Alginate Fiber-Enhanced Poly(vinyl alcohol) Hydrogels with Superior Lubricating Property and Biocompatibility
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PVA/SA/NaCl Hydrogels
2.3. Characterization
2.4. Mechanical Measurements
2.5. Friction Test
2.6. The Evaluation of Hydrogel Bioactivity
2.6.1. Cell Proliferation
2.6.2. Cell Viability Assay
2.6.3. Cell Morphology
3. Results and Discussion
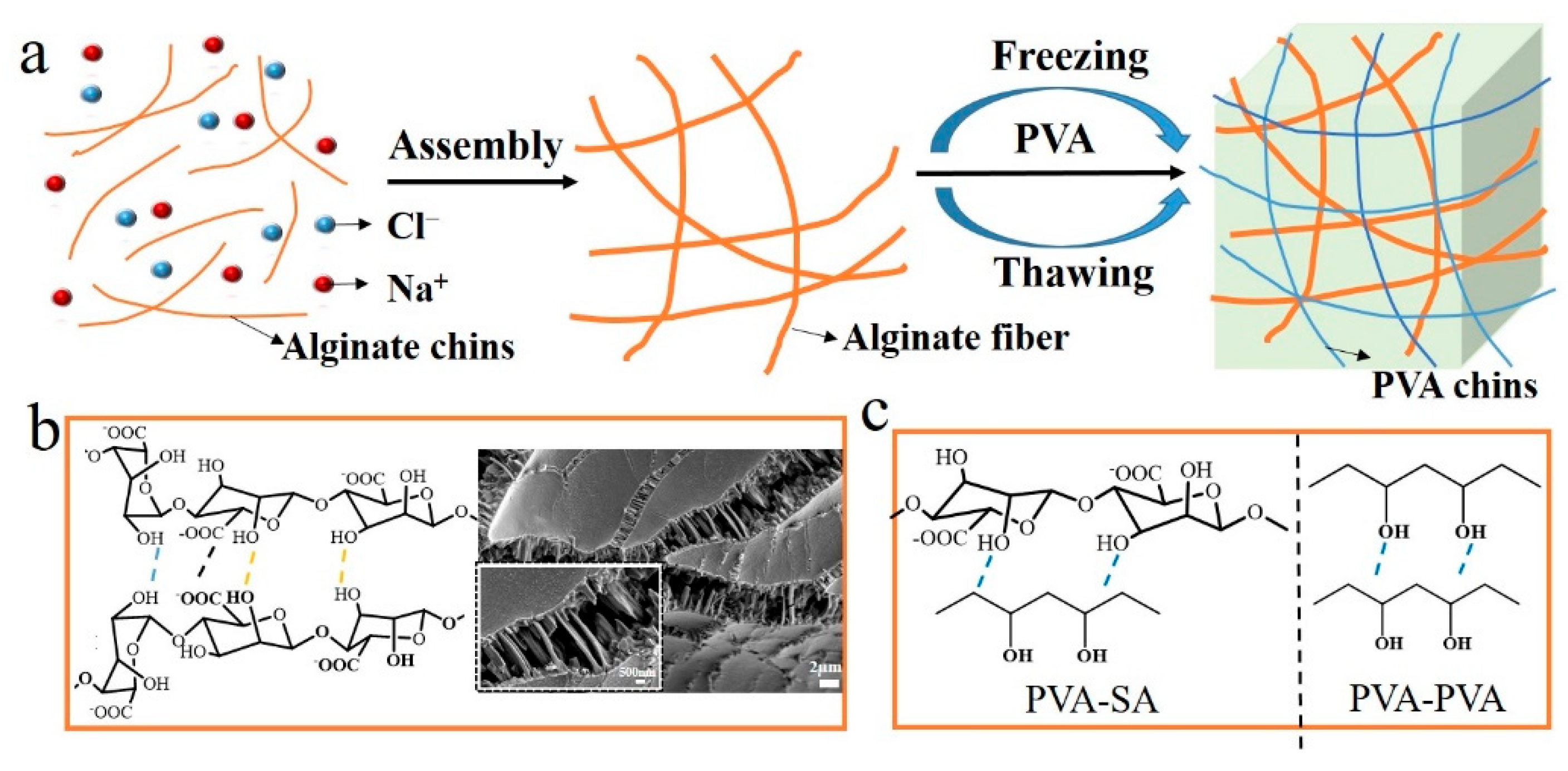
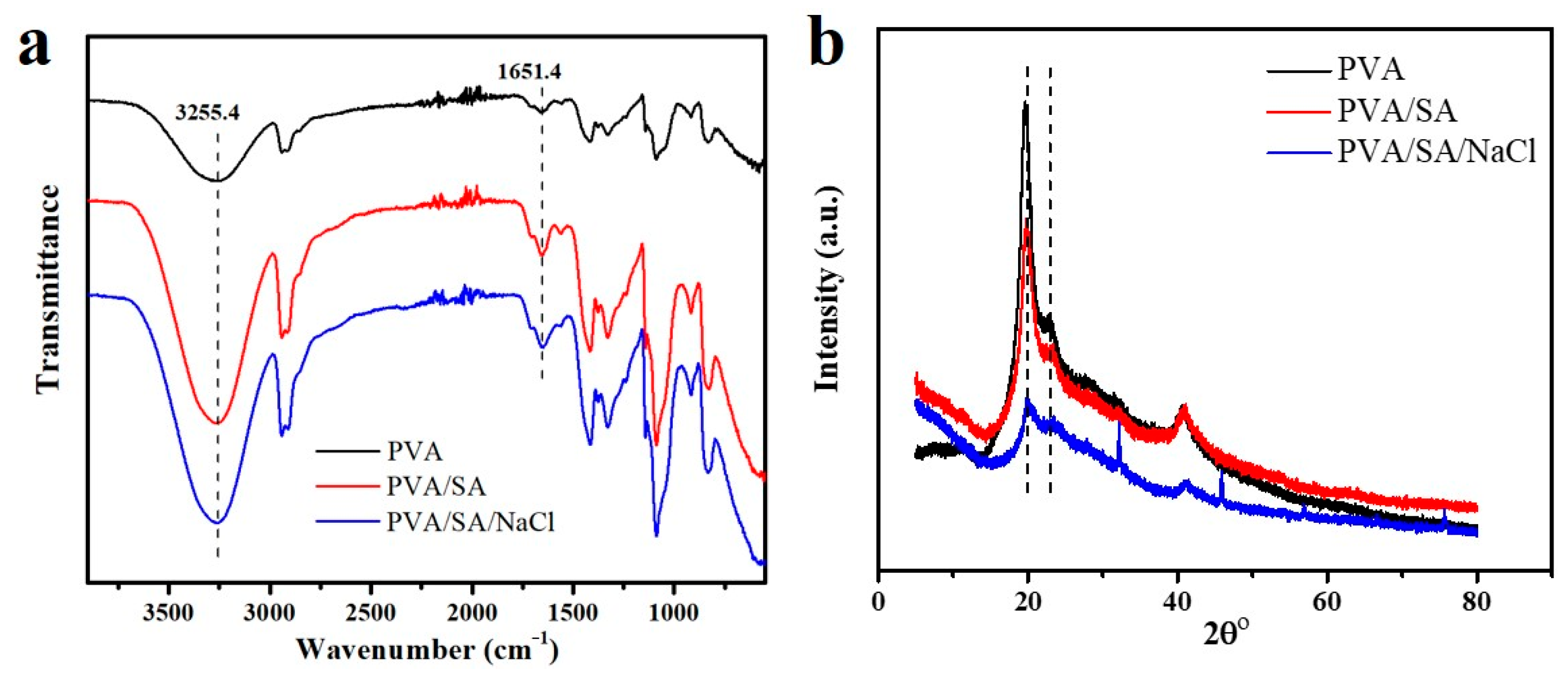
3.1. Preparation and Characterization of PVA/SA/NaCl Hydrogels
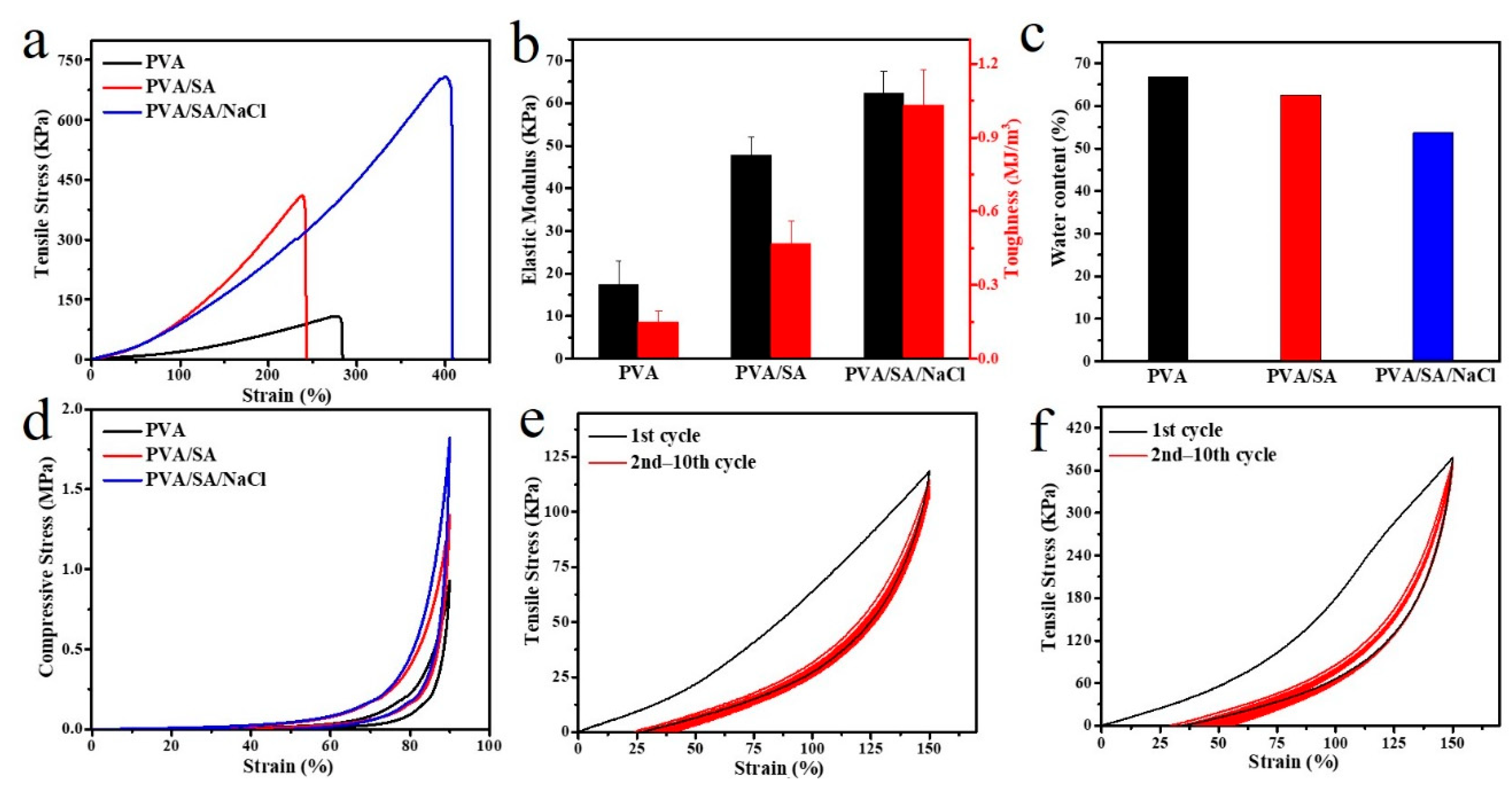
3.2. Mechanical Properties of PVA/SA/NaCl Hydrogels
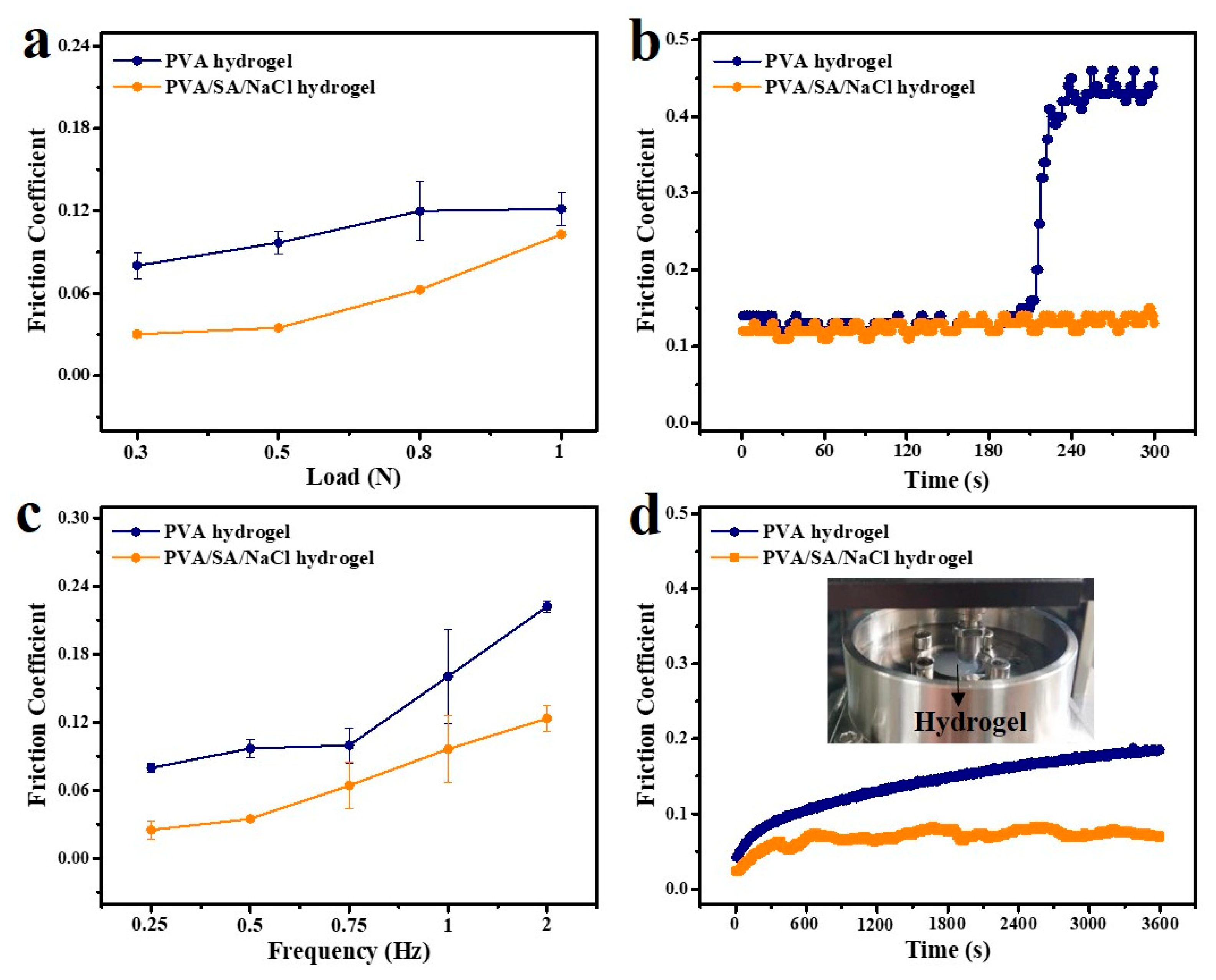
3.3. Lubrication Properties of PVA/SA/NaCl Hydrogels
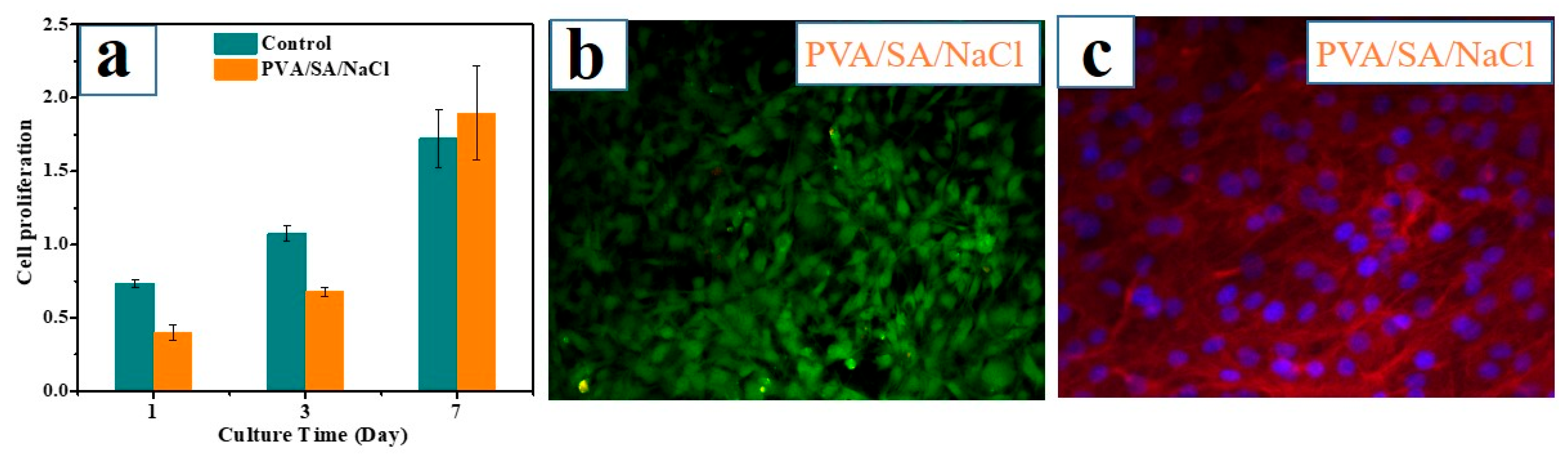
3.4. Evaluation of Hydrogel Bioactivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, L.; Zhao, X.; Xu, C.; Kotov, N.A. Water-Rich Biomimetic Composites with Abiotic Self-Organizing Nanofiber Network. Adv. Mater. 2017, 30, 1870007. [Google Scholar] [CrossRef]
- Means, A.K.; Grunlan, M.A. Modern Strategies to Achieve Tissue-Mimetic, Mechanically Robust Hydrogels. ACS Macro Lett. 2019, 8, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.Y.; Liu, W.G. Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog. Polym. Sci. 2017, 71, 1–25. [Google Scholar] [CrossRef]
- Qu, M.; Liu, H.; Yan, C.; Ma, S.; Cai, M.; Ma, Z.; Zhou, F. Layered Hydrogel with Controllable Surface Dissociation for Durable Lubrication. Chem. Mater. 2020, 32, 7805–7813. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Y.; Gao, L.; Bai, T.; Wang, W.; Cui, Y.; Liu, W. A Mechanically Strong, Highly Stable, Thermoplastic, and Self-Healable Supramolecular Polymer Hydrogel. Adv. Mater. 2015, 27, 3566–3571. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, M.; Zhao, W.; Chen, L.; Liu, H.; Jin, X.; Ma, A.; Zhang, G.; Jiang, D.; Chen, W. Supramolecularly Mediated Robust, Anti-Fatigue, and Strain-Sensitive Macromolecular Microsphere Composite Hydrogels. Macromol. Mater. Eng. 2020, 305, 2000080. [Google Scholar] [CrossRef]
- Li, J.Y.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Hua, M.; Wu, S.; Ma, S.; Zhang, Y.; Zhang, L.; Yu, B.; Cai, M.; He, X.; Zhou, F. Continuously growing multi-layered hydrogel structures with seamless interlocked interface. Matter 2022, 5, 634–653. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.Y.; Kang, J.H.; Kim, H.S.; Shin, U.S. Design of thermoresponsive hydrogels by controlling the chemistry and imprinting of drug molecules within the hydrogel for enhanced loading and smart delivery of drugs. Mol. Syst. Des. Eng. 2021, 6, 286–292. [Google Scholar] [CrossRef]
- Xu, R.N.; Zhang, Y.L.; Ma, S.H.; Ma, Z.F.; Yu, B.; Cai, M.R.; Zhou, F. A Universal Strategy for Growing a Tenacious Hydrogel Coating from a Sticky Initiation Layer. Adv. Mater. 2022, 34, 2108889. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.C.; Wang, B.; Li, J.P.; Xu, J.; Li, J.; Zeng, J.S.; Gao, W.H.; Chen, K.F. A self-healing, recyclable and conductive gelatin/nanofibrillated cellulose/Fe3+ hydrogel based on multi-dynamic interactions for a multifunctional strain sensor. Mater. Horiz. 2022, 9, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhu, K.; Guo, W.; Wu, D.; Quan, X.; Huang, X.; Liu, S.; Li, Y.; Fang, H.; Qiu, Y.; et al. Adhesive and Hydrophobic Bilayer Hydrogel Enabled On-Skin Biosensors for High-Fidelity Classification of Human Emotion. Adv. Funct. Mater. 2022, 32, 2200457. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Y.; Zheng, X.; Yi, Y.; Chen, X.; Li, Y.; Sun, D.; Zhang, L. Multifunctional load-bearing hybrid hydrogel with combined drug release and photothermal conversion functions. NPG Asia. Mater. 2020, 12, 18–28. [Google Scholar] [CrossRef]
- Jing, L.; Li, H.; Tay, R.Y.; Sun, B.; Tsang, S.H.; Cometto, O.; Lin, J.J.; Teo, E.H.T.; Tok, A.I.Y. Biocompatible Hydroxylated Boron Nitride Nanosheets/Poly(vinyl alcohol) Interpenetrating Hydrogels with Enhanced Mechanical and Thermal Response. Acs Nano 2017, 11, 3742–3751. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.L.; Luo, F.; Hong, W.; Cui, K.; Huang, Y.; Zhang, H.J.; King, D.R.; Kurokawa, T.; Nakajima, T.; Gong, J.P. Bulk Energy Dissipation Mechanism for the Fracture of Tough and Self-Healing Hydrogels. Macromolecules 2017, 50, 2923–2931. [Google Scholar] [CrossRef]
- King, D.R.; Sun, T.L.; Huang, Y.; Kurokawa, T.; Nonoyama, T.; Crosby, A.J.; Gong, J.P. Extremely tough composites from fabric reinforced polyampholyte hydrogels. Mater. Horiz. 2015, 2, 584–591. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, Y.; Lin, P.; Jia, Z.; Zhang, Y.; Liu, F.; Yu, B.; Zhou, F. Extremely Tough Hydrogels with Cotton Fibers Reinforced. Adv. Eng. Mater. 2020, 22, 2000508. [Google Scholar] [CrossRef]
- Li, J.; Gao, L.; Xu, R.; Ma, S.; Ma, Z.; Liu, Y.; Wu, Y.; Feng, L.; Cai, M.; Zhou, F. Fibers reinforced composite hydrogels with improved lubrication and load-bearing capacity. Friction 2022, 10, 54–67. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Chen, Y.; Han, X.; Jiang, F. Cellulose Nanofibrils Enhanced, Strong, Stretchable, Freezing-Tolerant Ionic Conductive Organohydrogel for Multi-Functional Sensors. Adv. Funct. Mater. 2020, 30, 2003430. [Google Scholar] [CrossRef]
- Li, J.; Suo, Z.; Vlassak, J.J. Stiff, strong, and tough hydrogels with good chemical stability. J. Mater. Chem. B 2014, 2, 6708–6713. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xiong, D.S. Microstructure and friction properties of PVA/PVP hydrogels for articular cartilage repair as function of polymerization degree and polymer concentration. Wear 2013, 305, 280–285. [Google Scholar] [CrossRef]
- Zhang, X.; Sheng, N.; Wang, L.; Tan, Y.; Liu, C.; Xia, Y.; Nie, Z.; Sui, K. Supramolecular nanofibrillar hydrogels as highly stretchable, elastic and sensitive ionic sensors. Mater. Horiz. 2019, 6, 326–333. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, S.H.; Wei, Q.B.; Ye, Q.; Yu, B.; van der Gucht, J.; Zhou, F. The Weak Interaction of Surfactants with Polymer Brushes and Its Impact on Lubricating Behavior. Macromolecules 2015, 48, 6186–6196. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Ren, X.; Gao, G. Alginate fiber toughened gels similar to skin intelligence as ionic sensors. Carbohydr. Polym. 2020, 235, 116018. [Google Scholar] [CrossRef]
- Xu, R.; Ma, S.; Lin, P.; Yu, B.; Zhou, F.; Liu, W. High Strength Astringent Hydrogels Using Protein as the Building Block for Physically Cross-linked Multi-Network. ACS Appl. Mater. Interfaces 2018, 10, 7593–7601. [Google Scholar] [CrossRef]
- Ma, R.; Xiong, D.; Miao, F.; Zhang, J.; Peng, Y. Novel PVP/PVA hydrogels for articular cartilage replacement. Mat. Sci. Eng. C 2009, 29, 1979–1983. [Google Scholar] [CrossRef]
- Rong, M.; Liu, H.; Scaraggi, M.; Bai, Y.; Bao, L.; Ma, S.; Ma, Z.; Cai, M.; Dini, D.; Zhou, F. High Lubricity Meets Load Capacity: Cartilage Mimicking Bilayer Structure by Brushing up Stiff Hydrogels from Subsurface. Adv. Funct. Mater. 2020, 30, 2004062. [Google Scholar] [CrossRef]
- Zhang, R.; Feng, Y.; Ma, S.; Cai, M.; Yang, J.; Yu, B.; Zhou, F. Tuning the Hydration and Lubrication of the Embedded Load-Bearing Hydrogel Fibers. Langmuir 2017, 33, 2069–2075. [Google Scholar] [CrossRef]


| Hydrogels | PVA (g) | SA (g) | NaCl (g) | Water (mL) |
|---|---|---|---|---|
| PVA | 5 | / | / | 20 |
| PVA/SA | 5 | 0.6 | / | 20 |
| PVA/SA/NaCl | 5 (25%) | 0.6 | 0.4 | 20 |
| 3 (15%) | 0.6 | 0.4 | 20 | |
| 4 (20%) | 0.6 | 0.4 | 20 | |
| 6 (30%) | 0.6 | 0.4 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Zhao, W.; Ning, F.; Zhen, J.; Qiang, H.; Zhang, Y.; Liu, F.; Jia, Z. Alginate Fiber-Enhanced Poly(vinyl alcohol) Hydrogels with Superior Lubricating Property and Biocompatibility. Polymers 2022, 14, 4063. https://doi.org/10.3390/polym14194063
Zhang R, Zhao W, Ning F, Zhen J, Qiang H, Zhang Y, Liu F, Jia Z. Alginate Fiber-Enhanced Poly(vinyl alcohol) Hydrogels with Superior Lubricating Property and Biocompatibility. Polymers. 2022; 14(19):4063. https://doi.org/10.3390/polym14194063
Chicago/Turabian StyleZhang, Ran, Wenhui Zhao, Fangdong Ning, Jinming Zhen, Huifen Qiang, Yujue Zhang, Fengzhen Liu, and Zhengfeng Jia. 2022. "Alginate Fiber-Enhanced Poly(vinyl alcohol) Hydrogels with Superior Lubricating Property and Biocompatibility" Polymers 14, no. 19: 4063. https://doi.org/10.3390/polym14194063
APA StyleZhang, R., Zhao, W., Ning, F., Zhen, J., Qiang, H., Zhang, Y., Liu, F., & Jia, Z. (2022). Alginate Fiber-Enhanced Poly(vinyl alcohol) Hydrogels with Superior Lubricating Property and Biocompatibility. Polymers, 14(19), 4063. https://doi.org/10.3390/polym14194063






