Enhanced Mechanical and Thermal Properties of Chain-Extended Waterborne Polyurethane Coatings with Cellulose Acetate Butyrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of WPU Dispersions and Films
2.3. Characterization
2.3.1. Characterization of WPU Dispersions
2.3.2. Characterization of WPU Films
3. Results and Discussion
3.1. Structure Analysis
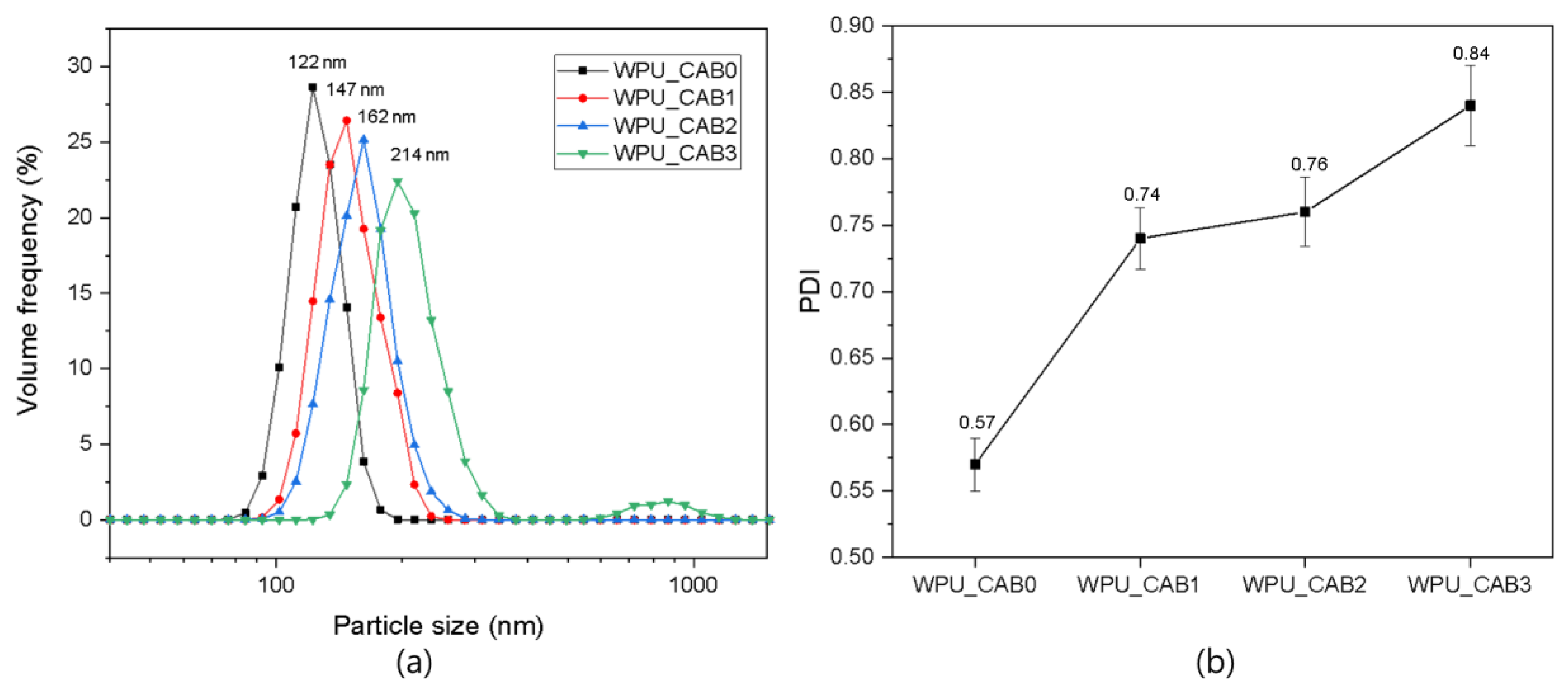
3.2. Properties of WPU Dispersions
3.3. Properties of WPU Films
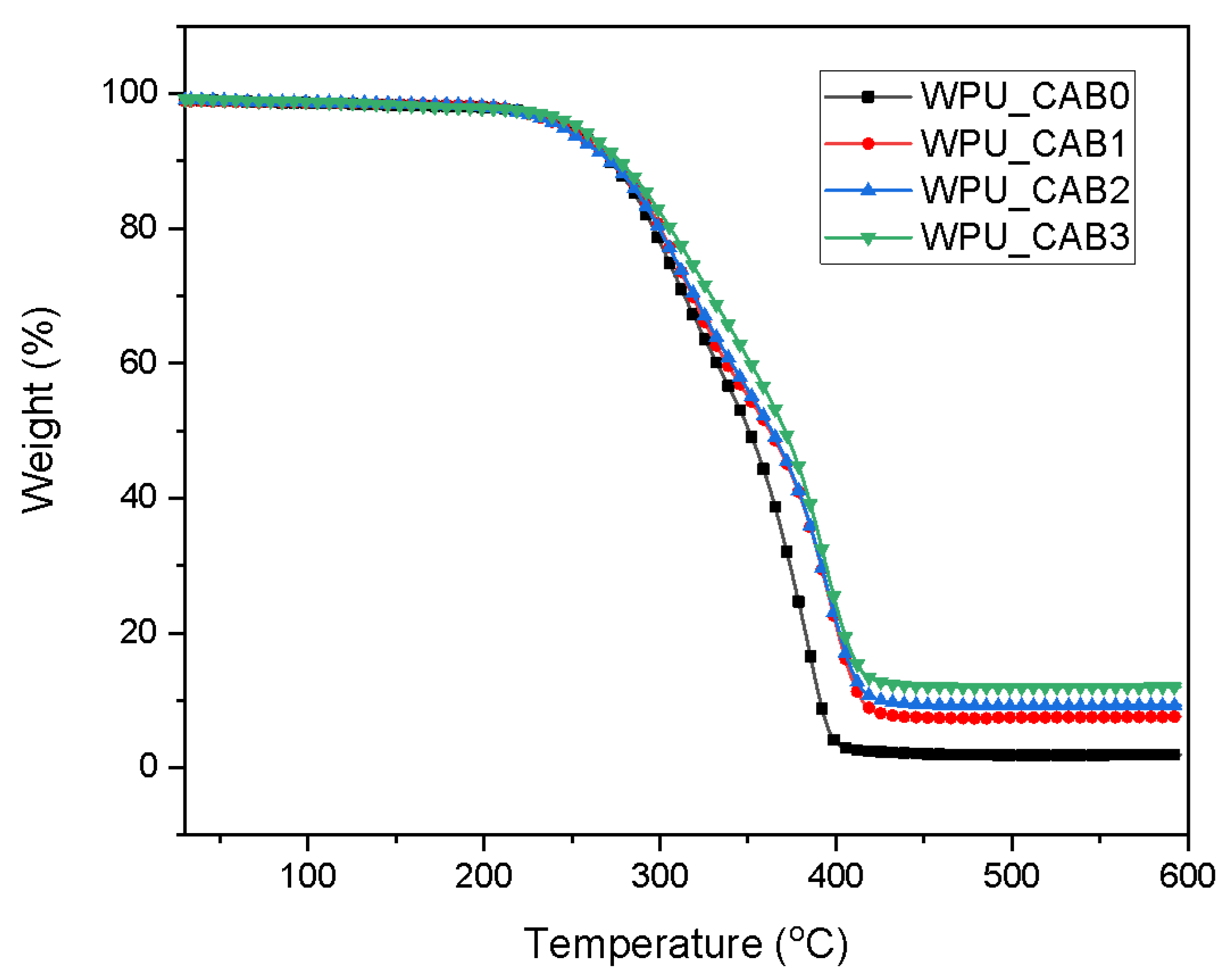
3.3.1. Thermal Properties
3.3.2. Mechanical Properties
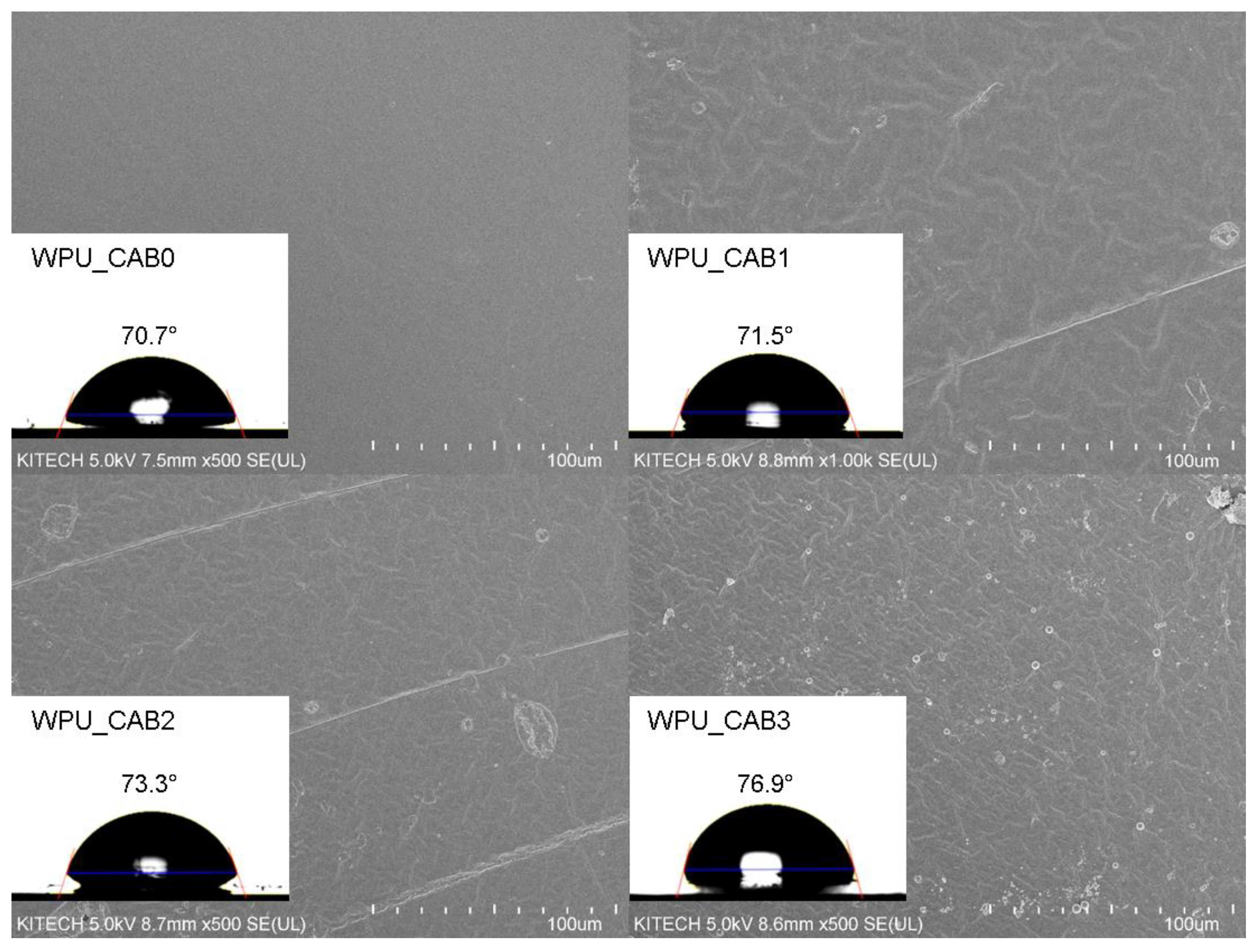
3.3.3. Surface Morphologies and Water Contact Angles
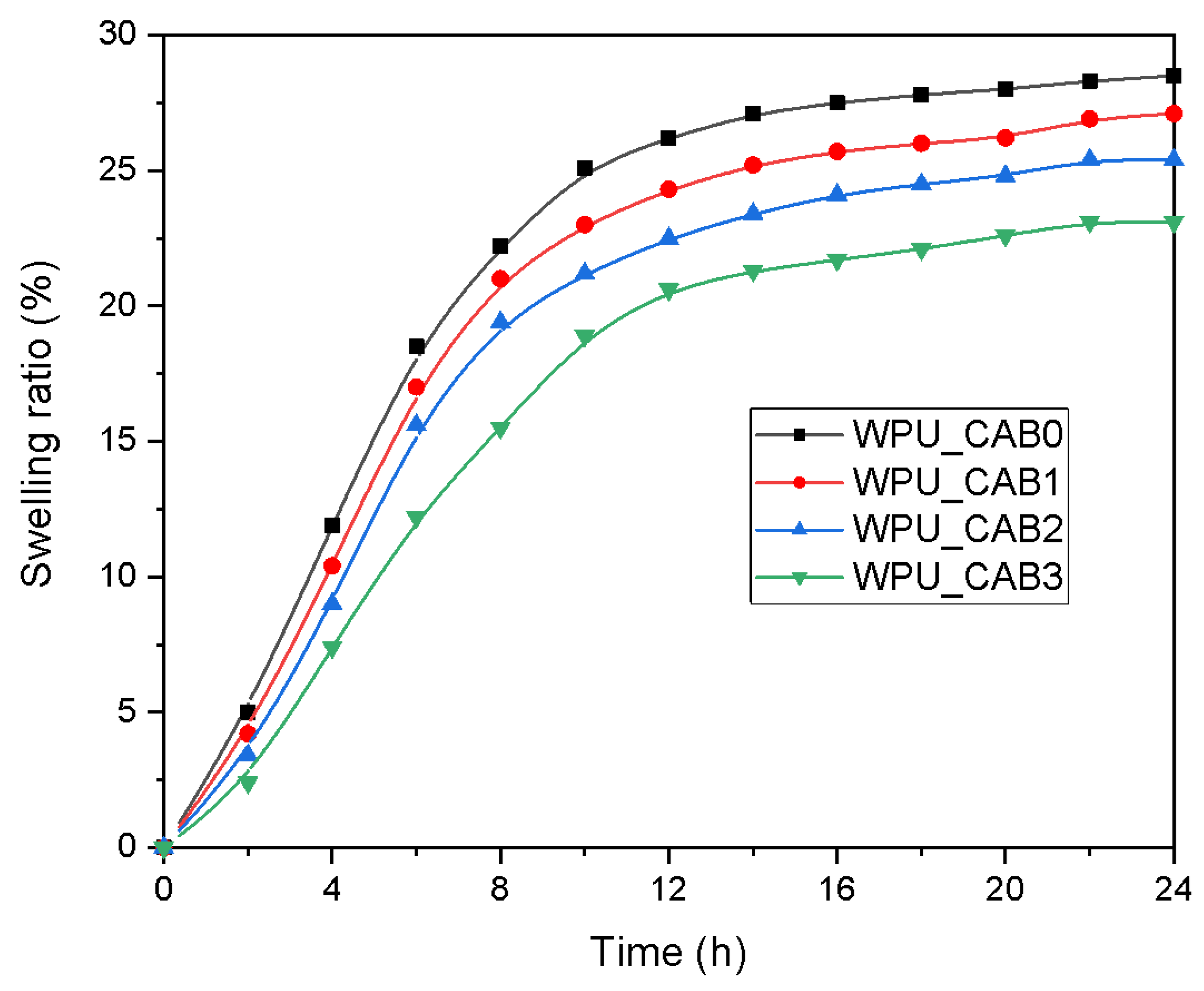
3.3.4. Swelling Behavior
3.3.5. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally friendly anticorrosive polymeric coatings. Appl. Sci. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Abdel-Wakil, W.S.; Kamoun, E.A.; Fahmy, A.; Hassan, W.; Abdelhai, F.; Salama, T.M. Assessment of vinyl acetate polyurethane-based graft terpolymers for emulsion coatings: Synthesis and characterization. J. Macromol. Sci. A 2020, 57, 229–243. [Google Scholar] [CrossRef]
- Jiang, S.; Van Dyk, A.; Maurice, A.; Bohling, J.; Fasano, D.; Brownell, S. Design colloidal particle morphology and self-assembly for coating applications. Chem. Soc. Rev. 2017, 46, 3792–3807. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Taylor, L.S.; Edgar, K.J. The role of polymers in oral bioavailability enhancement; a review. Polymer 2015, 77, 399–415. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, P.; Tanwar, S.; Varshney, G.; Yadav, S. Assessment of Bio-Based Polyurethanes: Perspective on Applications and Bio-Degradation. Macromol 2022, 2, 284–314. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.; Ghazali, S.; Islam, M.R.; Jeyaratnam, N.; Yuvaraj, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 114453–114482. [Google Scholar] [CrossRef]
- Wu, Q.; Henriksson, M.; Liu, X.; Berglund, L.A. A high strength nanocomposite based on microcrystalline cellulose and polyurethane. Biomacromolecules 2007, 8, 3687–3692. [Google Scholar] [CrossRef]
- Travinskaya, T.; Savelyev, Y.; Mishchuk, E. Waterborne polyurethane-based starch containing materials: Preparation, properties and study of degradability. Polym. Anal. Degrad. Stab. 2014, 101, 102–108. [Google Scholar] [CrossRef]
- Lee, D.I.; Kim, S.H.; Lee, D.S. Synthesis of self-healing waterborne polyurethane systems chain extended with chitosan. Polymers 2019, 11, 503. [Google Scholar] [CrossRef]
- Graninger, G.; Kumar, S.; Garrett, G.; Falzon, B.G. Effect of shear forces on dispersion-related properties of microcrystalline cellulose-reinforced EVOH composites for advanced applications. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106103. [Google Scholar] [CrossRef]
- Zheng, B.; Ge, S.; Wang, S.; Shao, Q.; Jiao, C.; Liu, M.; Das, R.; Dong, B.; Guo, Z. Effect of γ-aminopropyltriethoxysilane on the properties of cellulose acetate butyrate modified acrylic waterborne coatings. React. Funct. Polym. 2020, 154, 104657. [Google Scholar] [CrossRef]
- Jiao, S.Z.; Sun, Z.C.; Li, F.R.; Yan, M.J.; Cao, M.J.; Li, D.S.; Liu, Y.; Li, L.H. Preparation and application of conductive polyaniline-coated thermally expandable microspheres. Polymers 2018, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, L.; Jin, S.; Wang, Y.; Lan, G.; Chen, Y. Study on cellulose acetate butyrate/plasticizer systems by molecular dynamics simulation and experimental characterization. Polymers 2020, 12, 1272. [Google Scholar] [CrossRef] [PubMed]
- Monisha, S.; Selvasekarapandian, S.; Mathavan, T.; Benial, A.M.F.; Manoharan, S.; Karthikeyan, S. Preparation and characterization of biopolymer electrolyte based on cellulose acetate for potential applications in energy storage devices. J. Mater. Sci. Mater. Electron. 2016, 27, 9314–9324. [Google Scholar] [CrossRef]
- Azeh, Y.; Olatunji, G.A.; Adekola, F.A. Synthesis and characterization of cellulose nanoparticles and its derivatives using a combination of spectro-analytical techniques. Int. J. Nanotechnol. Med. Eng. 2017, 2, 65–94. [Google Scholar] [CrossRef]
- Das, S.; Pandey, P.; Mohanty, S.; Nayak, S.K. Insight on castor oil-based polyurethane and nanocomposites: Recent trends and development. Polym. Plast. Technol. Eng. 2017, 56, 1556–1585. [Google Scholar] [CrossRef]
- Rymarczyk, J.; Carewska, M.; Appetecchi, G.B.; Zane, D.; Alessandrini, F.; Passerini, S. A novel ternary polymer electrolyte for LMP batteries based on thermal cross-linked poly (urethane acrylate) in presence of a lithium salt and an ionic liquid. Eur. Polym. J. 2008, 44, 2153–2161. [Google Scholar] [CrossRef]
- Yen, F.S.; Hong, J.L. Hydrogen-bond interactions between ester and urethane linkages in small model compounds and polyurethanes. Macromolecules 1997, 30, 7927–7938. [Google Scholar] [CrossRef]
- Nanda, A.K.; Wicks, D.A. The influence of the ionic concentration, concentration of the polymer, degree of neutralization and chain extension on aqueous polyurethane dispersions prepared by the acetone process. Polymer 2006, 47, 1805–1811. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, Y.; Kang, M.; Wang, J.; Wang, X.; Feng, Y.; Yin, N.; Li, Q. The effects of the molecular weight and structure of polycarbonatediols on the properties of waterborne polyurethanes. Prog. Org. Coat. 2015, 82, 46–56. [Google Scholar] [CrossRef]
- Xu, Y.; Petrovic, Z.; Das, S.; Wilkes, G.L. Morphology and properties of thermoplastic polyurethanes with dangling chains in ricinoleate-based soft segments. Polymer 2008, 49, 4248–4258. [Google Scholar] [CrossRef]
- Choi, S.S.; Hong, J.P.; Seo, Y.S.; Chung, S.M.; Nah, C. Fabrication and characterization of electrospun polybutadiene fibers crosslinked by UV irradiation. J. Appl. Polym. Sci. 2006, 101, 2333–2337. [Google Scholar] [CrossRef]
- Cao, X.; Ge, X.; Chen, H.; Li, W. Effects of trimethylol propane and AAS salt on properties of waterborne polyurethane with low gloss. Prog. Org. Coat. 2017, 107, 5–13. [Google Scholar] [CrossRef]
- Sun, Z.; Fan, H.; Chen, Y.; Huang, J. Synthesis of self-matting waterborne polyurethane coatings with excellent transmittance. Polym. Int. 2018, 67, 78–84. [Google Scholar] [CrossRef]




| Sample | PBAT (mol) | IDPI (mol) | DMPA (mol) | CAB (mol) | EDA (mol) |
|---|---|---|---|---|---|
| WPU_CAB0 | 0.05 | 0.1 | 0.025 | - | 0.025 |
| WPU_CAB1 | 0.05 | 0.1 | 0.025 | 1 × 10−3 | 0.021 |
| WPU_CAB2 | 0.05 | 0.1 | 0.025 | 2 × 10−3 | 0.016 |
| WPU_CAB3 | 0.05 | 0.1 | 0.025 | 3 × 10−3 | 0.012 |
| Sample | Storage Stability (Month) | Average Particle Size (nm) | DPI | Viscosity (cP) |
|---|---|---|---|---|
| WPU_CAB0 | >6 | 122 | 0.57 | 286 |
| WPU_CAB1 | >6 | 147 | 0.74 | 124 |
| WPU_CAB2 | >6 | 162 | 0.76 | 72 |
| WPU_CAB3 | >6 | 214 | 0.84 | 58 |
| Sample | Temperature (°C) | |||
|---|---|---|---|---|
| 150 | 250 | 350 | 500 | |
| WPU_CAB0 | 98.3 | 94.8 | 50.4 | 1.9 |
| WPU_CAB1 | 98.5 | 94.4 | 55.1 | 7.4 |
| WPU_CAB2 | 98.5 | 94.1 | 56.0 | 9.2 |
| WPU_CAB3 | 98.3 | 95.6 | 60.7 | 11.9 |
| Sample | 300% Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| WPU_CAB0 | 2.5 ± 0.1 | 9.5 ± 0.2 | 846 ± 22 |
| WPU_CAB1 | 3.0 ± 0.1 | 13.2 ± 0.4 | 837 ± 28 |
| WPU_CAB2 | 3.6 ± 0.2 | 15.9 ± 0.6 | 806 ± 31 |
| WPU_CAB3 | 2.7 ± 0.2 | 11.6 ± 0.5 | 924 ± 30 |
| Sample | WPU_CAB0 | WPU_CAB1 | WPU_CAB2 | WPU_CAB3 |
|---|---|---|---|---|
| Transmittance (%) | 88.7 | 91.2 | 93.1 | 93.4 |
| Gloss (o) | 86 | 76 | 73 | 68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.-R.; Kim, H.-C.; Kim, J.-S.; So, J.-H.; Chang, Y.-W.; Kim, D.-H. Enhanced Mechanical and Thermal Properties of Chain-Extended Waterborne Polyurethane Coatings with Cellulose Acetate Butyrate. Polymers 2022, 14, 4062. https://doi.org/10.3390/polym14194062
Kwon Y-R, Kim H-C, Kim J-S, So J-H, Chang Y-W, Kim D-H. Enhanced Mechanical and Thermal Properties of Chain-Extended Waterborne Polyurethane Coatings with Cellulose Acetate Butyrate. Polymers. 2022; 14(19):4062. https://doi.org/10.3390/polym14194062
Chicago/Turabian StyleKwon, Yong-Rok, Hae-Chan Kim, Jung-Soo Kim, Ju-Hee So, Young-Wook Chang, and Dong-Hyun Kim. 2022. "Enhanced Mechanical and Thermal Properties of Chain-Extended Waterborne Polyurethane Coatings with Cellulose Acetate Butyrate" Polymers 14, no. 19: 4062. https://doi.org/10.3390/polym14194062
APA StyleKwon, Y.-R., Kim, H.-C., Kim, J.-S., So, J.-H., Chang, Y.-W., & Kim, D.-H. (2022). Enhanced Mechanical and Thermal Properties of Chain-Extended Waterborne Polyurethane Coatings with Cellulose Acetate Butyrate. Polymers, 14(19), 4062. https://doi.org/10.3390/polym14194062







