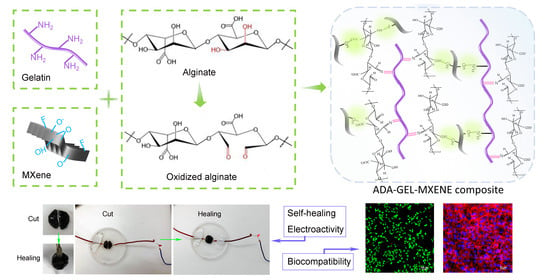
Electroactive Oxidized Alginate/Gelatin/MXene (Ti3C2Tx) Composite Hydrogel with Improved Biocompatibility and Self-Healing Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
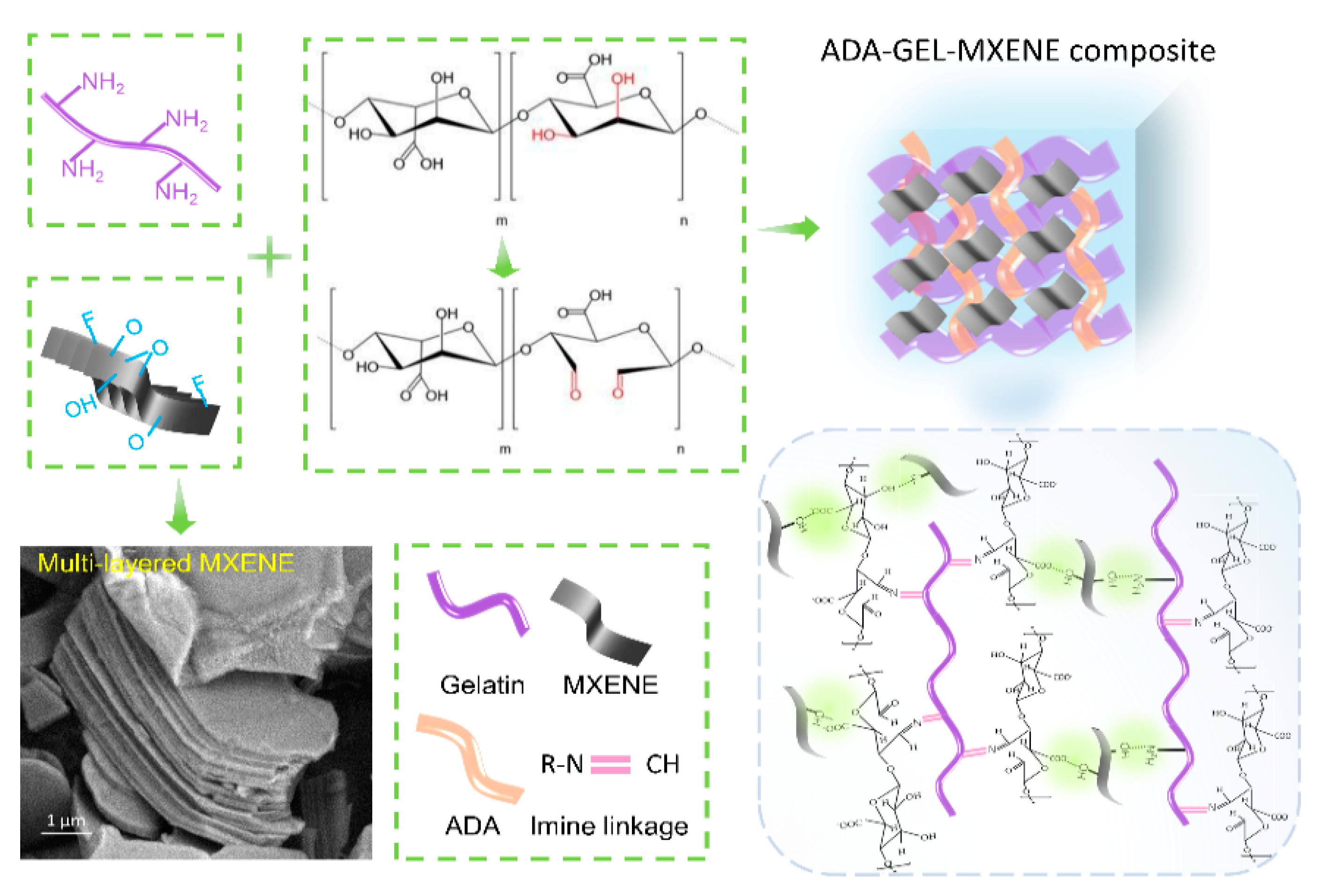
2.2. Preparation of Alginate-Di-Aldehyde (ADA)
2.3. Preparation of ADA-GEL (AG) Hydrogel
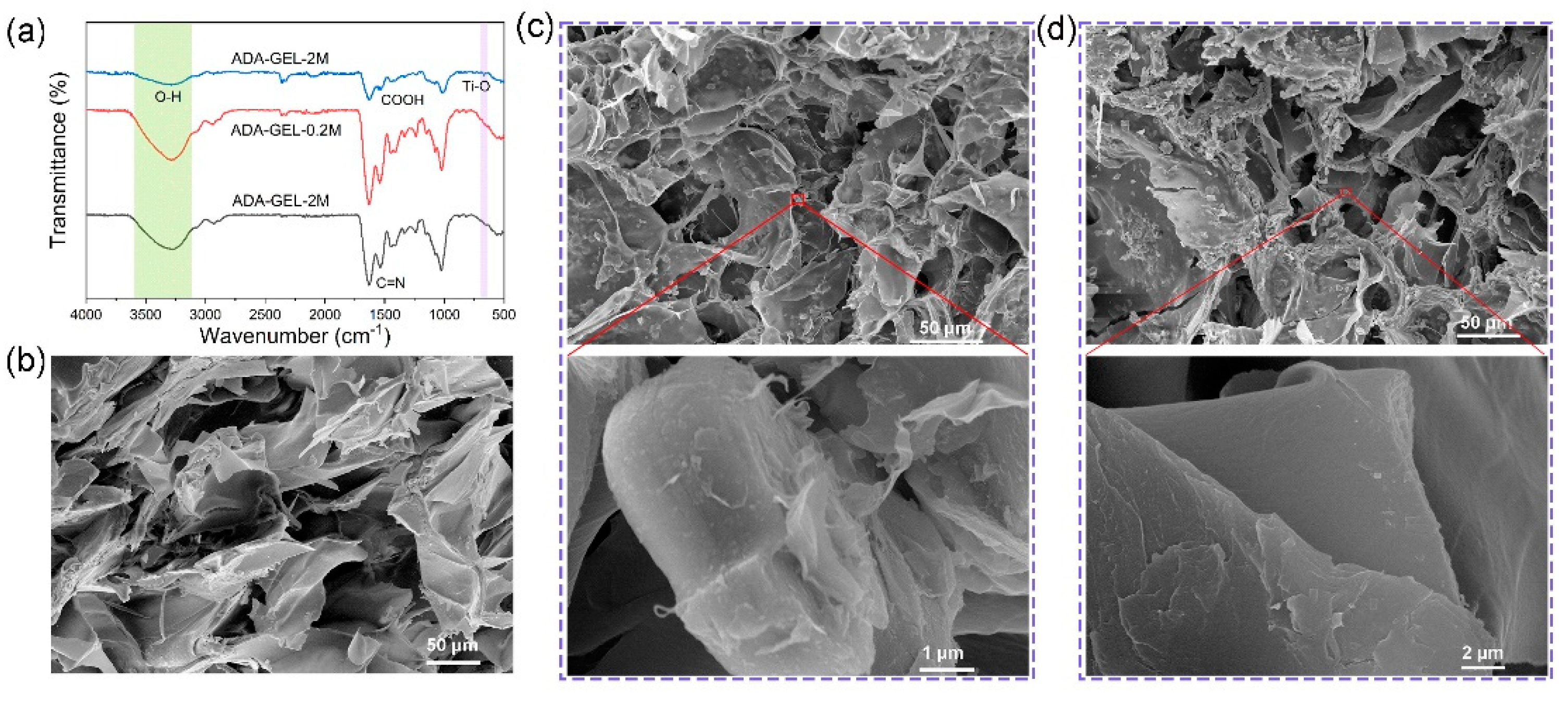
2.4. Characterization of Hydrogel
2.5. Cell Study
2.6. Data Analysis
3. Results and Discussion
3.1. Preparation and Micro-Structures of MXene-ADA-GEL Hydrogels
3.2. Characterizations of the Rheology, Self-Healing Property, Extrusion Capacity and Electroactivity of MXene-ADA-GEL Hydrogels
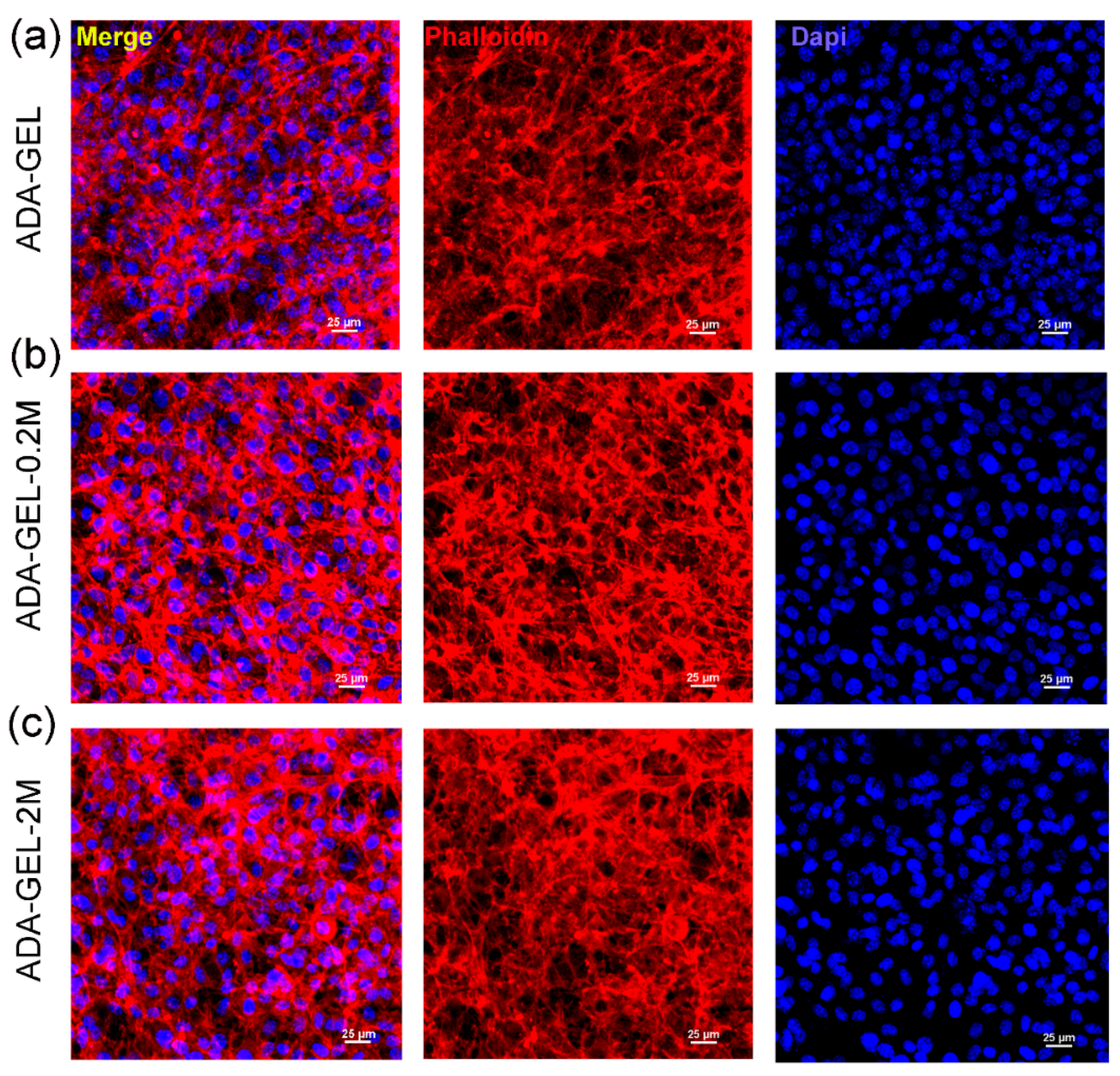
3.3. Cell Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Xiang, J.; Wen, X.; Du, X.; Wang, Y.; Du, Z.; Cheng, X.; Wang, S. Multifunctional skin-inspired resilient MXene-embedded nanocomposite hydrogels for wireless wearable electronics. Compos. Part A Appl. Sci. Manuf. 2022, 155, 106835. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, J.; Yang, C.-W.; Wan, Y.; Tang, H.-L.; Aljarb, A.A.; Chen, C.; Fu, J.-H.; Wei, X.; Huang, K.-W. Mixed-dimensional MXene-hydrogel heterostructures for electronic skin sensors with ultrabroad working range. Sci. Adv. 2020, 6, eabb5367. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Alsehli, M.H.; Omer, A.M.; Afifi, T.H.; Sabet, M.M.; Mohy-Eldin, M.S.; Hassan, M.A. Development of Polyvinyl Alcohol/Kaolin Sponges Stimulated by Marjoram as Hemostatic, Antibacterial, and Antioxidant Dressings for Wound Healing Promotion. Int. J. Mol. Sci. 2021, 22, 13050. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Guo, J.; Sun, L.; Zhang, X.; Zhao, Y. Microfluidic generation of microsprings with ionic liquid encapsulation for flexible electronics. Research 2019, 2019, 6906275. [Google Scholar] [CrossRef]
- Krisnadi, F.; Nguyen, L.L.; Ma, J.; Kulkarni, M.R.; Mathews, N.; Dickey, M.D. Directed Assembly of Liquid Metal-Elastomer Conductors for Stretchable and Self-Healing Electronics. Adv. Mater. 2020, 32, 2001642. [Google Scholar] [CrossRef]
- Ahmed, A.; Guan, Y.-S.; Hassan, I.; Ling, C.; Li, Z.; Mosa, I.; Phadke, G.; Selvaganapathy, P.R.; Chang, S.; Ren, S. Multifunctional smart electronic skin fabricated from two-dimensional like polymer film. Nano Energy 2020, 75, 105044. [Google Scholar] [CrossRef]
- Lu, Y.; Qu, X.; Zhao, W.; Ren, Y.; Si, W.; Wang, W.; Wang, Q.; Huang, W.; Dong, X. Highly stretchable, elastic, and sensitive MXene-based hydrogel for flexible strain and pressure sensors. Research 2020, 2020, 2038560. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, H.; Jin, X.; Liu, H.; Ma, A.; Yan, H.; Chen, L.; Chen, W. Highly compliant and low strain hysteresis sensory electronic skins based on solution processable hybrid hydrogels. J. Mater. Chem. C 2021, 9, 1822–1828. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Hedenqvist, M.S.; Chen, C.; Cai, C.; Li, H.; Liu, H.; Fu, J. Multifunctional conductive hydrogels and their applications as smart wearable devices. J. Mater. Chem. B 2021, 9, 2561–2583. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Vialli, N.; Fuh, J.Y.; Lu, W.F. Conductive collagen/polypyrrole-b-polycaprolactone hydrogel for bioprinting of neural tissue constructs. Int. J. Bioprint. 2019, 5, 229. [Google Scholar] [CrossRef]
- Dong, R.; Zhao, X.; Guo, B.; Ma, P.X. Self-healing conductive injectable hydrogels with antibacterial activity as cell delivery carrier for cardiac cell therapy. ACS Appl. Mater. Interfaces 2016, 8, 17138–17150. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Polley, C.; Shi, F.; Schneidereit, D.; Ashton, M.D.; Friedrich, O.; Kolb, J.F.; Hardy, J.G.; Detsch, R.; Seitz, H. Electrically Conductive and 3D-Printable Oxidized Alginate-Gelatin Polypyrrole: PSS Hydrogels for Tissue Engineering. Adv. Healthc. Mater. 2021, 10, 2001876. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, M.; Ma, C.; Wang, Y.; Li, X.; Yu, G. A conductive self-healing hybrid gel enabled by metal-ligand supramolecule and nanostructured conductive polymer. Nano Lett. 2015, 15, 6276–6281. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, L.-Y.; Jing, L.; Li, K.; Yang, H.; Li, Y.; Chen, P.-Y. Carbon nanotube-integrated conductive hydrogels as multifunctional robotic skin. Carbon 2020, 161, 784–793. [Google Scholar] [CrossRef]
- Li, M.; Tu, Q.; Long, X.; Zhang, Q.; Jiang, H.; Chen, C.; Wang, S.; Min, D. Flexible conductive hydrogel fabricated with polyvinyl alcohol, carboxymethyl chitosan, cellulose nanofibrils, and lignin-based carbon applied as strain and pressure sensor. Int. J. Biol. Macromol. 2021, 166, 1526–1534. [Google Scholar] [CrossRef]
- Zhang, B.; He, J.; Shi, M.; Liang, Y.; Guo, B. Injectable self-healing supramolecular hydrogels with conductivity and photo-thermal antibacterial activity to enhance complete skin regeneration. Chem. Eng. J. 2020, 400, 125994. [Google Scholar] [CrossRef]
- He, P.; He, J.; Huo, Z.; Li, D. Microfluidics-based fabrication of flexible ionic hydrogel batteries inspired by electric eels. Energy Storage Mater. 2022, 49, 348–359. [Google Scholar] [CrossRef]
- Dechiraju, H.; Jia, M.; Luo, L.; Rolandi, M. Ion-Conducting Hydrogels and Their Applications in Bioelectronics. Adv. Sustain. Syst. 2022, 6, 2100173. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2T x MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Li, Y.; Lan, J.; Yan, B.; Shi, L.; Ran, R. High-Strength, Self-Healable, Temperature-Sensitive, MXene-Containing Composite Hydrogel as a Smart Compression Sensor. ACS Appl. Mater. Interfaces 2019, 11, 47350–47357. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, J.; Song, J.; Gao, C.; Wang, Z.; Song, C.; Wu, Y.; Liu, Y. Self-Healing Ti3C2 MXene/PDMS Supramolecular Elastomers Based on Small Biomolecules Modification for Wearable Sensors. ACS Appl. Mater. Interfaces 2020, 12, 45306–45314. [Google Scholar] [CrossRef] [PubMed]
- Bednarzig, V.; Karakaya, E.; Egaña, A.L.; Teßmar, J.; Boccaccini, A.R.; Detsch, R. Advanced ADA-GEL bioink for bioprinted artificial cancer models. Bioprinting 2021, 23, e00145. [Google Scholar] [CrossRef]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Sarker, B.; Singh, R.; Silva, R.; Roether, J.A.; Kaschta, J.; Detsch, R.; Schubert, D.W.; Cicha, I.; Boccaccini, A.R. Evaluation of fibroblasts adhesion and proliferation on alginate-gelatin crosslinked hydrogel. PLoS ONE 2014, 9, e107952. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lei, X.; Huang, Z.; Rong, Z.; Li, H.; Xie, Y.; Duan, L.; Xiong, J.; Wang, D.; Zhu, S. Bioprinted Gelatin-Recombinant Type III Collagen Hydrogel Promotes Wound Healing. Int. J. Bioprint. 2022, 8, 517. [Google Scholar] [CrossRef]
- Heid, S.; Becker, K.; Byun, J.; Biermann, I.; Neščáková, Z.; Zhu, H.; Groll, J.; Boccaccini, A.R. Bioprinting with bioactive alginate dialdehyde-gelatin (ADA-GEL) composite bioinks: Time-dependent in-situ crosslinking via addition of calcium-silicate particles tunes in vitro stability of 3D bioprinted constructs. Bioprinting 2022, 26, e00200. [Google Scholar] [CrossRef]
- Zhu, H.; Monavari, M.; Zheng, K.; Distler, T.; Ouyang, L.; Heid, S.; Jin, Z.; He, J.; Li, D.; Boccaccini, A.R. 3D Bioprinting of Multifunctional Dynamic Nanocomposite Bioinks Incorporating Cu-Doped Mesoporous Bioactive Glass Nanoparticles for Bone Tissue Engineering. Small 2022, 18, 2104996. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, J.; Chen, M.; Wang, A.; Huang, A.; Xu, X.; Xu, J.; Wong, C.-P. An environmentally adaptive quasi-solid-state zinc-ion battery based on magnesium vanadate hydrate with commercial-level mass loading and anti-freezing gel electrolyte. J. Mater. Chem. A 2020, 8, 8397–8409. [Google Scholar] [CrossRef]
- Huang, J.; Huang, X.; Wu, P. One stone for three birds: One-step engineering highly elastic and conductive hydrogel electronics with multilayer MXene as initiator, crosslinker and conductive filler simultaneously. Chem. Eng. J. 2022, 428, 132515. [Google Scholar] [CrossRef]
- Zavahir, S.; Sobolčiak, P.; Krupa, I.; Han, D.S.; Tkac, J.; Kasak, P. Ti3C2Tx MXene-Based Light-Responsive Hydrogel Composite for Bendable Bilayer Photoactuator. Nanomaterials 2020, 10, 1419. [Google Scholar] [CrossRef]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive electrodes produced by oxidant-free polymerization of pyrrole between the layers of 2D titanium carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, M.; Ma, Z.; Xu, X.; Seraji, S.M.; Yu, B.; Sun, Z.; Wang, H.; Song, P. A reactive copper-organophosphate-MXene heterostructure enabled antibacterial, self-extinguishing and mechanically robust polymer nanocomposites. Chem. Eng. J. 2022, 430, 132712. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Liu, L.; Fu, L.; Yu, B.; Lv, Y.; Yang, F.; Song, P. Strengthening, toughing and thermally stable ultra-thin MXene nanosheets/polypropylene nanocomposites via nanoconfinement. Chem. Eng. J. 2019, 378, 122267. [Google Scholar] [CrossRef]
- Peng, W.; Han, L.; Huang, H.; Xuan, X.; Pan, G.; Wan, L.; Lu, T.; Xu, M.; Pan, L. A direction-aware and ultrafast self-healing dual network hydrogel for a flexible electronic skin strain sensor. J. Mater. Chem. A 2020, 8, 26109–26118. [Google Scholar] [CrossRef]
- Yang, B.; Yao, F.; Hao, T.; Fang, W.; Ye, L.; Zhang, Y.; Wang, Y.; Li, J.; Wang, C. Development of Electrically Conductive Double-Network Hydrogels via One-Step Facile Strategy for Cardiac Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 474–488. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Litowczenko, J.; Tadyszak, K.; Natu, V.; Aparicio, C.; Peplińska, B.; Barsoum, M.W.; Otyepka, M.; Scheibe, B. Unique cellular network formation guided by heterostructures based on reduced graphene oxide-Ti3C2Tx MXene hydrogels. Acta Biomater. 2020, 115, 104–115. [Google Scholar] [CrossRef]
- Sha, B.; Zhao, S.; Gu, M.; Zhao, G.; Wang, L.; Bi, G.-Q.; Du, Z. Multi-Functionalized Self-Bonding MXene for Minimal-invasive Jet-injected Neural Interface and Tissue Healing. bioRxiv 2021. [Google Scholar] [CrossRef]
- Basara, G.; Saeidi-Javash, M.; Ren, X.; Bahcecioglu, G.; Wyatt, B.C.; Anasori, B.; Zhang, Y.; Zorlutuna, P. Electrically conductive 3D printed Ti3C2Tx MXene-PEG composite constructs for cardiac tissue engineering. Acta Biomater. 2020, 139, 179–189. [Google Scholar] [CrossRef]
- Zhou, L.; Zheng, H.; Liu, Z.; Wang, S.; Liu, Z.; Chen, F.; Zhang, H.; Kong, J.; Zhou, F.; Zhang, Q. Conductive antibacterial hemostatic multifunctional scaffolds based on Ti3C2T x MXene nanosheets for promoting multidrug-resistant bacteria-infected wound healing. ACS Nano 2021, 15, 2468–2480. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Dai, W.; Wang, L.; Yao, C.; Wang, C.; Gu, B.; Li, D.; He, J. Electroactive Oxidized Alginate/Gelatin/MXene (Ti3C2Tx) Composite Hydrogel with Improved Biocompatibility and Self-Healing Property. Polymers 2022, 14, 3908. https://doi.org/10.3390/polym14183908
Zhu H, Dai W, Wang L, Yao C, Wang C, Gu B, Li D, He J. Electroactive Oxidized Alginate/Gelatin/MXene (Ti3C2Tx) Composite Hydrogel with Improved Biocompatibility and Self-Healing Property. Polymers. 2022; 14(18):3908. https://doi.org/10.3390/polym14183908
Chicago/Turabian StyleZhu, Hui, Weitao Dai, Liming Wang, Cong Yao, Chenxi Wang, Bingsong Gu, Dichen Li, and Jiankang He. 2022. "Electroactive Oxidized Alginate/Gelatin/MXene (Ti3C2Tx) Composite Hydrogel with Improved Biocompatibility and Self-Healing Property" Polymers 14, no. 18: 3908. https://doi.org/10.3390/polym14183908
APA StyleZhu, H., Dai, W., Wang, L., Yao, C., Wang, C., Gu, B., Li, D., & He, J. (2022). Electroactive Oxidized Alginate/Gelatin/MXene (Ti3C2Tx) Composite Hydrogel with Improved Biocompatibility and Self-Healing Property. Polymers, 14(18), 3908. https://doi.org/10.3390/polym14183908