. 2009, 27, 461–467. [Google Scholar] [CrossRef]
- Kato, K.; Lee, S.; Nagata, F. Efficient enzyme encapsulation inside sol-gel silica sheets prepared by poly-L-lysine as a catalyst. J. Asian Ceram. Soc. 2020, 8, 396–406. [Google Scholar] [CrossRef]
- Reátegui, E.; Kasinkas, L.; Kniesz, K.; Lefebvre, M.A.; Aksan, A. Silica–PEG gel immobilization of mammalian cells. J. Mater. Chem. B 2014, 2, 7440–7448. [Google Scholar] [CrossRef]
- Benson, J.J.; Sakkos, J.K.; Radian, A.; Wackett, L.P.; Aksan, A. Enhanced biodegradation of atrazine by bacteria encapsulated in organically modified silica gels. J. Colloid Interf. Sci. 2018, 510, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Homburg, S.V.; Venkanna, D.; Kraushaar, K.; Kruse, O.; Kroke, E.; Patel, A.V. Entrapment and growth of Chlamydomonas reinhardtii in biocompatible silica, Hydrogels. Colloids Surf. B Biointerfaces 2019, 173, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kapusuz, D.; Durucan, C. Exploring encapsulation mechanism of DNA and mononucleotides in sol-gel derived silica. J. Biomater. Appl. 2017, 32, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hernán, J.P.; Torres, B.; López, A.J.; Rams, J. The Role of the Sol-Gel Synthesis Process in the Biomedical Field and Its Use to Enhance the Performance of Bioabsorbable Magnesium Implants. Gels 2022, 8, 426. [Google Scholar] [CrossRef]
- Barik, T.K.; Sahu, B.; Swain, V. Nanosilica—from medicine to pest control. Parasitol. Res. 2008, 103, 253–258. [Google Scholar] [CrossRef]
- Ceniceros-Reyes, M.; Rodríguez, F.; Perera-Mercado, Y.; Saucedo-Salazar, E.; Díaz Barriga-Castro, E. Application of the Low Vacuum Scanning Electron Microscope to the Study of Glass-Ceramic spherical materials. Acta Microsc. 2014, 23, 78–84. [Google Scholar]
- Figueira, R.B. Hybrid Sol–gel Coatings for Corrosion Mitigation: A Critical Review. Polymers 2020, 12, 689. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Venkataraman, M.; Kremenakova, D.; Militky, J.; Zhou, Y. Progress in Sol-Gel Technology for the Coatings of Fabrics. Materials 2020, 13, 1838. [Google Scholar] [CrossRef]
- Startek, K.; Szczurek, A.; Tran, T.N.L.; Krzak, J.; Bachmatiuk, A.; Lukowiak, A. Structural and Functional Properties of Fluorinated Silica Hybrid Barrier Layers on Flexible Polymeric Foil. Coatings 2021, 11, 573. [Google Scholar] [CrossRef]
- Szczurek, A.; Barcikowski, M.; Leluk, K.; Babiarczuk, B.; Kaleta, J.; Krzak, J. Improvement of interaction in a composite structure by using a sol-gel functional coating on carbon fibers. Materials 2017, 10, 990. [Google Scholar] [CrossRef]
- Baskin, C.C.; Thompson, K.; Baskin, J.M. Mistakes in germination ecology and how to avoid them. Seed Sci. Res. 2006, 16, 165–168. [Google Scholar] [CrossRef]
- Bewleyl, J.D. Seed Germination and Dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-X.; Gong, H.-J.; Yin, J.-L. Role of Silicon in Mediating Salt Tolerance in Plants: A Review. Plants 2019, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Khan, A.L.; Lee, I.-J. Silicon: A duo synergy for regulating crop growth and hormonal signaling under abiotic stress conditions. Crit. Rev. Biotechnol. 2016, 36, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses, Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ansary, M.M.U.; Keya, S.S.; Abdelrahman, M.; Miah, M.G.; Tran, L.-S.P. Silicon in mitigation of abiotic stress-induced oxidative damage in plants. Crit. Rev. Biotechnol. 2021, 41, 918–934. [Google Scholar] [CrossRef]






| Sample | Description |
|---|---|
| 5 | Seed after 5 min in SiO2 sol in ultrasonic cleaner |
| 15 | Seed after 10 min in SiO2 sol in ultrasonic cleaner |
| 30 | Seed after 30 min in SiO2 sol in ultrasonic cleaner |
| 5\5 | Seed after 5 min in SiO2 sol in ultrasonic cleaner and 5 min in EtOH |
| 5\10 | Seed after 5 min in SiO2 sol in ultrasonic cleaner and 10 min in EtOH |
| 5M | Seed after 5 min in SiO2 sol on magnetic stirrer |
| 5M5 | Seed after 5 min in SiO2 sol on magnetic stirrer and 5 min in EtOH |
| 025EtOH | Seed after 5 min in SiO2 sol diluted with EtOH (1:4) by volume ratios |
| 025H2O | Seed after 5 min in SiO2 sol diluted with H2O (1:4) by volume ratios |
| 05EtOH | Seed after 5 min in SiO2 sol diluted with EtOH (1:2) by volume ratios |
| 05H2O | Seed after 5 min in SiO2 sol diluted with H2O (1:2) by volume ratios |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borak, B. Sol-Gel Coatings with Azofoska Fertilizer Deposited onto Pea Seeds. Polymers 2022, 14, 4119. https://doi.org/10.3390/polym14194119
Borak B. Sol-Gel Coatings with Azofoska Fertilizer Deposited onto Pea Seeds. Polymers. 2022; 14(19):4119. https://doi.org/10.3390/polym14194119
Chicago/Turabian StyleBorak, Beata. 2022. "Sol-Gel Coatings with Azofoska Fertilizer Deposited onto Pea Seeds" Polymers 14, no. 19: 4119. https://doi.org/10.3390/polym14194119
APA StyleBorak, B. (2022). Sol-Gel Coatings with Azofoska Fertilizer Deposited onto Pea Seeds. Polymers, 14(19), 4119. https://doi.org/10.3390/polym14194119






