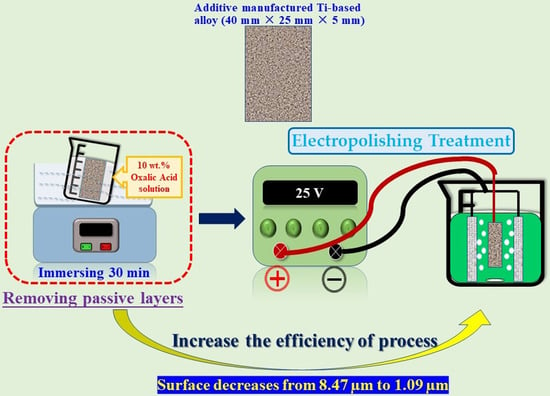
The Effect of Oxalic Acid as the Pre-Activator for the Electropolishing of Additive Manufactured Titanium-Based Materials and Its Characterization
Abstract
1. Introduction
2. Experimental
2.1. Materials and Electropolish Parameters
2.2. Microstructural Characterization
2.3. Electrochemical Properties Measurements
3. Results and Discussion
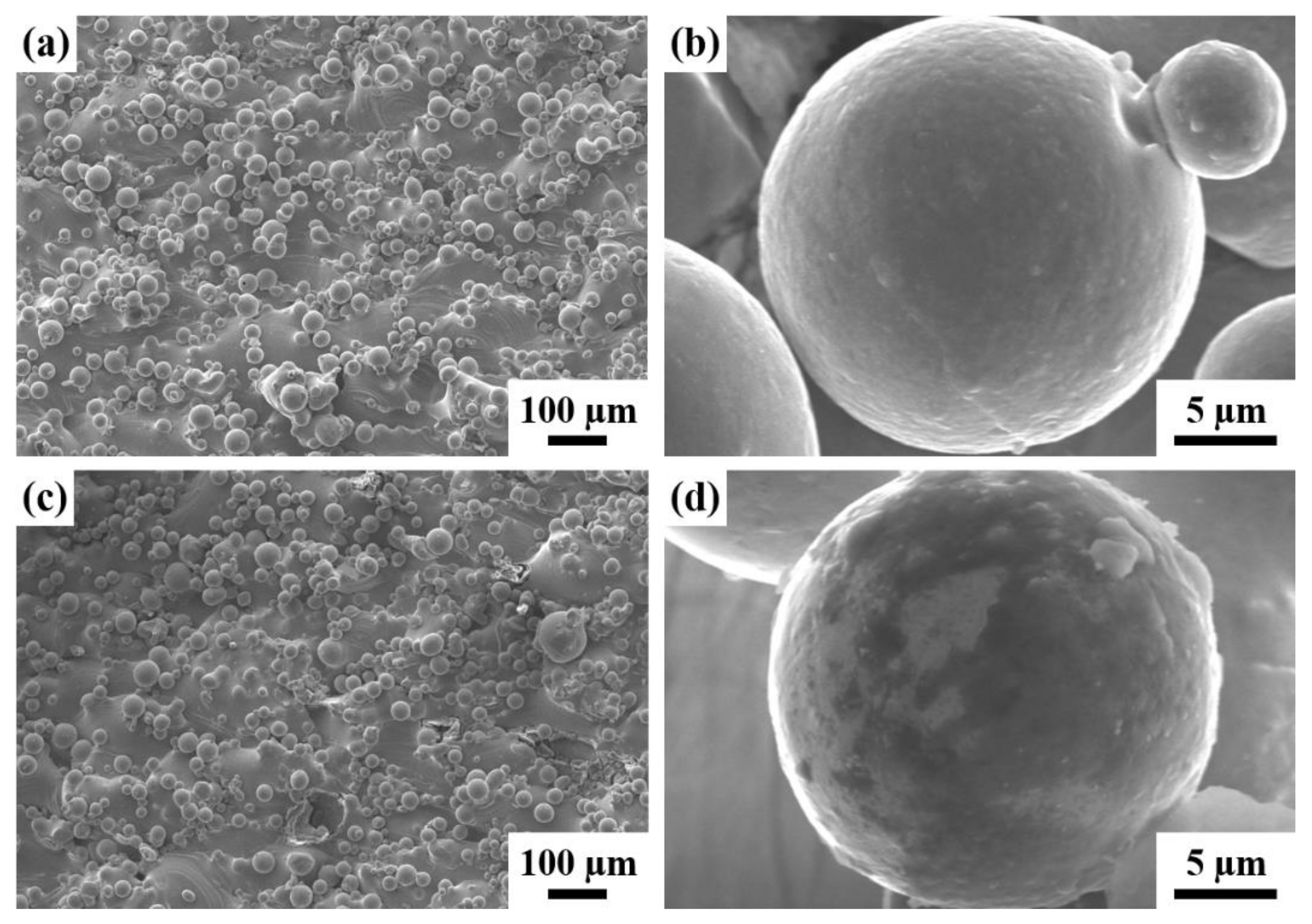
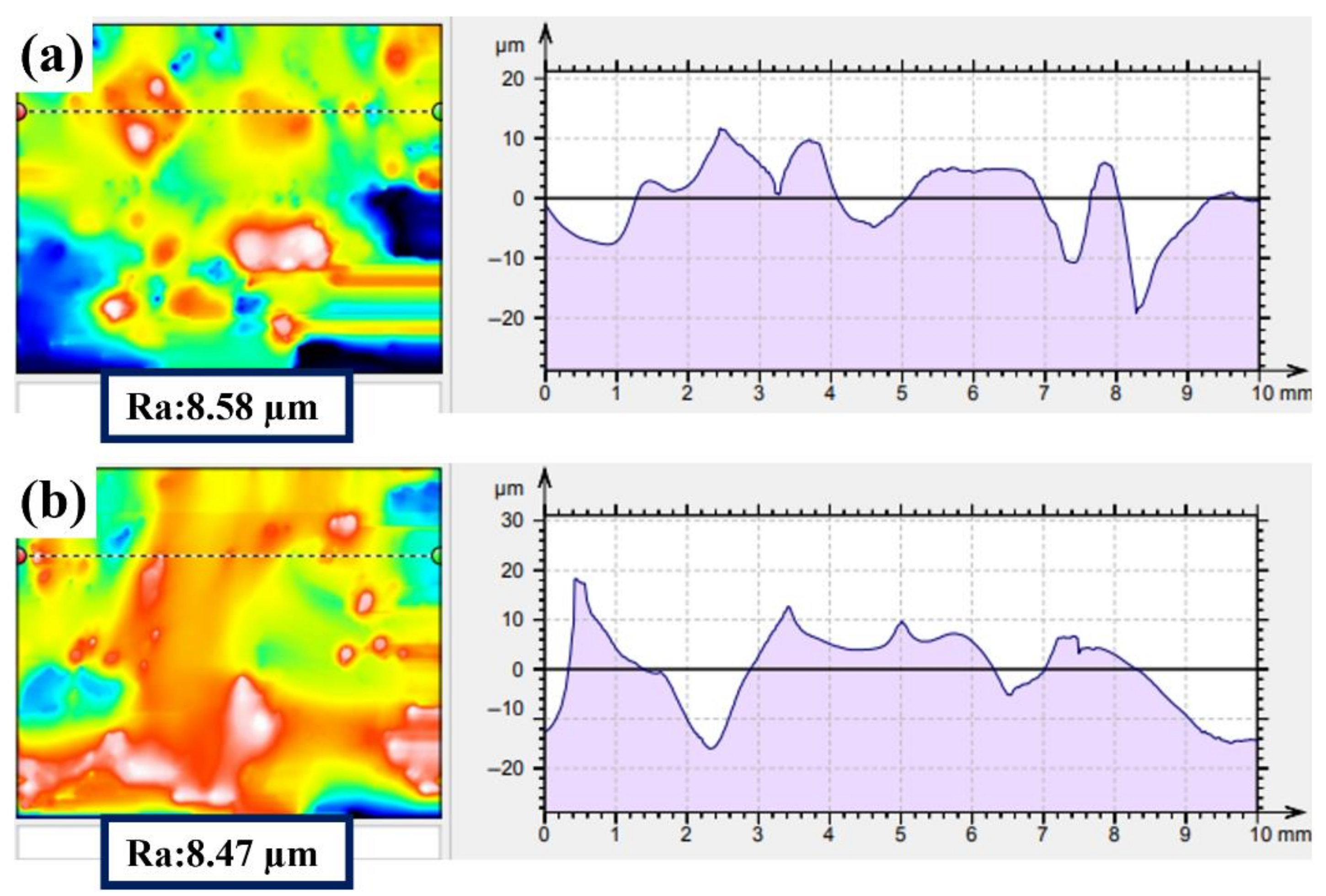
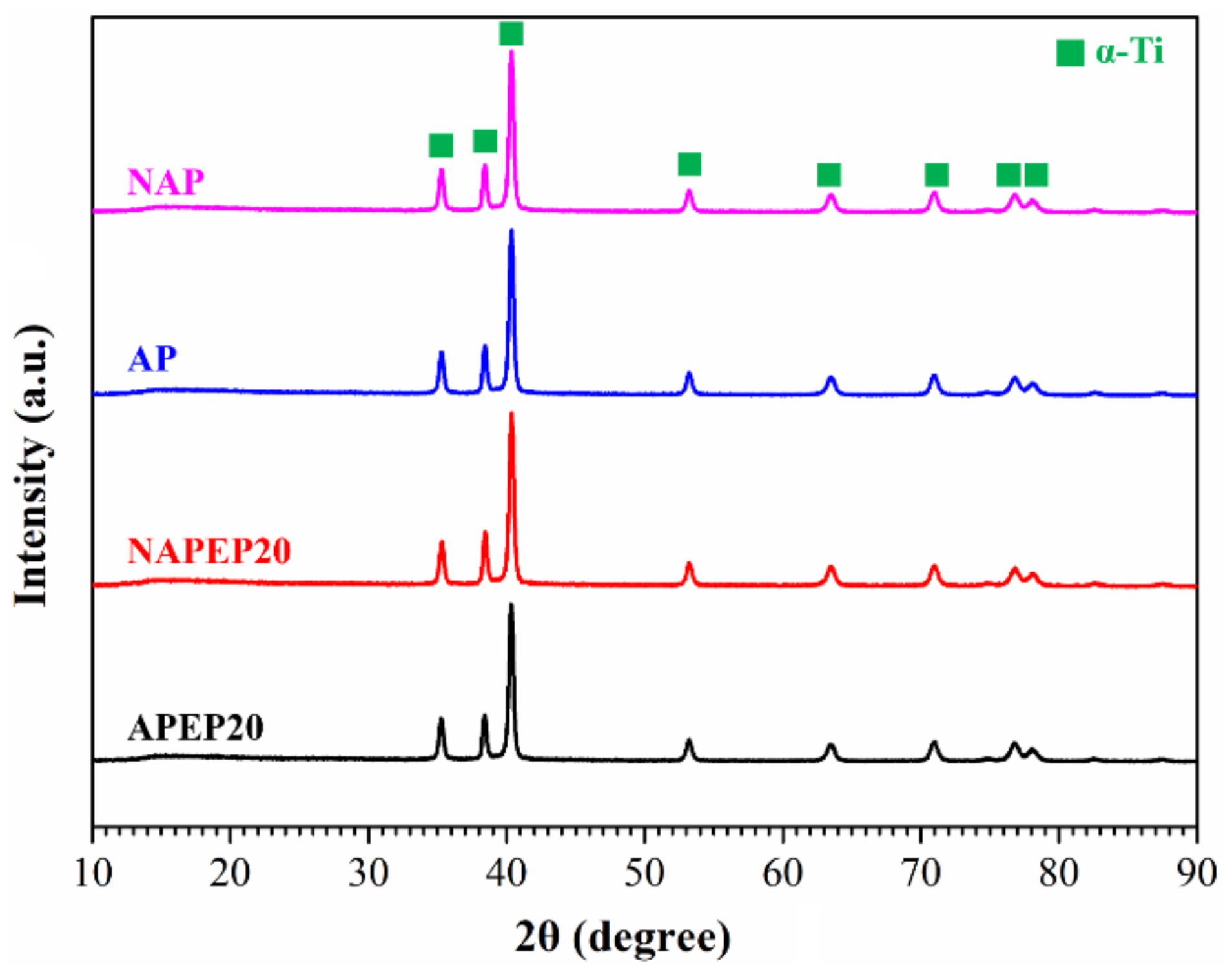
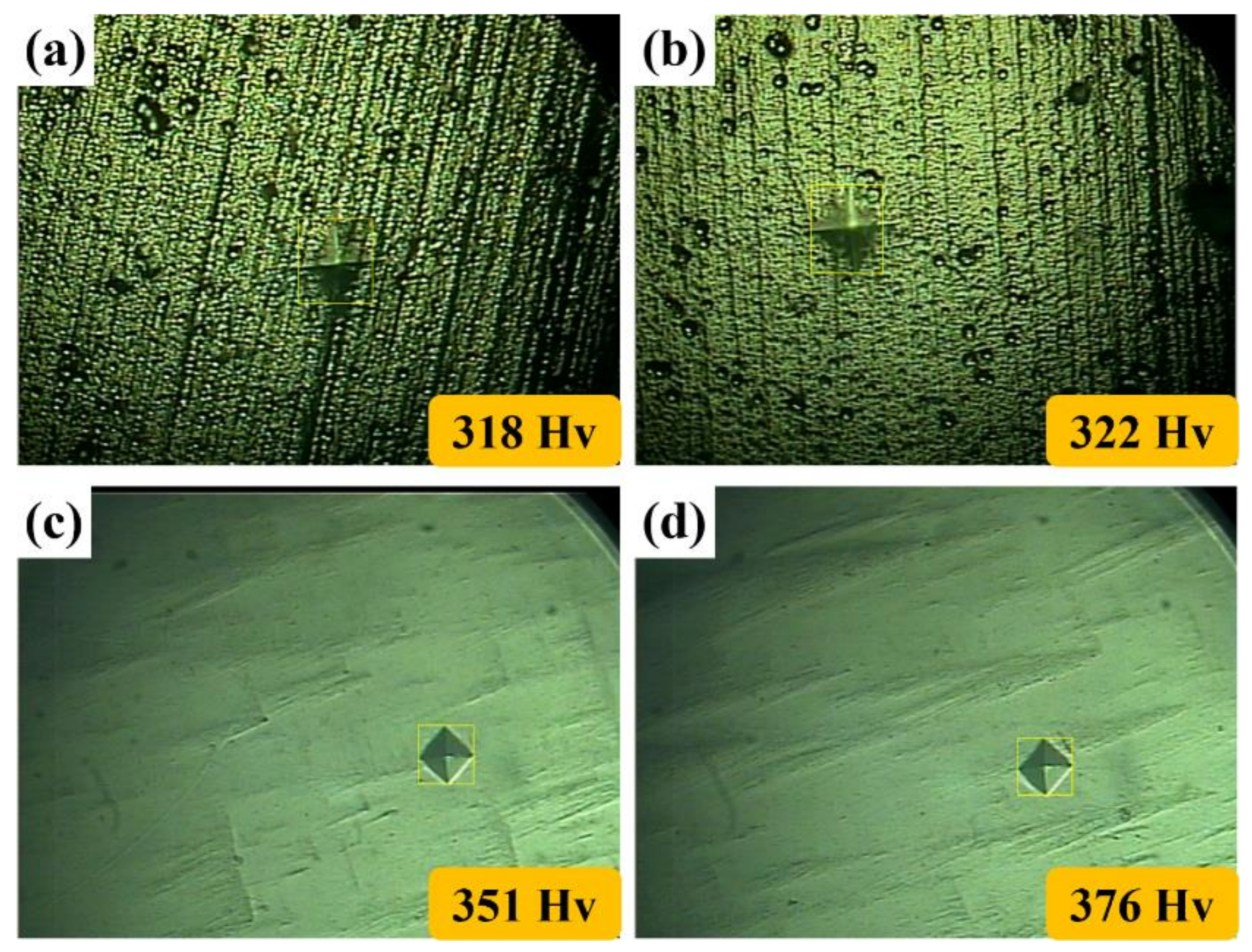
3.1. The Properties of the Pre-Activation Specimens
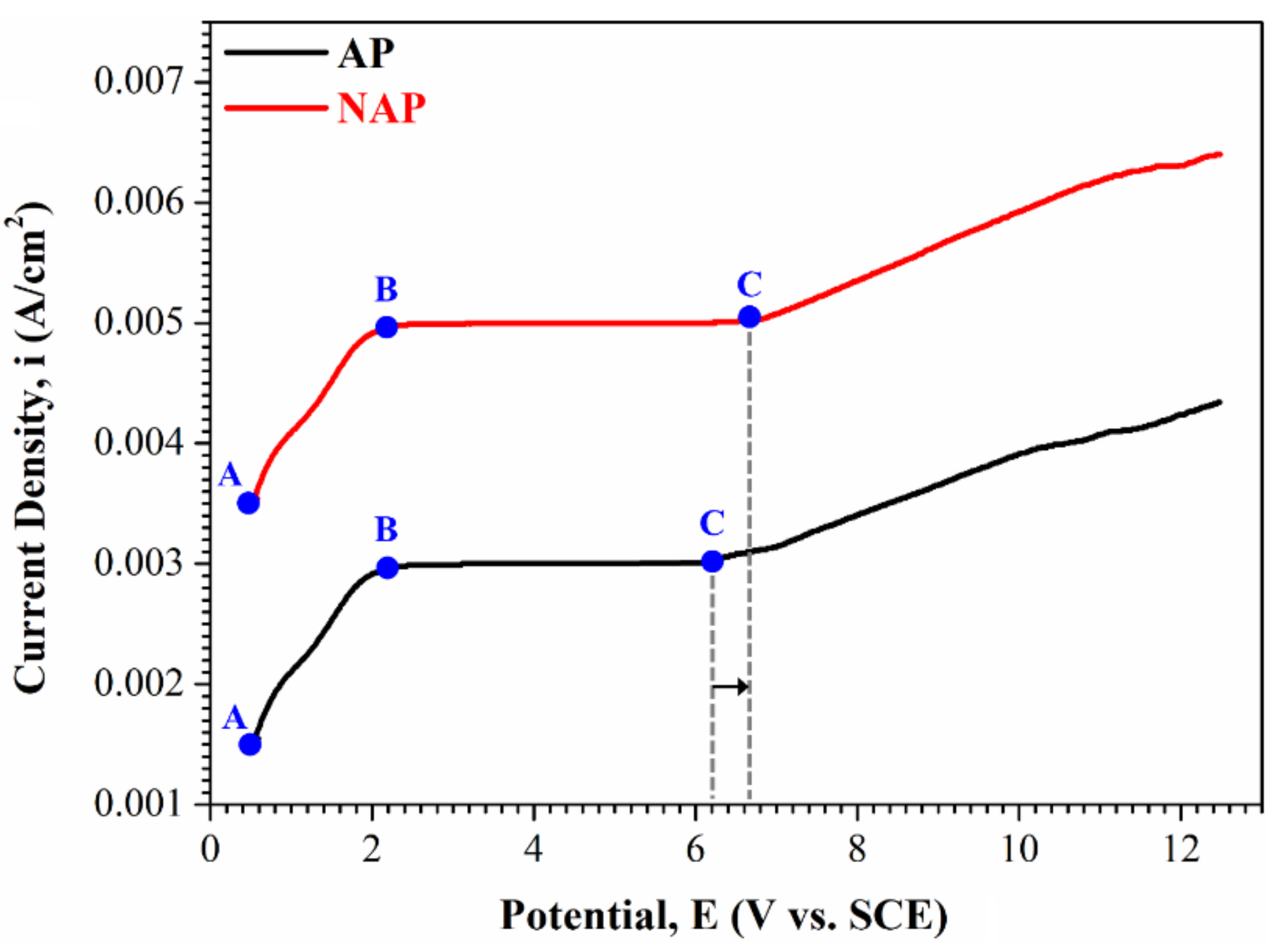
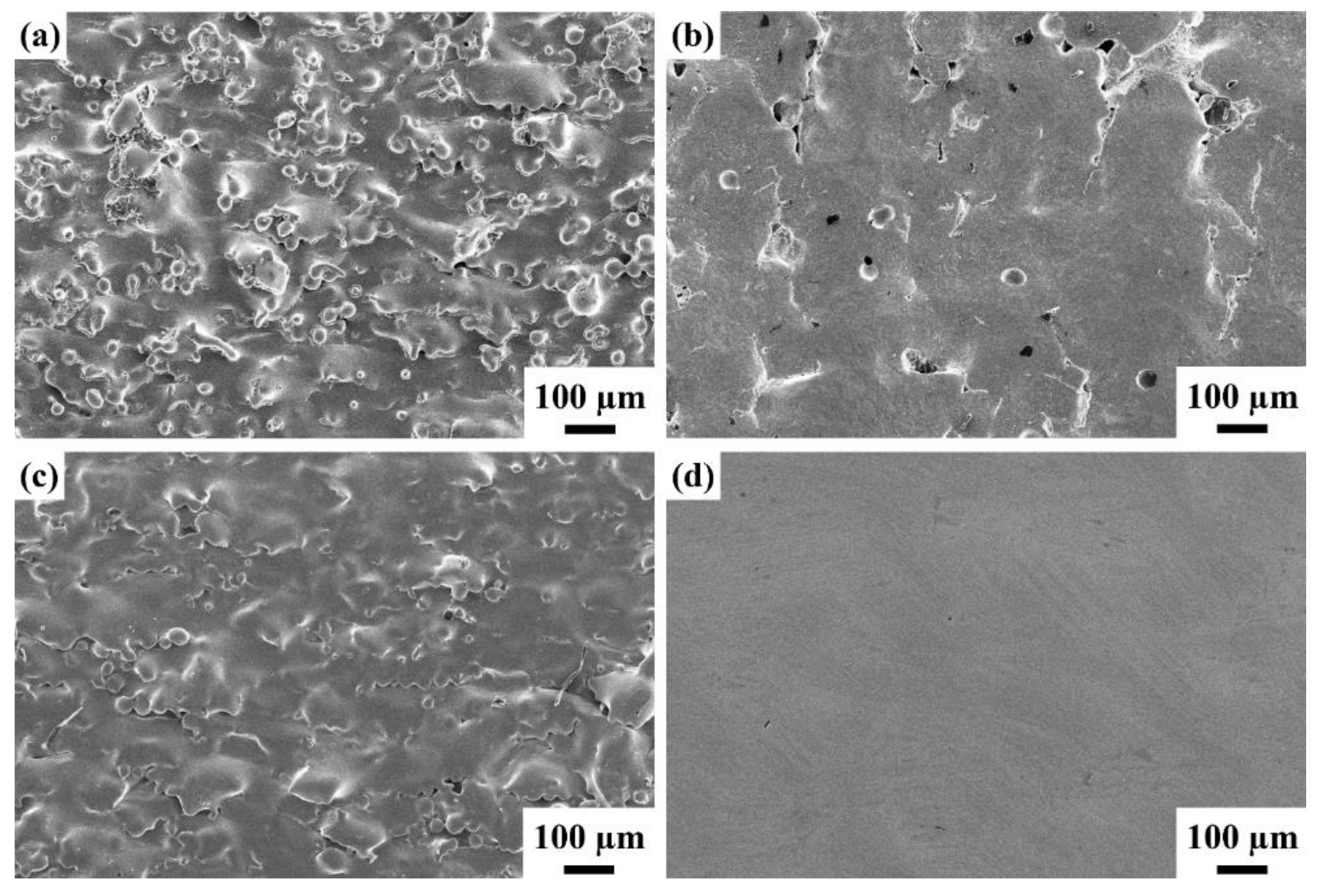
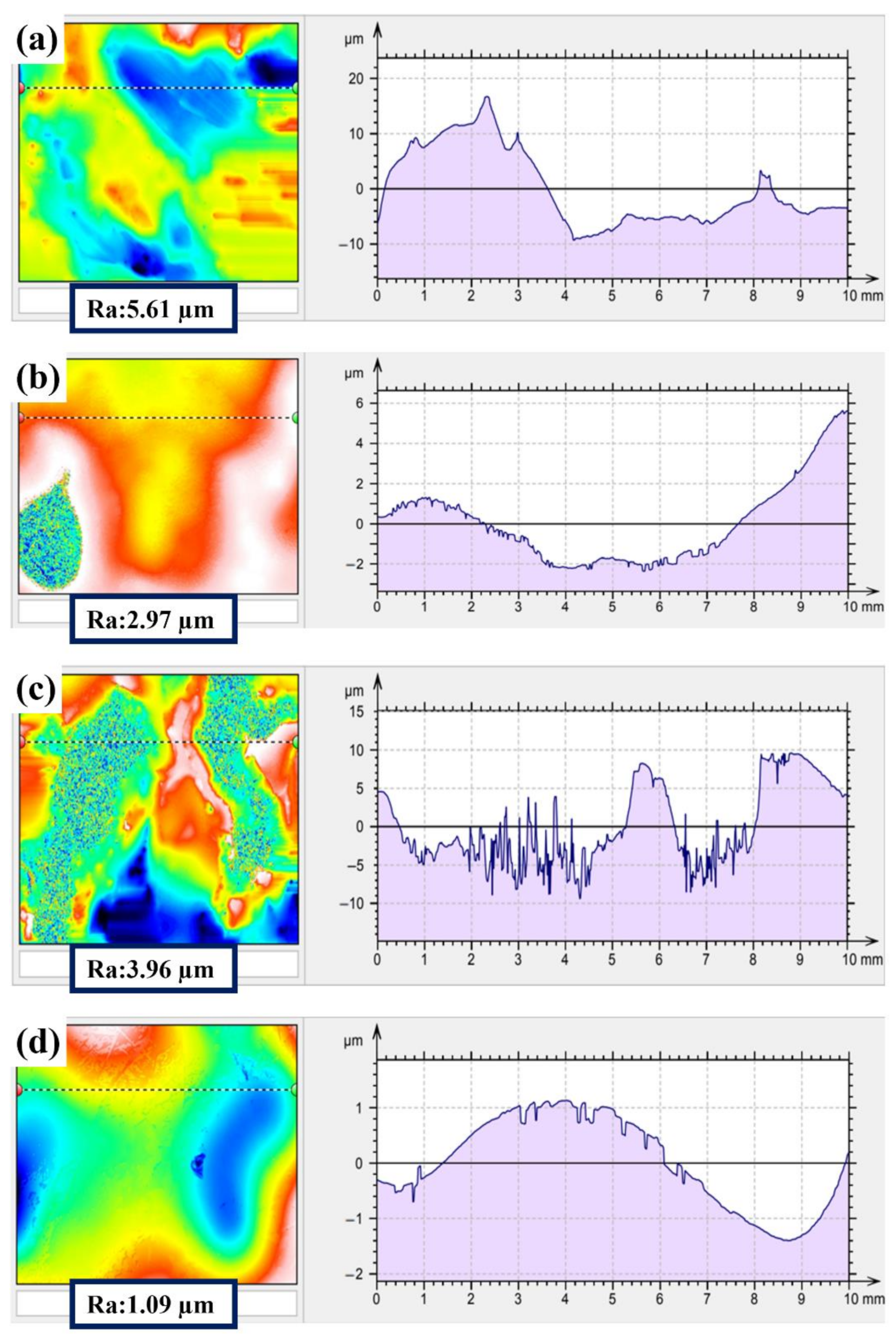
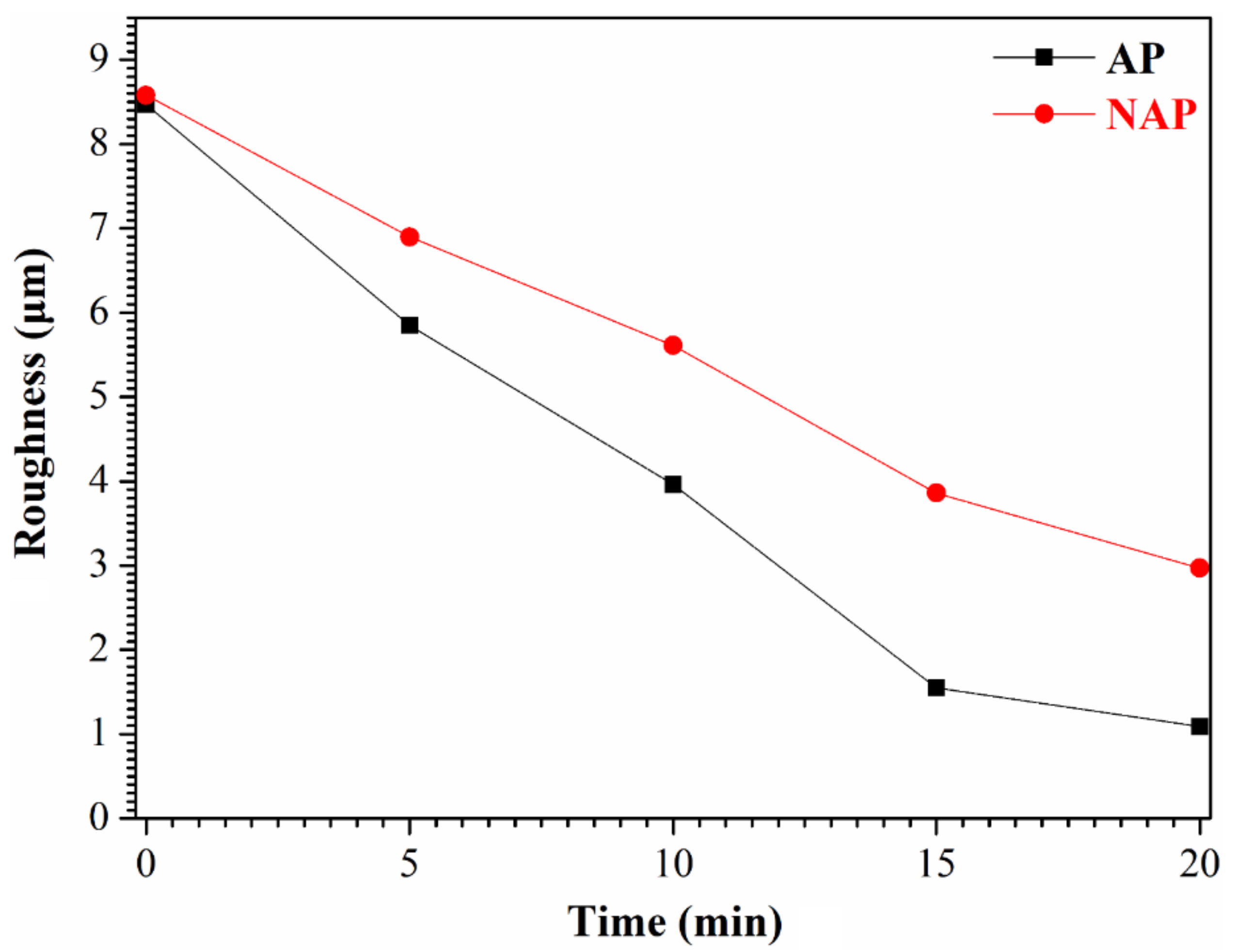
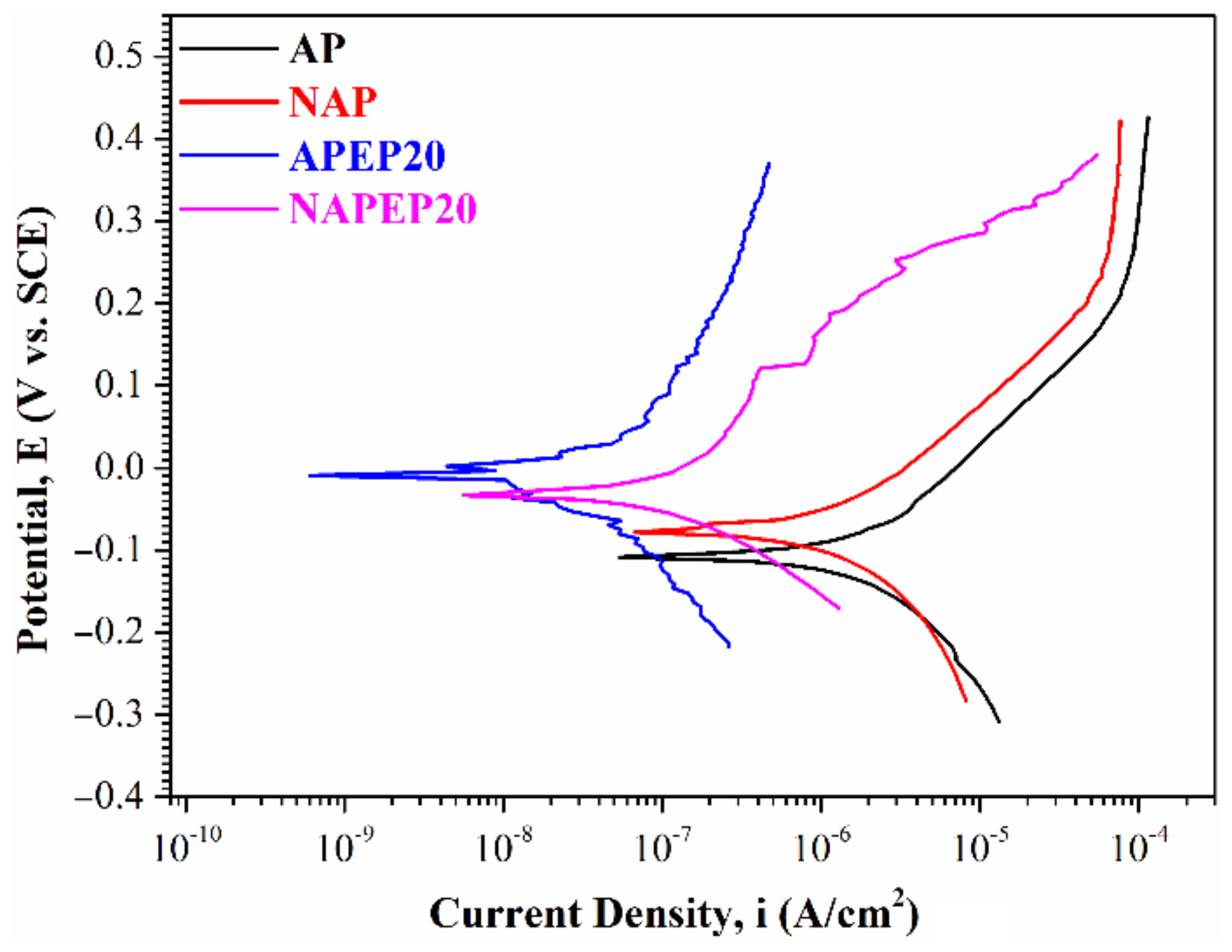
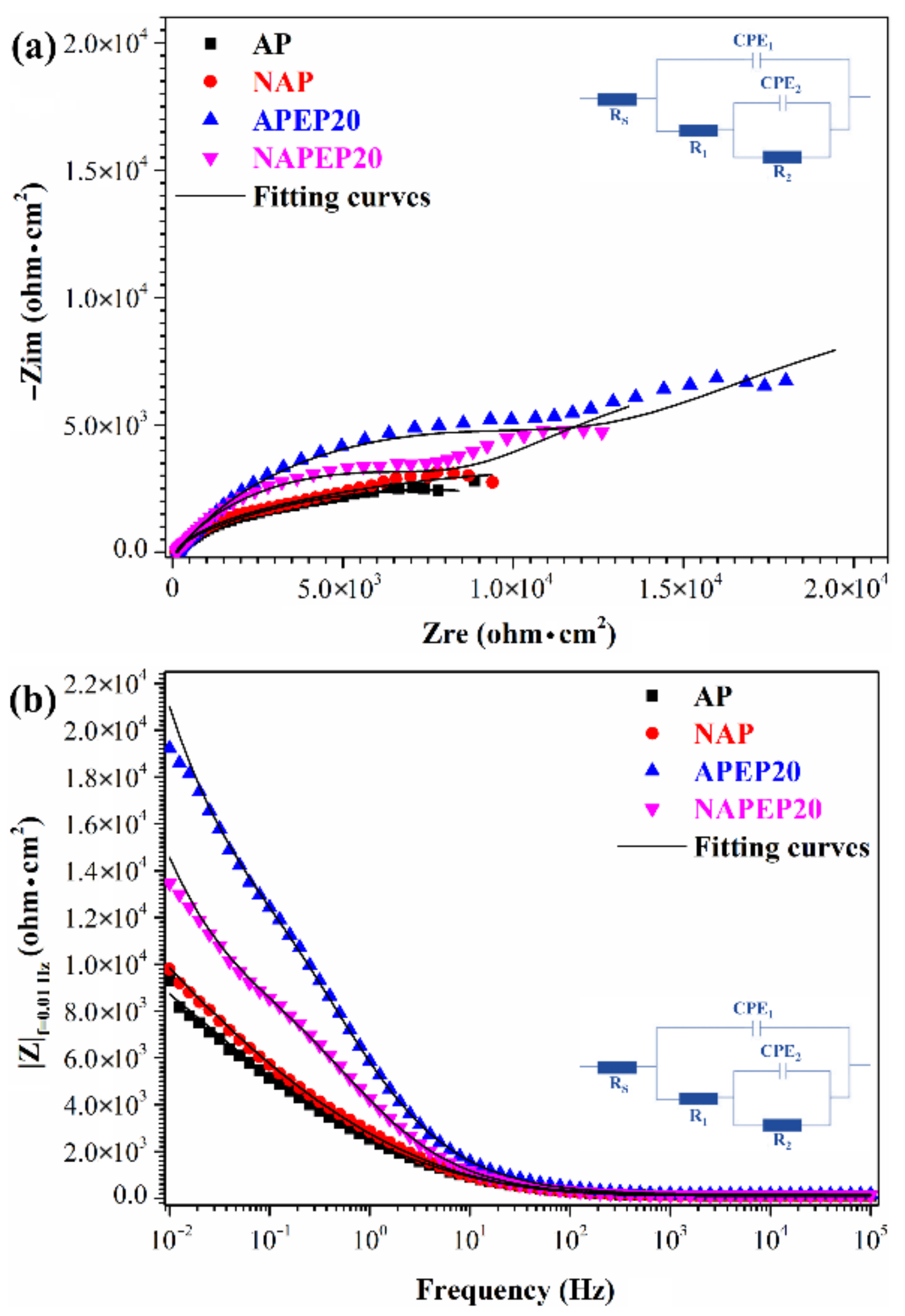
3.2. The Properties of the Electropolished Specimens
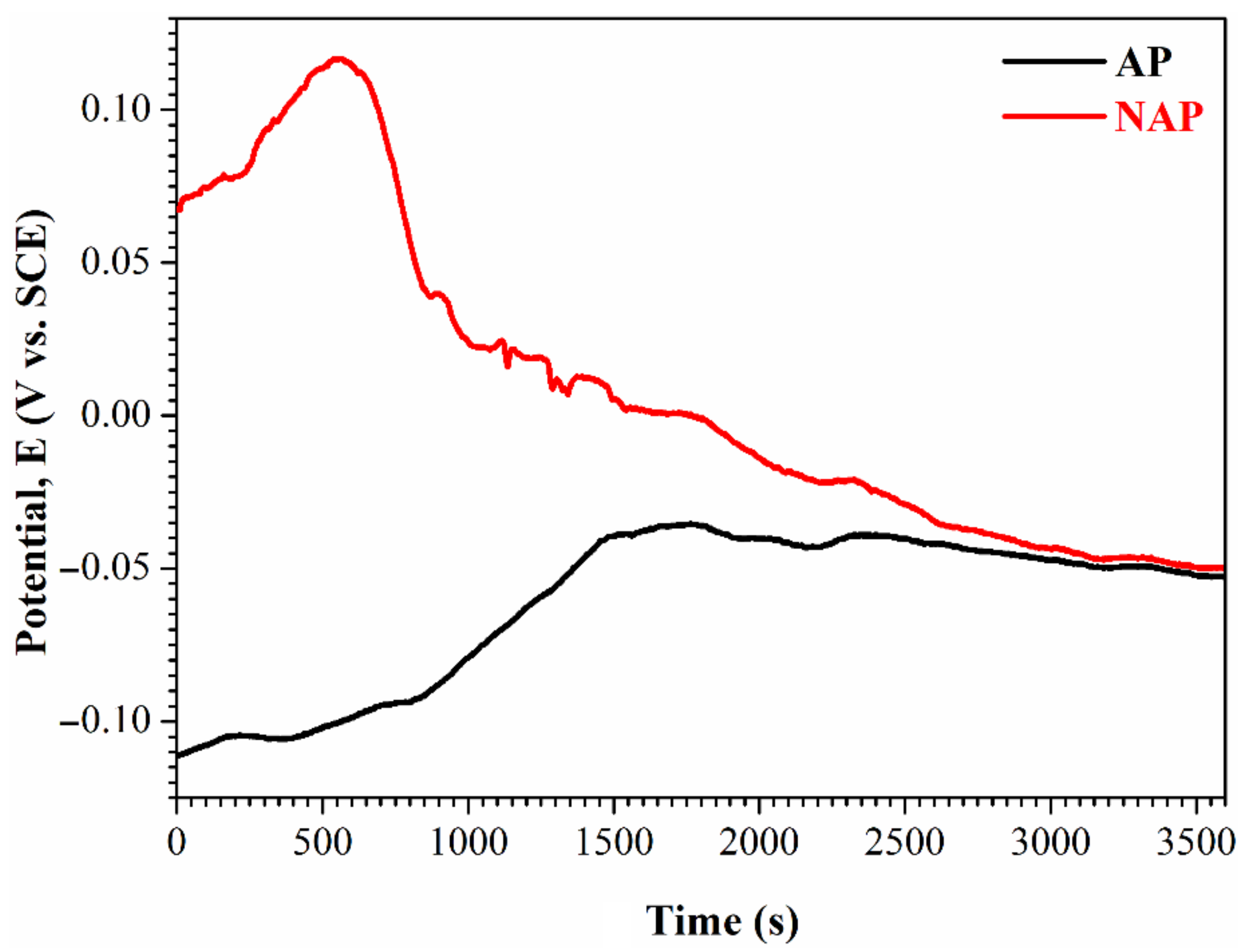
3.3. Comparison of the NAPEP and APEP Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donachie, M.J. Titanium: A Technical Guide; ASM international: Almere, The Netherlands, 2000. [Google Scholar]
- Venkateswarlu, K.; Rameshbabu, N.; Sreekanth, D.; Bose, A.C.; Muthupandi, V.; Babu, N.K.; Subramanian, S. Role of electrolyte additives on in-vitro electrochemical behavior of micro arc oxidized titania films on Cp Ti. Appl. Surf. Sci. 2012, 258, 6853–6863. [Google Scholar]
- Kim, H.M.; Kokubo, T.; Fujibayashi, S.; Nishiguchi, S.; Nakamura, T. Bioactive macroporous titanium surface layer on titanium substrate. J. Biomed. Mater. Res. 2000, 52, 553–557. [Google Scholar] [CrossRef]
- Kumar, A.M.; Sudhagar, P.; Ramakrishna, S.; Kang, Y.S.; Kim, H.; Gasem, Z.M.; Rajendran, N. Evaluation of chemically modified Ti–5Mo–3Fe alloy surface: Electrochemical aspects and in vitro bioactivity on MG63 cells. Appl. Surf. Sci. 2014, 307, 52–61. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Nie, F.; Zheng, Y.; Cheng, Y.; Wei, S.; Valiev, R. Enhanced in vitro biocompatibility of ultrafine-grained titanium with hierarchical porous surface. Appl. Surf. Sci. 2011, 257, 5634–5640. [Google Scholar] [CrossRef]
- Wohlers, T.; Gornet, T. Wohlers Report: Additive Manufacturing and 3D Printing State of the Industry Annual Worldwide Progress Report; ASTM International: Washington, DC, USA, 2011. [Google Scholar]
- Annalisa, A.; Tullio, M. The Electropolishing of Additively Manufactured Parts in Titanium: State of the Art. Adv. Eng. Mater. 2021, 23, 2100545. [Google Scholar]
- Greitemeier, D.; Donne, C.D.; Syassen, F.; Eufinger, J.; Melz, T. Effect of surface roughness on fatigue performance of additive manufactured Ti–6Al–4V. Mater. Sci. Technol. 2016, 32, 629–634. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, S.J.; Wang, H.L.; Hou, W.T.; Yang, R.; Sercombe, T.B.; Zhang, L.C. Microstructure, defects and mechanical behavior of beta-type titanium porous structures manufactured by electron beam melting and selective laser melting. Acta Mater. 2016, 113, 56–67. [Google Scholar] [CrossRef]
- Critchlow, G.W.; Brewis, D.M. Review of surface pretreatments for titanium alloys. Int. J. Adhes. Adhes. 1995, 15, 161–172. [Google Scholar] [CrossRef]
- Rafi, H.K.; Karthik, N.V.; Gong, H.; Starr, T.L.; Stucker, B.E. Microstructures and Mechanical Properties of Ti6Al4V Parts Fabricated by Selective Laser Melting and Electron Beam Melting. J. Mater. Eng. Perform. 2013, 22, 3872–3883. [Google Scholar] [CrossRef]
- Wagner, C. Contribution to the Theory of Electropolishing. J. Electrochem. Soc. 1954, 101, 225–228. [Google Scholar] [CrossRef]
- Matlosz, M. Modeling of impedance mechanisms in electropolishing. Electrochim. Acta 1995, 40, 393–401. [Google Scholar] [CrossRef]
- Hoar, T.P.; Mears, D.C.; Rothwell, G.P. The relationships between anodic passivity, brightening and pitting. Corros. Sci. 1965, 5, 279–289. [Google Scholar] [CrossRef]
- Tsoeunyane, G.M.; Mathe, N.; Tshabalala, L.; Makhatha, M.E. Electropolishing of Additively Manufactured Ti-6Al-4V Surfaces in Nontoxic Electrolyte Solution. Adv. Mater. Sci. Eng. 2022, 2022, 6987353. [Google Scholar] [CrossRef]
- Liu, L.; Wu, S.Q.; Gu, H.F.; Yang, Y.; Wang, H.; Huang, A.J. Effect of The Number of Hydroxyls and Carbons on the Solubility of Sodium Chloride in Alcohol and The Electropolishing Behavior of Ti-6Al-4V Alloy in Alcohol-Salt System. J. Electrochem. Soc. 2021, 168, 093503. [Google Scholar] [CrossRef]
- Han, W.; Fang, F.Z. Orientation effect of electropolishing characteristics of 316L stainless steel fabricated by laser powder bed fusion. Front. Mech. Eng. 2021, 16, 580–592. [Google Scholar] [CrossRef]
- Han, W.; Fang, F.Z. Investigation of electropolishing characteristics of tungsten in ecofriendly sodium hydroxide aqueous solution. Adv. Manuf. 2020, 8, 265–278. [Google Scholar] [CrossRef]
- Han, W.; Fang, F.Z. Eco-friendly NaCl-based electrolyte for electropolishing 316L stainless steel. J. Manuf. Process. 2020, 58, 1257–1269. [Google Scholar] [CrossRef]
- Han, W.; Fang, F.Z. Fundamental aspects and recent developments in electropolishing. Int. J. Mach. Tools Manuf. 2019, 139, 1–23. [Google Scholar] [CrossRef]
- Tajima, K.; Hironaka, M.; Chen, K.K.; Nagamatsu, Y.; Kakigawa, H.; Kozono, Y. Electropolishing of CP Titanium and Its Alloys in an Alcoholic Solution-based Electrolyte. Dent. Mater. J. 2008, 27, 258–265. [Google Scholar] [CrossRef]
- Piotrowski, O.; Madore, C.; Landolt, D. The Mechanism of Electropolishing of Titanium in Methanol-Sulfuric Acid Electrolytes. J. Electrochem. Soc. 1998, 145, 2362–2369. [Google Scholar] [CrossRef]
- Piotrowski, O.; Madore, C.; Landolt, D. Electropolishing of Titanium and Titanium Alloys in Perchlorate-Free Electrolytes. Plating surf. finish. 1998, 85, 115–119. [Google Scholar]
- Piotrowski, O.; Madore, C.; Landolt, D. Electropolishing of tantalum in sulfuric acid-methanol electrolytes. Electrochim. Acta 1999, 44, 3389–3399. [Google Scholar] [CrossRef]
- Lebedeva, O.; Snytko, V.; Kuznetsova, I.; Kalmykov, K.; Kultin, D.; Root, N.; Philippova, S.; Dunaev, S.; Zakharov, A.; Kustov, L. Impact of Pretreatment of Metal Glass Fe70Cr15B15 on Anodization in 1-butyl-3-methylimidazolium Tetrafluoroborate Ionic Liquid. Metals 2020, 10, 583. [Google Scholar] [CrossRef]
- Cui, C.; Gu, A.; Xiao, M.; Xu, L.; Li, H.; Yang, Z.; Dong, T.; Gao, H.; Jiang, F. A novel in-situ activation method on titanium alloys for electroless plating. Mater. Lett. 2020, 276, 128275. [Google Scholar] [CrossRef]
- Li, L.F.; Caenen, P.; Daerden, M.; Vaes, D.; Meers, G.; Dhondt, C.; Celis, J.P. Mechanism of single and multiple step pickling of 304 stainless steel in acid electrolytes. Corros. Sci. 2005, 47, 1307–1324. [Google Scholar] [CrossRef]
- Fekry, A.M. The influence of chloride and sulphate ions on the corrosion behavior of Ti and Ti-6Al-4V alloy in oxalic acid. Electrochim. Acta 2009, 54, 3480–3489. [Google Scholar] [CrossRef]
- Bandeira, R.M.; Rêgo, G.C.; Picone, C.A.; Drunen, J.V.; Correr, W.R.; Casteletti, L.C.; Machado, S.A.A.; Germano, T.F. Alternating current oxidation of Ti–6Al–4V alloy in oxalic acid for corrosion resistant surface finishing. SN Appl. Sci. 2020, 2, 1092. [Google Scholar] [CrossRef]
- Jian, S.Y.; Chang, K.L. Effect of cerium ion on the microstructure and properties of permanganate conversion coating on LZ91 magnesium alloy. Appl. Surf. Sci. 2020, 509, 144767. [Google Scholar] [CrossRef]
- Jian, S.Y.; Yang, C.Y.; Chang, J.K. Robust corrosion resistance and self-healing characteristics of a novel Ce/Mn conversion coatings on EV31 magnesium alloys. Appl. Surf. Sci. 2020, 510, 145385. [Google Scholar] [CrossRef]
- Jian, S.Y.; Tzeng, Y.C.; Ger, M.D.; Chang, K.L.; Shi, G.N.; Huang, W.H.; Chen, C.Y.; Wu, C.C. The study of corrosion behavior of manganese-based conversion coating on LZ91 magnesium alloy: Effect of addition of pyrophosphate and cerium. Mater. Des. 2020, 192, 108707. [Google Scholar] [CrossRef]
- Jian, S.Y.; Chang, J.K.; Lin, K.F.; Chen, T.Y.; Yuan, J.H.; Ger, M.D. Characteristic V-Mn conversion coating in reinforcing corrosion resistance of electroless Ni-P coated LZ91 Mg alloy. Surf. Coat. Technol. 2020, 394C, 125724. [Google Scholar] [CrossRef]
- Padhi, D.; Yahalom, J.; Gandikota, S.; Dixit, G. Planarization of Copper Thin Films by Electropolishing in Phosphoric Acid for ULSI Applications. J. Electrochem. Soc. 2003, 150, G10. [Google Scholar] [CrossRef]
- Safdar, A.; Wei, L.Y.; Snis, A.; Lai, Z. Evaluation of microstructural development in electron beam melted Ti-6Al-4V. Mater. Charact. 2012, 65, 8–15. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, L.C.; Zhang, J.X.; Zhang, X.; Ni, Q.Z.; Chen, Y.; Wu, M.L.; Yang, C. Corrosion behavior of selective laser melted Ti-6Al-4V alloy in NaCl solution. Corros. Sci. 2016, 111, 484–489. [Google Scholar] [CrossRef]
- Wei, K.H.; Wang, Y.S.; Liu, C.P.; Chen, K.W.; Wang, Y.L.; Cheng, Y.L. The influence of abrasive particle size in copper chemical mechanical planarization. Surf. Coat. Technol. 2013, 231, 543–545. [Google Scholar] [CrossRef]
- Seo, J.; Yoon, K.S.; Moon, J.; Kim, K.; Sigmund, W.; Paik, U. Effects of physico-chemical properties between poly(ethyleneimine) and silica abrasive on copper chemical mechanical planarization. Microelectron. Eng. 2014, 113, 50–54. [Google Scholar] [CrossRef]
- Hung, C.C.; Lee, W.H.; Wang, Y.S.; Chen, Y.R. Measurement Techniques of Sheet Resistance on Copper Defects after Chemical Mechanical Polishing. Jpn. Soc. Appl. Phys. 2008, 47, 989. [Google Scholar] [CrossRef]
- Chang, R.; Cao, Y.; Spanos, C. Modeling the electrical effects of metal dishing due to CMP for on-chip interconnect optimization. IEEE Trans. Electron. Devices 2004, 51, 1577–1583. [Google Scholar] [CrossRef]
- Wang, Y.B.; Li, H.F.; Zheng, Y.F.; Li, M. Corrosion performances in simulated body fluids and cytotoxicity evaluation of Fe-based bulk metallic glasses. Mater. Sci. Eng. C 2012, 32, 599–606. [Google Scholar] [CrossRef]
- Liu, W.; Cao, F.; Chen, A.; Chang, L.; Zhang, J.; Cao, C. Corrosion behaviour of AM60 magnesium alloys containing Ce or La under thin electrolyte layers. Part 1: Microstructural characterization and electrochemical behaviour. Corros. Sci. 2010, 52, 627–638. [Google Scholar] [CrossRef]
| Names | Activation Process | Electropolishing Process | Operation Time (min) |
|---|---|---|---|
| NAP | N/A | N/A | N/A |
| AP | √ | N/A | N/A |
| NAPEP10 | N/A | √ | 10 |
| NAPEP20 | N/A | √ | 20 |
| APEP10 | √ | √ | 10 |
| APEP20 | √ | √ | 20 |
| AP | NAP | APEP20 | NAPEP20 | |
|---|---|---|---|---|
| Ecorr (V vs. SCE) | −0.107 | −0.078 | −0.009 | −0.033 |
| icorr (A/cm2) | 8.66 × 10−6 | 2.42 × 10−6 | 1.25 × 10−7 | 1.18 × 10−8 |
| Rs (ohm·cm2) | 94.3 | 95.1 | 114.8 | 114.5 |
| CPE1 (sn·μohm−1·cm−2) | 74.7 | 67.8 | 40.6 | 53.7 |
| CPE1- n | 0.69 | 0.69 | 0.69 | 0.69 |
| R1 (ohm·cm2) | 2422 | 3293 | 12,350 | 8274 |
| CPE2 (sn·μohm−1·cm−2) | 551.2 | 503.7 | 491.8 | 497.2 |
| CPE2- n | 0.64 | 0.66 | 0.66 | 0.67 |
| R2 (ohm·cm2) | 8672 | 9826 | 32,500 | 24,410 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Lee, C.-Y.; Ger, M.-D.; Jian, S.-Y.; Hung, J.-C.; Yang, P.-J.; Kao, C.-H.; Ferng, Y.-C.; Huang, Y.-S.; Jen, K.-K. The Effect of Oxalic Acid as the Pre-Activator for the Electropolishing of Additive Manufactured Titanium-Based Materials and Its Characterization. Polymers 2022, 14, 4198. https://doi.org/10.3390/polym14194198
Chen C-H, Lee C-Y, Ger M-D, Jian S-Y, Hung J-C, Yang P-J, Kao C-H, Ferng Y-C, Huang Y-S, Jen K-K. The Effect of Oxalic Acid as the Pre-Activator for the Electropolishing of Additive Manufactured Titanium-Based Materials and Its Characterization. Polymers. 2022; 14(19):4198. https://doi.org/10.3390/polym14194198
Chicago/Turabian StyleChen, Chun-Hao, Chia-Yu Lee, Ming-Der Ger, Shun-Yi Jian, Jung-Chou Hung, Po-Jen Yang, Chun-Hsiang Kao, Yi-Cherng Ferng, Ying-Sun Huang, and Kuo-Kuang Jen. 2022. "The Effect of Oxalic Acid as the Pre-Activator for the Electropolishing of Additive Manufactured Titanium-Based Materials and Its Characterization" Polymers 14, no. 19: 4198. https://doi.org/10.3390/polym14194198
APA StyleChen, C.-H., Lee, C.-Y., Ger, M.-D., Jian, S.-Y., Hung, J.-C., Yang, P.-J., Kao, C.-H., Ferng, Y.-C., Huang, Y.-S., & Jen, K.-K. (2022). The Effect of Oxalic Acid as the Pre-Activator for the Electropolishing of Additive Manufactured Titanium-Based Materials and Its Characterization. Polymers, 14(19), 4198. https://doi.org/10.3390/polym14194198