Coagulation Mechanism and Compressive Strength Characteristics Analysis of High-Strength Alkali-Activated Slag Grouting Material
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.1.1. Portland Cement (PC)
2.1.2. Granulated Blast-Furnace Slag (GBFS)
2.1.3. Alkali Activator
2.2. Experimental Methods
2.2.1. Experimental Mix Proportion
2.2.2. Preparation and Curing of Specimens
2.2.3. Compressive Strength Test
2.2.4. Condensation Behavior Test
2.2.5. X-ray Diffraction (XRD) Analysis
2.2.6. Scanning Electron Microscopy (SEM) Analysis
3. Experimental Results and Discussion
3.1. Compressive Strength Analysis
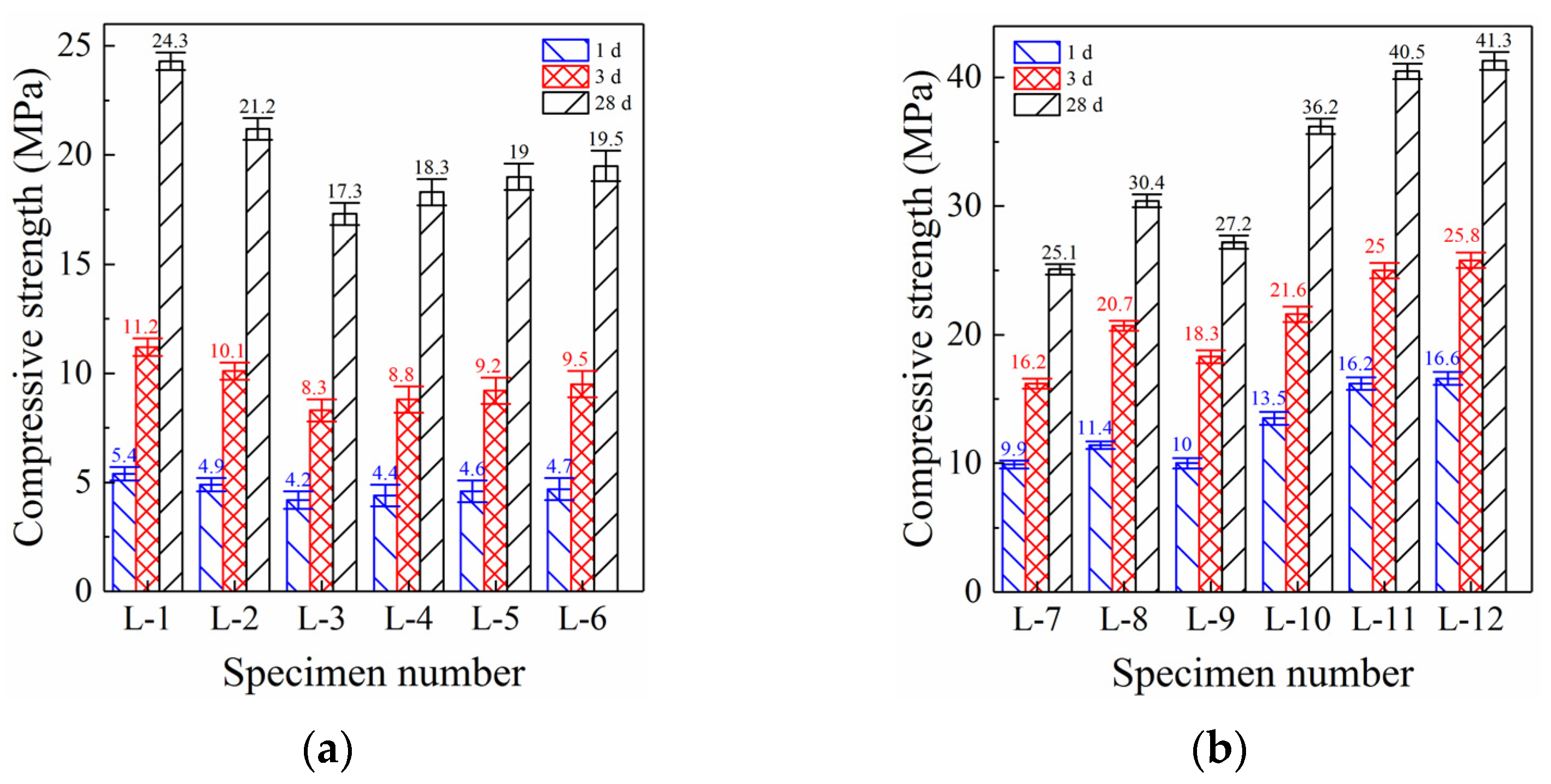
3.1.1. Compressive Strength of PC Grouting Materials
3.1.2. Compressive Strength of GBFS Alkali-Activated Grouting Materials
3.1.3. Effect of LSS on Compressive Strength
3.2. Condensation Behavior Analysis
3.2.1. Condensation Behavior of PC Grouting Materials
3.2.2. Condensation Behavior of GBFS Grouting Materials
3.2.3. Effect of LSS on Condensation Behavior
3.3. XRD Analysis
3.3.1. XRD Analysis of PC Grouting Materials
3.3.2. XRD Analysis of GBFS Alkali-Activated Grouting Materials
3.4. SEM Analysis
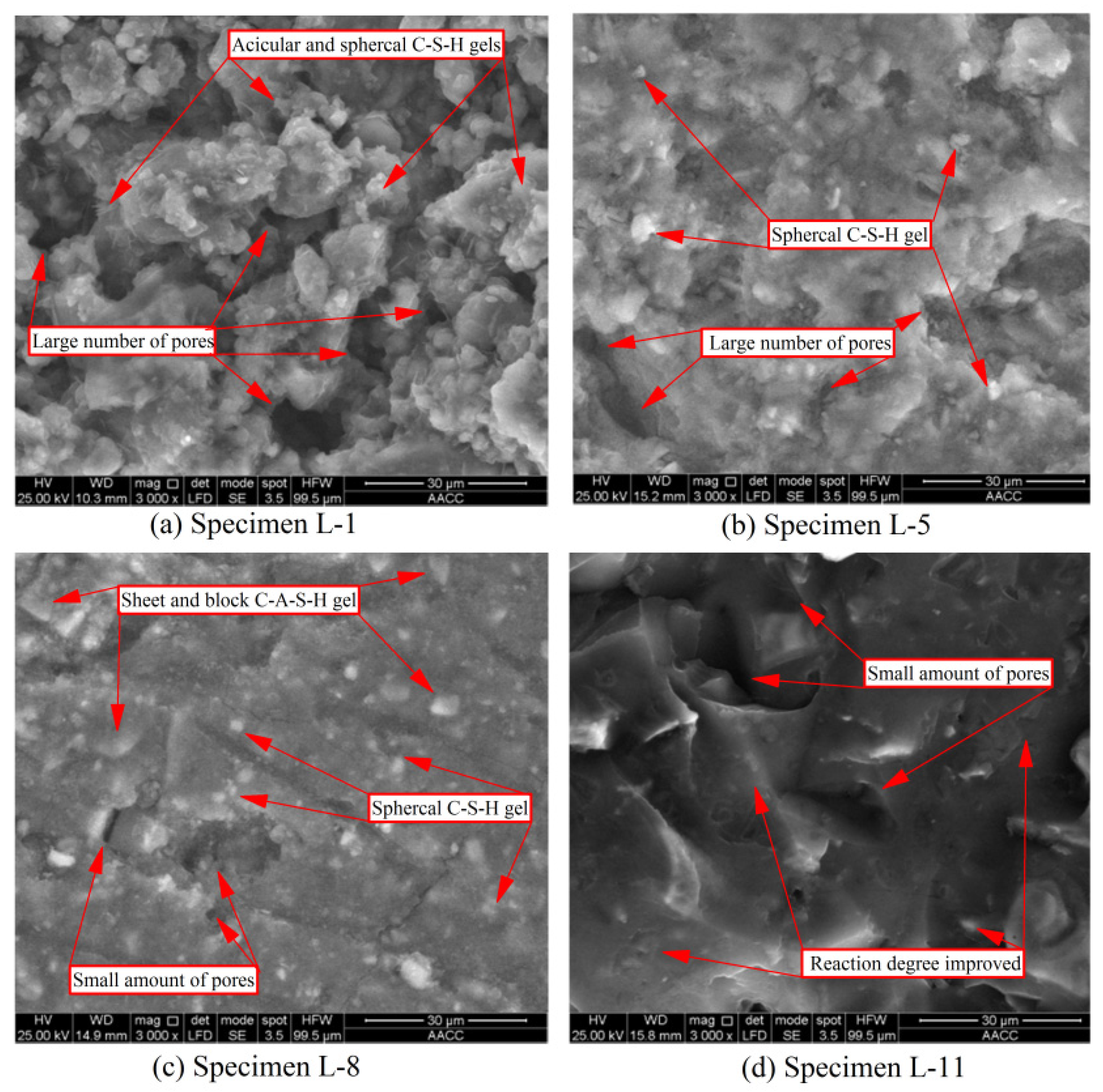
3.4.1. Microstructure of PC Grouting Materials
3.4.2. Microstructure of GBFS Alkali-Activated Grouting Materials
4. Conclusions
- The early compressive strength of cement grouting material was clearly insufficient. Moreover, both the strength development and setting were slow, making it difficult to achieve early reinforcement and the water plugging effect. Cement grouting material was especially unsuitable for use as grouting material in rescue engineering. Although the addition of LSS significantly accelerated the setting, it could not significantly improve the compressive strength. XRD analysis identified the formation of albite as the cause of the accelerated condensation.
- The early compressive strength of GBFS alkali-activated grouting material was significantly better than that of PC specimens (1 d strength increased by 120%), and its setting time was also significantly faster (IST shortened by 33.3%). With an increase in sodium hydroxide content, the compressive strength first increased and then decreased (maximum increase of 21.1%), while the setting time continued to shorten. XRD analysis identified the formation of aluminosilicate minerals as the main reason for the excellent mechanical properties and accelerated coagulation rate. SEM analysis showed that the crystal structure of aluminosilicate minerals was flat and smooth, making the microstructure denser and more complete.
- Based on GBFS alkali-activated grouting material, the addition of LSS can further improve the compressive strength and shorten the setting time. With an increase in LSS content, the compressive strength first increased significantly and then remained unchanged (maximum increase of 35.9%), while the setting time first decreased significantly and then remained unchanged (IST shortened by 58.1%). The optimal mix proportion was 40 g sodium hydroxide and 300 g LSS. XRD analysis showed that the crystallinity and formation of silicate and aluminosilicate were significantly improved after the addition of LSS. SEM analysis showed that the degree of polymerization after LSS addition and the microstructure of the specimen were significantly improved.
- This paper realized the preparation of early and high-strength slag alkali-activated grouting materials. However, extensive and in-depth research on hardness, fracture toughness, and impact strength is still needed to ensure their performance stability in all aspects.
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Q.; Wu, Q.; Xue, Y.; Kong, P.; Gong, B. Analysis of Overlying Strata Movement and Disaster-Causing Effects of Coal Mining Face under the Action of Hard Thick Magmatic Rock. Processes 2018, 6, 150. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, B. Study on the development laws of bed-separation under the hard-thick magmatic rock and its fracture disaster-causing mechanism. Geotech. Geol. Eng. 2018, 36, 1525–1543. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, Y.; Wang, H. Coupled disaster-causing mechanisms of strata pressure behavior and abnormal gas emissions in underground coal extraction. Environ. Earth Sci. 2015, 74, 6717–6735. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, Q.; Wu, Q.; Wang, P.; Zhang, C.; Gong, B. Study on distribution characteristics of mining stress and elastic energy under hard and thick igneous rocks. Geotech. Geol. Eng. 2018, 36, 3451–3466. [Google Scholar] [CrossRef]
- Jiang, L.; Sainoki, A.; Mitri, H. Influence of fracture-induced weakening on coal mine gateroad stability. Int. J. Rock Mech. Min. Sci. 2016, 88, 307–317. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, J.; Zhang, P. Breaking process and mining stress evolution characteristics of a high-position hard and thick stratum. Int. J. Min. Sci. Technol. 2016, 26, 563–569. [Google Scholar] [CrossRef]
- Wang, F.; Chen, S.; Gao, P.; Guo, Z.; Tao, Z. Research on Deformation Mechanisms of a High Geostress Soft Rock Roadway and Double-Shell Grouting Technology. Geofluids 2021, 6215959. [Google Scholar] [CrossRef]
- He, M.C.; Wang, Q.; Wu, Q.Y. Innovation and future of mining rock mechanics. J. Rock Mech. Geotech. Eng. 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Shu, X.; Zhao, Y.; Liu, Z.; Zhao, C. A study on the mix proportion of fiber-polymer composite reinforced cement-based grouting material. Constr. Build. Mater. 2022, 328, 127025. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J. Experiment and Application Study on High-Performance Grouting Material Used to Solve the Floor Heave Problem of Broken Soft Rocks. Front. Phys. 2022, 10, 829681. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, P.; Yan, W.; Wu, J.; Yan, F. Grouting Material Development and Dynamic GroutingTest of Broken Rock Mass. J. Mater. Civ. Eng. 2022, 34, 04022072-1. [Google Scholar] [CrossRef]
- Cui, Y.; Tan, Z.; Han, D.; Song, J. Investigation and application of a high performance grouting material in water-rich silty fine sand stratum. Constr. Build. Mater. 2022, 329, 127100. [Google Scholar] [CrossRef]
- Yun-ding, Z.; Kun, C. Research on Chemical Grouting Advance Support Technology of Silty Sand Tunnel with Low Moisture Content. Constr. Des. Proj. 2020, 145, 120–121. [Google Scholar]
- Song, W.; Zhu, Z.; Pu, S.; Wan, Y.; Huo, W.; Song, S.; Zhang, J.; Yao, K.; Hu, L. Synthesis and characterization of eco-friendly alkali-activated industrial solid waste-based two-component backfilling grouts for shield tunnelling. J. Clean. Prod. 2020, 266, 21974. [Google Scholar] [CrossRef]
- Liu, L.; Xie, M.; He, Y.; Li, Y.; Wei, A.; Cui, X.; Shi, C. Expansion behavior and microstructure change of alkali-activated slag grouting material in carbonate environment. Constr. Build. Mater. 2020, 262, 120593. [Google Scholar] [CrossRef]
- Zhang, S.; He, Y.; Zhang, H.; Chen, J.; Liu, L. Effect of fine sand powder on the rheological properties of one-part alkali-activated slag semi-flexible pavement grouting materials. Constr. Build. Mater. 2022, 333, 127328. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, Z.; Zhang, K.; Tang, X.; Luo, Y. Preparation and characterization of the greener alkali-activated grouting materials based on multi-index optimization. Constr. Build. Mater. 2021, 269, 121328. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, C.; Zhou, X.; Gao, G.; Feng, X. Full-scale physical modelling of fissure grouting in deep underground rocks. Tunn. Undergr. Space Technol. 2019, 89, 249–261. [Google Scholar] [CrossRef]
- Li, S.; Liu, R.; Zhang, Q.; Zhang, X. Protection against water or mud inrush in tunnels by grouting: A review. Int. J. Rock Mech. Geotechn. Eng. 2016, 8, 753–766. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Hou, Z.; Dong, Z. Improving strength of calcinated coal gangue geopolymer mortars via increasing calcium content. Constr. Build. Mater. 2018, 166, 760–768. [Google Scholar] [CrossRef]
- Shi, C.; Fernández-Jiménez, A.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- GB175-2020; Common Portland Cement. AQSIQ: Beijing, China, 2020.
- GB/T 18046-2017; Granulated Blast Furnace Slag Powder Used for Cement, Mortar and Concrete. AQSIQ: Beijing, China, 2017.
- GB/T 17671-2021; Method of Testing Cements—Determination of Strength. AQSIQ: Beijing, China, 2021.
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. AQSIQ: Beijing, China, 2011.
- Ren, J.; Zhao, Z.; Xu, Y.; Wang, S.; Chen, H.; Huang, J.; Xue, B.; Wang, J.; Chen, J.; Yang, C. High-Fluidization, Early Strength Cement Grouting Material Enhanced by Nano-SiO2: Formula and Mechanisms. Materials 2021, 14, 6144. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Kwon, H.M. Influence of Activator Na2O Concentration on Residual Strengths of Alkali-Activated Slag Mortar upon Exposure to Elevated Temperatures. Materials 2018, 11, 1296. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Li, D.; Cui, Y.; Feng, J.; Gao, Q.; Lu, T.; Zhang, Y.; Zhu, J. Compressive Strength Enhancement in Early Age Acid Activated Mortars: Mechanical Properties and Analysis. Crystals 2022, 12, 804. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Zhang, l.; Hou, Z.; Zhang, L.; Wu, S. Influence of calcium content on structure and strength of MSWI bottom ash-based geopolymer. Mag. Concr. Res. 2019, 71, 362–372. [Google Scholar] [CrossRef]
- Puertas, F.; González-Fonteboa, B.; González-Taboada, I.; Alonso, M.M.; Torres-Carrasco, M.; Rojo, G.; Martínez-Abella, F. Alkali-activated slag concrete: Fresh and hardened behavior. Cem. Concr. Compos. 2018, 85, 22–31. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, C.; Zhang, J.; Wan, S.; Zhang, Z.; Ou, Z. Alkali-silica reaction in waterglass-activated slag mortars incorporating fly ash and metakaolin. Cem. Concr. Res. 2018, 108, 10–19. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Zhang, L.; Li, J.; Hou, Z. The influence of curing methods on the strength of MSWI bottom ash-based alkali-activated mortars: The role of leaching of OH- and free alkali. Constr. Build. Mater. 2018, 186, 978–985. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Zhang, L.; Liu, X.; Liu, B. Effect of activated silica on polymerization mechanism and strength development of MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2019, 201, 90–99. [Google Scholar] [CrossRef]
- Bergold, S.; Goetz-Neunhoeffer, F.; Neubauer, J. Interaction of silicate and aluminate reaction in a synthetic cement system: Implications for the process of alite hydration. Cem. Concr. Res. 2017, 93, 32–44. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Hunpratub, S.; Thongbai, P.; Maensiri, S.; Sata, V.; Chindaprasirt, P. Effects of NaOH concentrations on physical and electrical properties of high calcium fly ash geopolymer paste. Cem. Concr. Compos. 2014, 45, 9–14. [Google Scholar] [CrossRef]
- Huang, G.; Yuan, L.; Ji, Y.; Liu, B.; Xu, Z. Cooperative action and compatibility between Portland cement and MSWI bottom ash alkali-activated double gel system materials. Constr. Build. Mater. 2019, 209, 445–453. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Sun, Y.; Lu, Z.; Zhang, X.; Zuo, L.; Feng, Y.; Qian, R.; Qi, Y.; Ji, Y.; et al. Influence of NaOH content on the alkali conversion mechanism in MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 248, 118582. [Google Scholar] [CrossRef]
- Huang, G.; Li, Y.; Zhang, Y.; Zhu, J.; Li, D.; Wang, B. Effect of Sodium Hydroxide, Liquid Sodium Silicate, Calcium Hydroxide, and Slag on the Mechanical Properties and Mineral Crystal Structure Evolution of Polymer Materials. Crystals 2021, 11, 1586. [Google Scholar] [CrossRef]
- Nedunuri, A.S.S.S.; Muhammad, S. Fundamental understanding of the setting behaviour of the alkali activated binders based on ground granulated blast furnace slag and fly ash. Constr. Build. Mater. 2021, 291, 123243. [Google Scholar] [CrossRef]
- Oderji, S.Y.; Chen, B.; Ahmad, M.R.; Shah, S.F.A. Fresh and hardened properties of one-part fly ash-based geopolymer binders cured at room temperature: Effect of slag and alkali activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Han, L.; Liu, Z.; Wang, D. Setting controlling of lithium slag-based geopolymer by activator and sodium tetraborate as a retarder and its effects on mortar properties. Cem. Concr. Compos. 2020, 110, 103598. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Chen, L.; Lu, Z.; Sun, Y.; Zhang, X.; Feng, Y.; Ji, Y.; Xu, Z. Use of pretreatment to prevent expansion and foaming in highperformance MSWI bottom ash alkali-activated mortars. Constr. Build. Mater. 2020, 245, 118471. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Lo, M. The fresh and engineering properties of alkali activated slag as a function of fly ash replacement and alkali concentration. Constr. Build. Mater. 2015, 84, 224–229. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- Walkley, B.; Nicolas, R.S.; Sani, M.; Bernal, S.A.; van Deventer, J.S.; Provis, J.L. Provise, Structural evolution of synthetic alkali-activated CaO-MgO-Na2O-Al2O3-SiO2 materials is influenced by Mg content. Cem. Concr. Res. 2017, 99, 155–171. [Google Scholar] [CrossRef]
- He, R.; Ye, H.; Ma, H.; Fu, C.; Jin, X.; Li, Z. Correlating the Chloride Diffusion Coefficient and Pore Structure of Cement-Based Materials Using Modified Noncontact Electrical Resistivity Measurement. J. Mater. Civ. Eng. 2019, 31, 04019006. [Google Scholar] [CrossRef]
- Wu, Q.; Li, X.; Wang, J.X.; Shi, W.; Wang, S. Size Distribution Model and Development Characteristics of Corrosion Pits in Concrete under Two Curing Methods. Materials 2019, 20, 1846. [Google Scholar] [CrossRef] [PubMed]
- Maraghechi, H.; Rajabipour, F.; Pantano, C.G.; Burgos, W.D. Effect of calciumon dissolution and precipitation reactions of amorphous silica at high alkalinity. Cem. Concr. Res. 2016, 87, 1–13. [Google Scholar] [CrossRef]
- Davoodabadi, M.; Liebscher, M.; Hampel, S. Multi-walled carbon nanotube dispersion methodologies in alkaline media and their influence on mechanical reinforcement of alkali-activated nanocomposites. Compos. Part B Eng. 2021, 209, 108559. [Google Scholar] [CrossRef]
- Xu, Z.; Yue, J.; Pang, G.; Li, R.; Zhang, P.; Xu, S. Influence of the Activator Concentration and Solid/Liquid Ratio on the Strength and Shrinkage Characteristics of Alkali-Activated Slag Geopolymer Pastes. Adv. Civ. Eng. 2021, 2021, 6631316. [Google Scholar] [CrossRef]




| Raw Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | Others | Loss |
|---|---|---|---|---|---|---|---|---|---|---|
| PC | 21.15 | 4.79 | 2.12 | 61.82 | 2.55 | 0.67 | 0.24 | 2.35 | 1.52 | 2.21 |
| GBFS | 32.42 | 20.85 | 0.69 | 33.79 | 6.36 | 1.32 | 0.77 | - | 2.31 | 0.73 |
| PC | GBFS | LSS | Sodium Hydroxide | Water | Liquid–Solid Ratio | |
|---|---|---|---|---|---|---|
| L-1 | 1000 | 0 | 0 | 0 | 600 | 0.6 |
| L-2 | 800 | 200 | 0 | 0 | 600 | 0.6 |
| L-3 | 600 | 400 | 0 | 0 | 600 | 0.6 |
| L-4 | 600 | 400 | 100 | 0 | 535 | 0.6 |
| L-5 | 600 | 400 | 200 | 0 | 470 | 0.6 |
| L-6 | 600 | 400 | 300 | 0 | 405 | 0.6 |
| L-7 | 0 | 1000 | 0 | 20 | 600 | 0.6 |
| L-8 | 0 | 1000 | 0 | 40 | 600 | 0.6 |
| L-9 | 0 | 1000 | 0 | 60 | 600 | 0.6 |
| L-10 | 0 | 1000 | 100 | 40 | 535 | 0.6 |
| L-11 | 0 | 1000 | 200 | 40 | 470 | 0.6 |
| L-12 | 0 | 1000 | 300 | 40 | 405 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Huang, G.; Cui, Y.; Wang, B.; Chang, B.; Yin, Q.; Zhang, S.; Wang, Q.; Feng, J.; Ge, M. Coagulation Mechanism and Compressive Strength Characteristics Analysis of High-Strength Alkali-Activated Slag Grouting Material. Polymers 2022, 14, 3980. https://doi.org/10.3390/polym14193980
Li M, Huang G, Cui Y, Wang B, Chang B, Yin Q, Zhang S, Wang Q, Feng J, Ge M. Coagulation Mechanism and Compressive Strength Characteristics Analysis of High-Strength Alkali-Activated Slag Grouting Material. Polymers. 2022; 14(19):3980. https://doi.org/10.3390/polym14193980
Chicago/Turabian StyleLi, Mingjing, Guodong Huang, Yi Cui, Bo Wang, Binbin Chang, Qiaoqiao Yin, Shuwei Zhang, Qi Wang, Jiacheng Feng, and Ming Ge. 2022. "Coagulation Mechanism and Compressive Strength Characteristics Analysis of High-Strength Alkali-Activated Slag Grouting Material" Polymers 14, no. 19: 3980. https://doi.org/10.3390/polym14193980
APA StyleLi, M., Huang, G., Cui, Y., Wang, B., Chang, B., Yin, Q., Zhang, S., Wang, Q., Feng, J., & Ge, M. (2022). Coagulation Mechanism and Compressive Strength Characteristics Analysis of High-Strength Alkali-Activated Slag Grouting Material. Polymers, 14(19), 3980. https://doi.org/10.3390/polym14193980








