Synthesis and Characterization of a Self-Polycondensation Diazaphthalanone Monomer and Its Polymers from Polycondensation Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
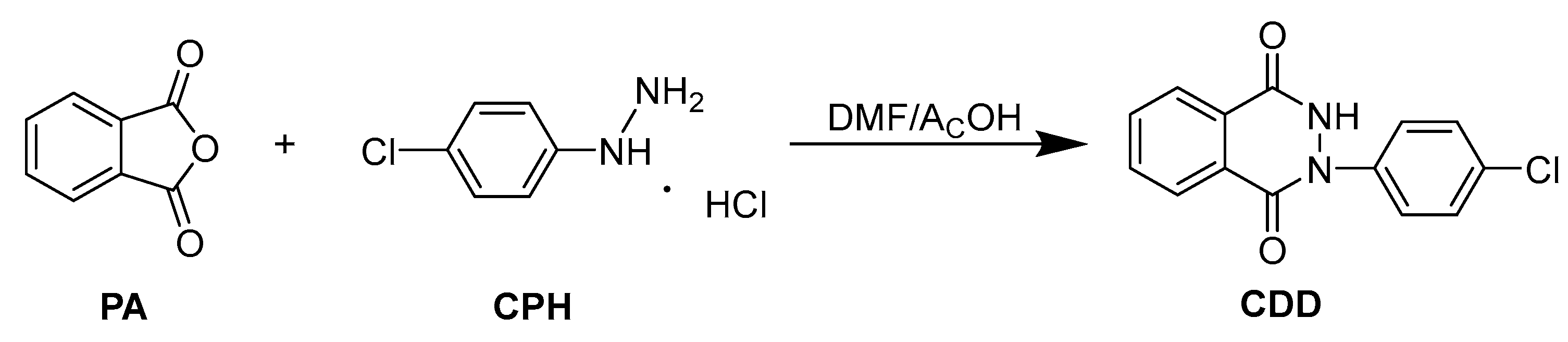
2.3. Monomer Synthesis
Synthesis of Novel Nitrogenous Heterocyclic Polyaromatic Ether Monomer CDD
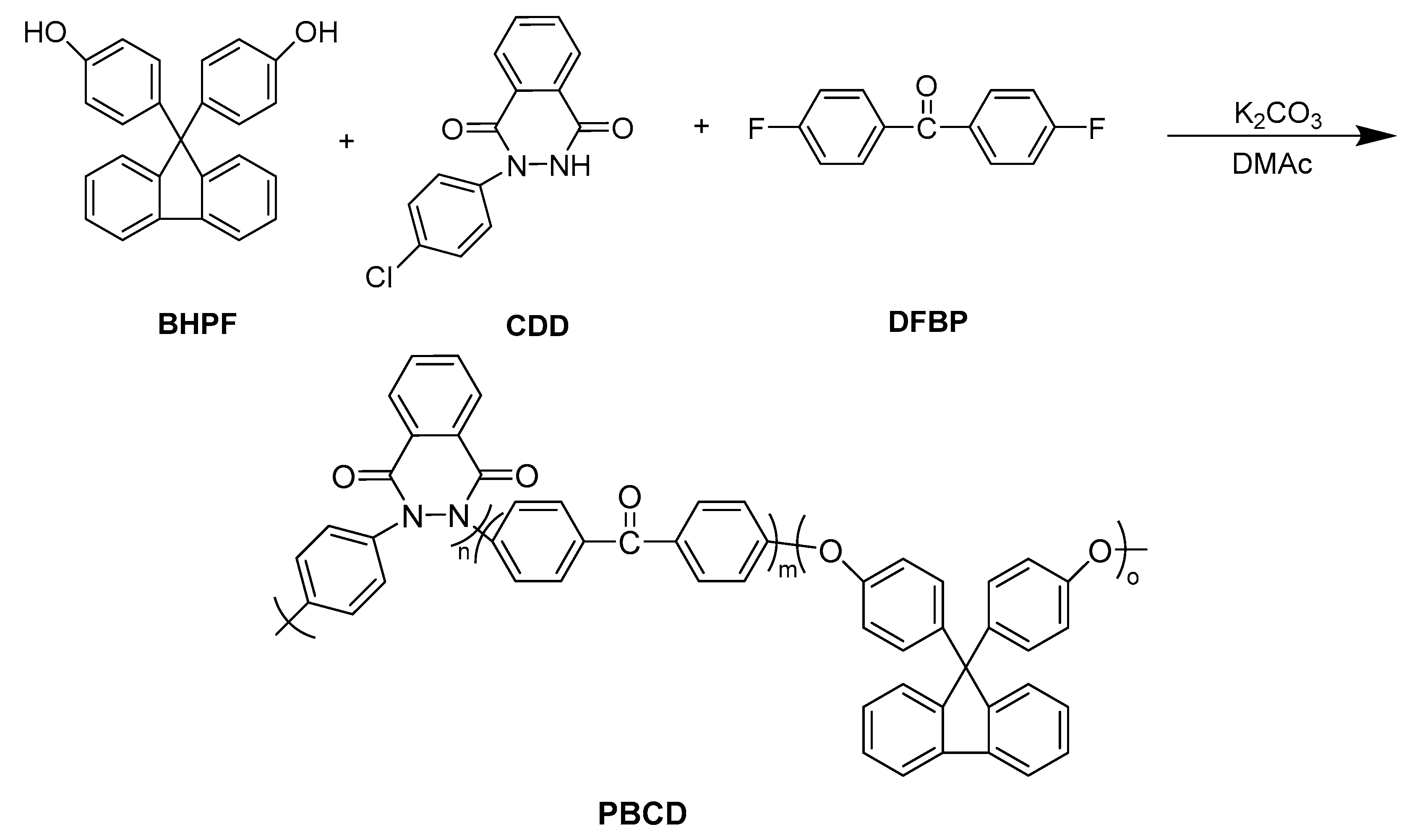
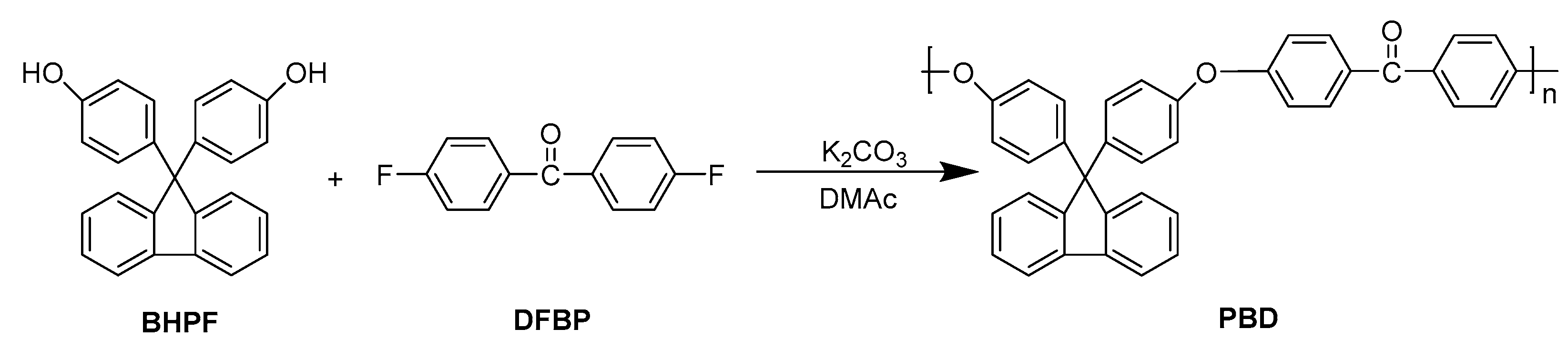
2.4. Polymer Synthesis
2.4.1. Self-Polycondensation of CDD-Synthesis of PCDD
2.4.2. Synthesis of Ternary Copolymerized Poly(aryl ether ketone)-Synthesis of PCBD
2.4.3. Synthesis of Binary Copolymerized Poly(aryl ether ketone)
3. Results and Discussion
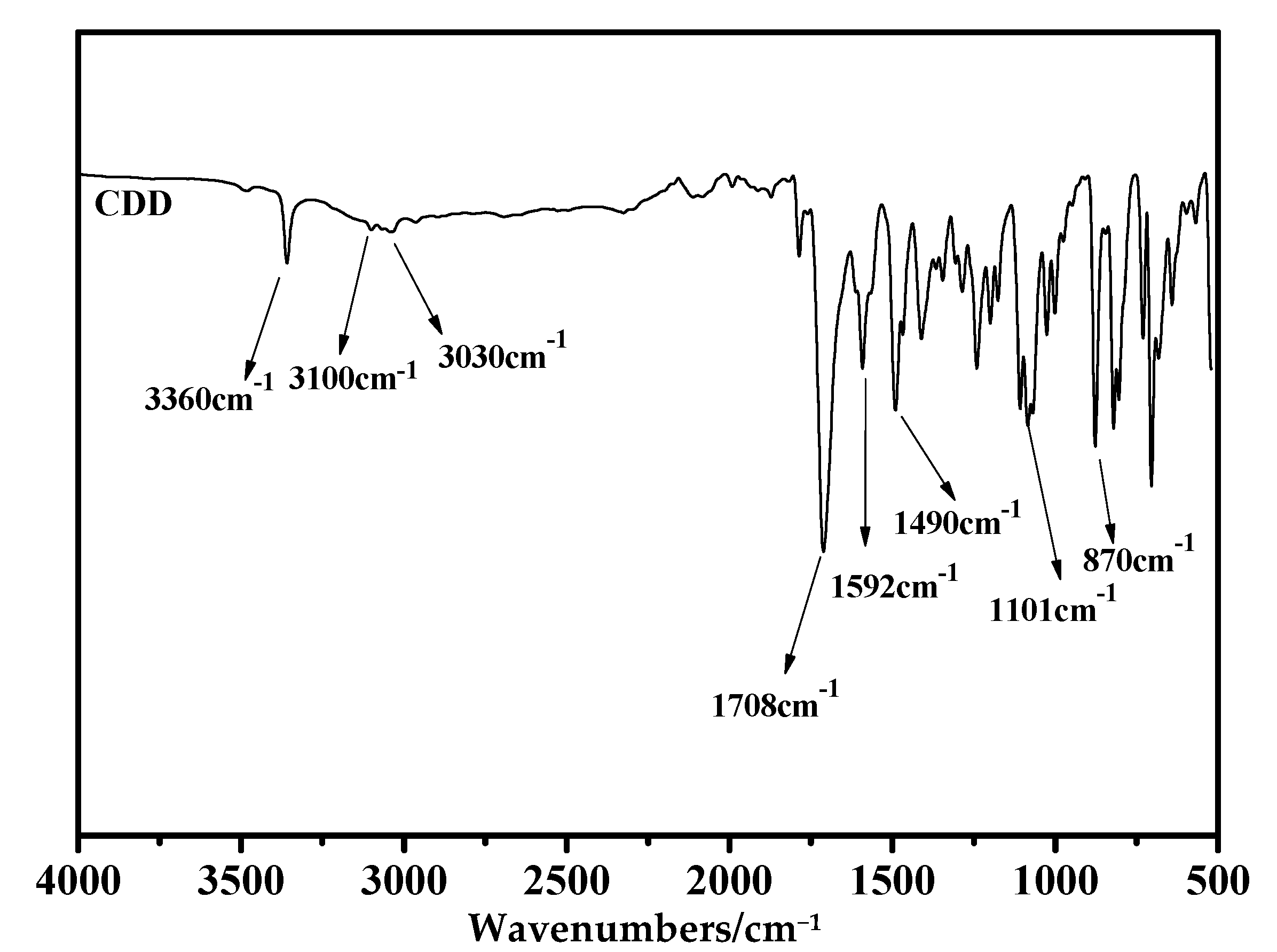
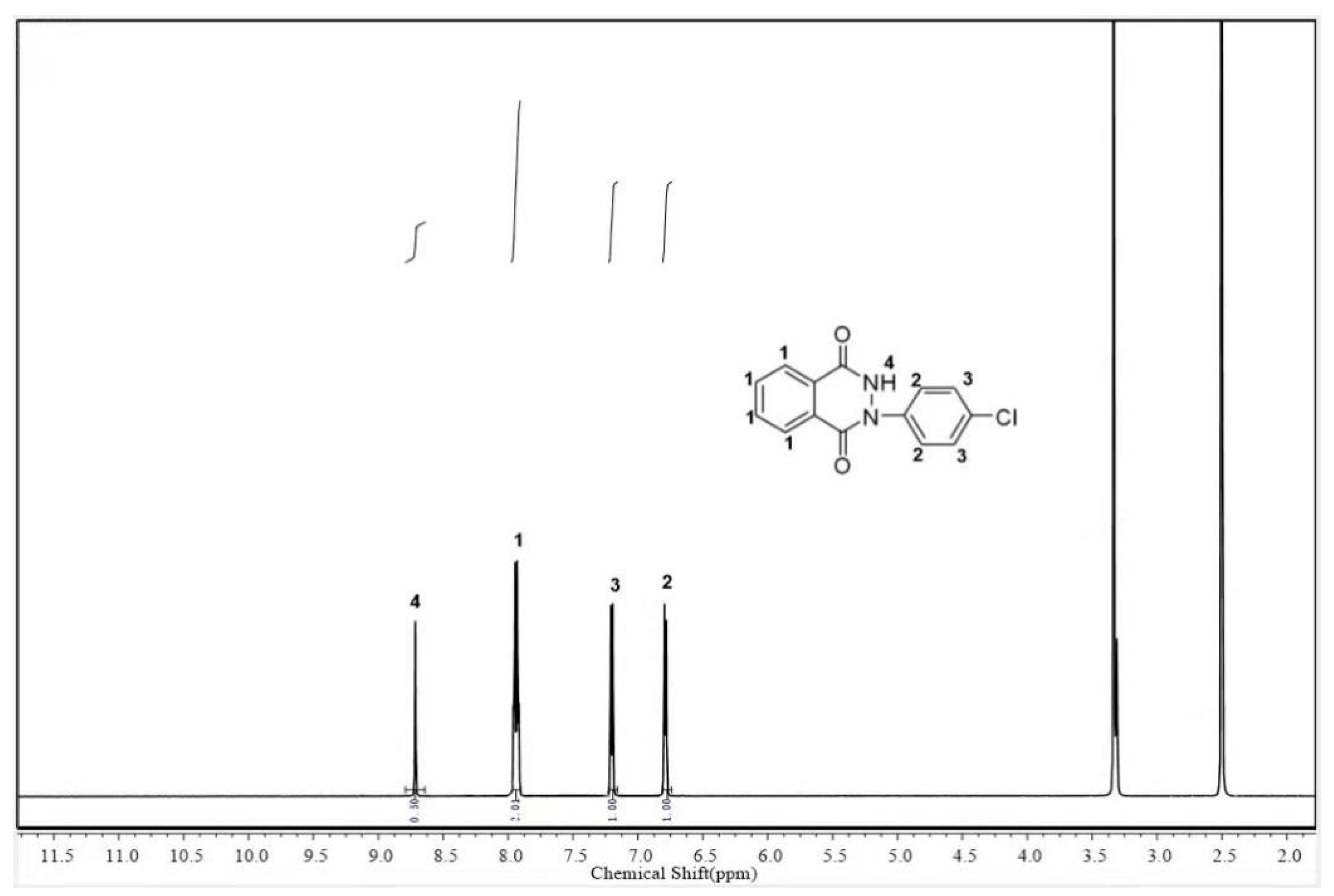
3.1. Structural Characterization of Novel Monomer CDD
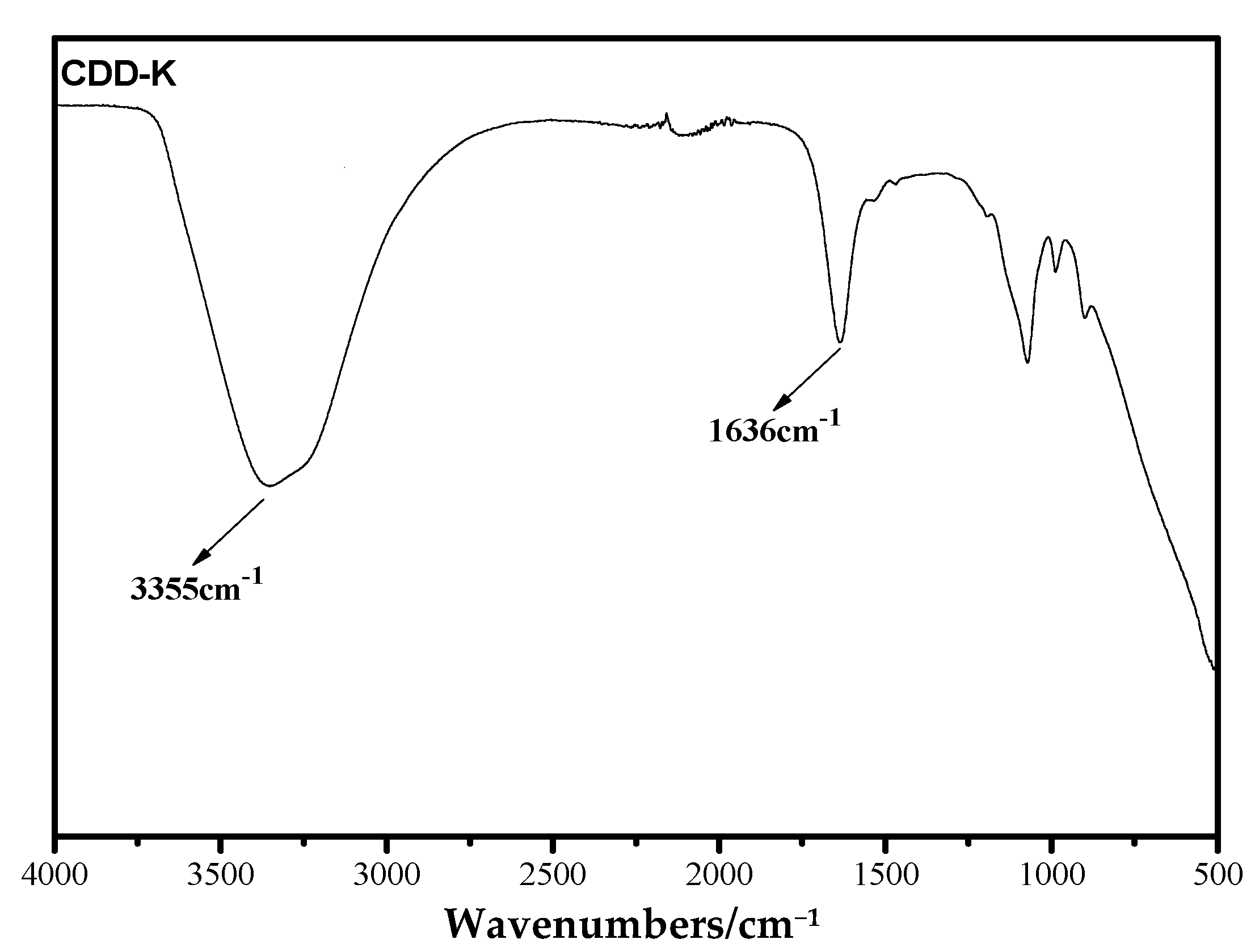
3.2. Study on Self-Condensation Polymerization of Novel Monomer CDD
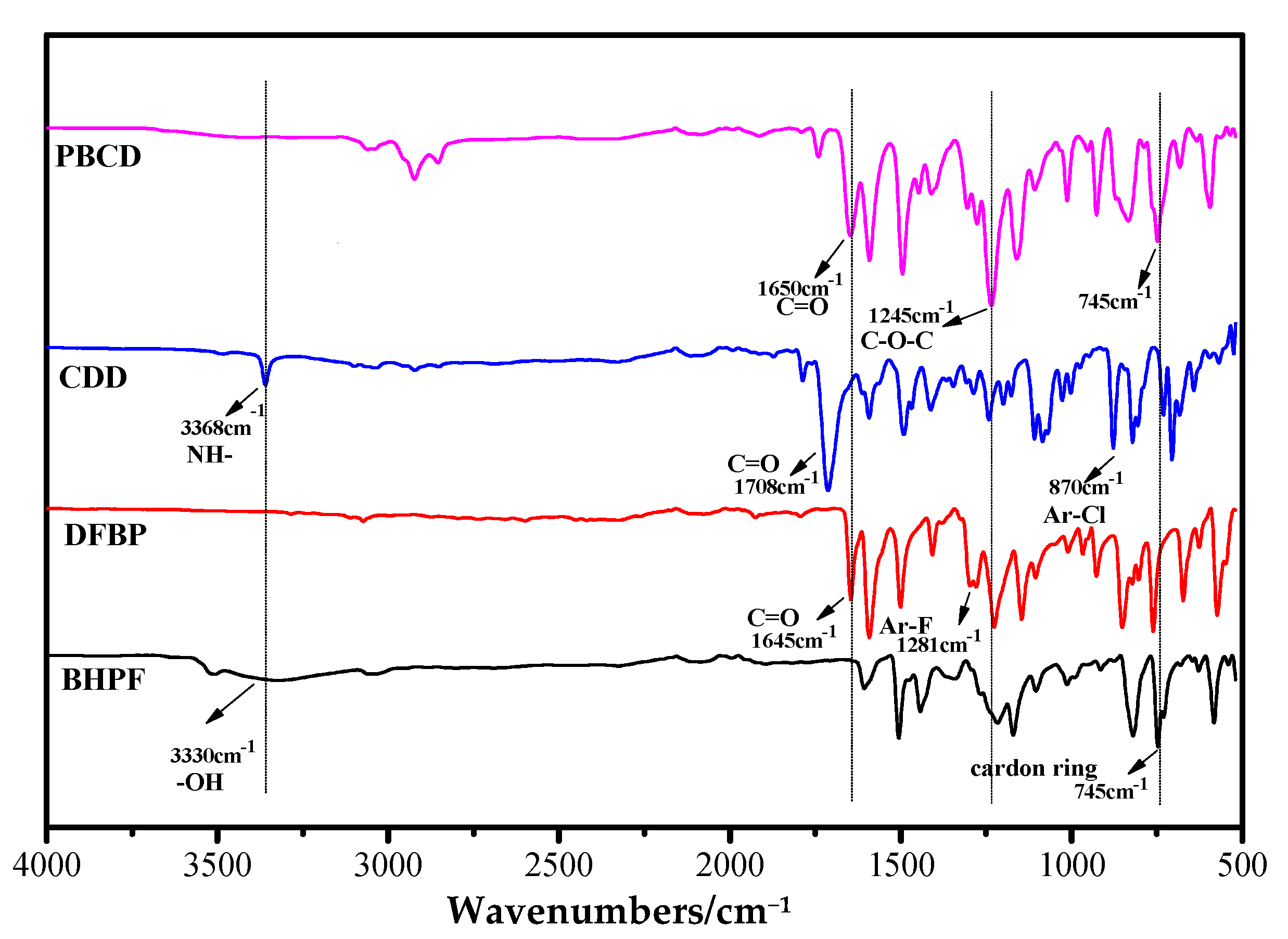
3.3. Study on Ternary Copolymerization of New Monomer CDD
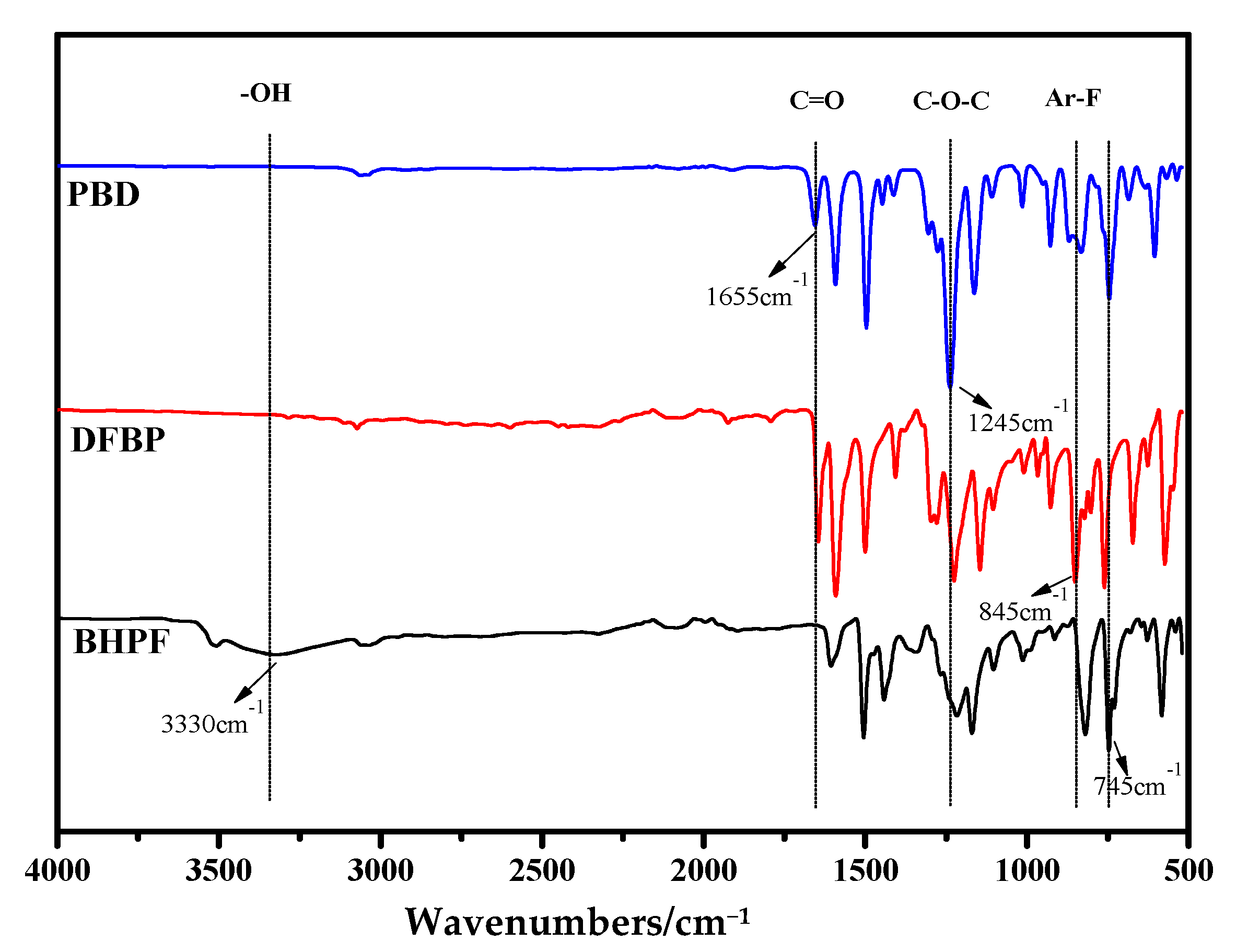
3.4. Study on Binary Copolymerization Polymerization
3.5. Analysis of Molecular Weight Test Results
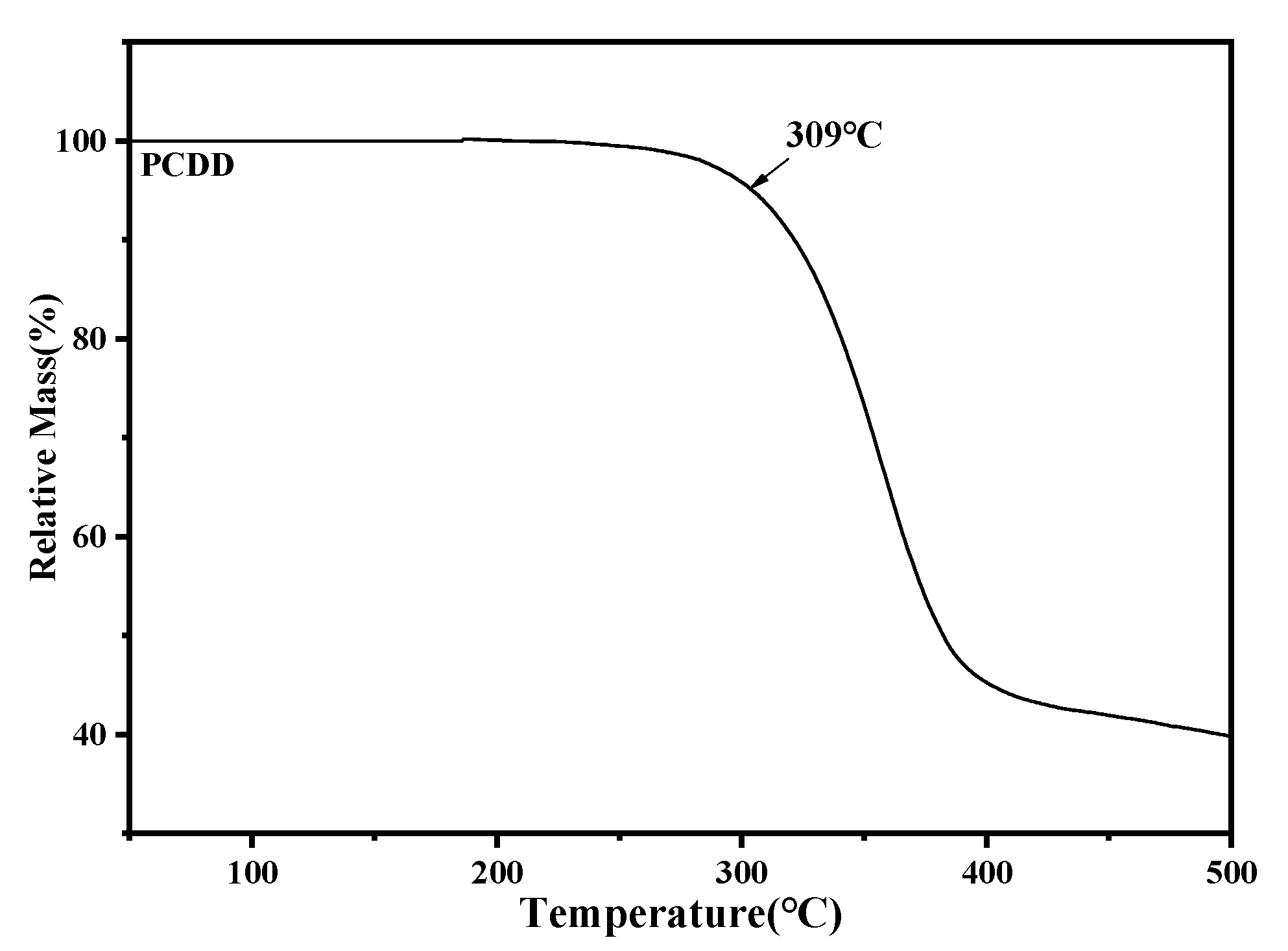
3.6. Study on Thermal Decomposition Temperature of Terpolymer
3.7. The Solubility of Terpolymer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PA | phthalic anhydride |
| CPH | 4-chlorophenylhydrazine hydrochloride |
| CDD | 2-(4-chlorophenyl)-2,3-dihydrophthalazine-1,4-dione |
| BHPF | 4,4′-(9H-fluorene-9,9-diyl) diphenol |
| DFBP | 4,4′-difluorobenzophenone |
| PCDD | homopolymer of 2-(4-chlorophenyl)-2,3-dihydrophthalazine-1,4-dione |
| PBCD | bisphenol fluorene-4,4-difluorobenzophenone-2-(4-chlorophenyl)-2,3-dihydrophthalazine-1,4-dione terpolymer (1:1:1) |
| PBD | bisphenol fluorene-4,4-difluorobenzophenone copolymer (1:1) |
References
- Sun, G.; Dong, G.; Kong, L.; Yan, X.; Tian, G.; Qi, S.; Wu, D. Robust polyimide nanofibrous membrane with porous-layer-coated morphology by in situ self-bonding and micro-crosslinking for lithium-ion battery separator. Nanoscale 2018, 10, 22439–22447. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Velasco, J.I. Multifunctional polymer foams with carbon nanoparticles. Prog. Polym. Sci. 2014, 39, 486–509. [Google Scholar] [CrossRef]
- Ni, H.J.; Liu, J.G.; Wang, Z.H.; Yang, S.Y. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y.; Ishida, H. Synthesis, characterization, and properties of new thermally curable polyetheresters containing benzoxazine moieties in the main chain. J. Polym. Sci. Part A Polym. Chem. 2010, 46, 414–420. [Google Scholar] [CrossRef]
- Tan, B.; Woods, H.M.; Licence, P.; Howdle, S.M.; Cooper, A.I. Synthesis and CO2 Solubility Studies of Poly(ether carbonate)s and Poly(ether ester)s Produced by Step Growth Polymerization. Macromolecules 2005, 38, 1691–1698. [Google Scholar] [CrossRef]
- Liu, Y.J.; Jian, X.G.; Liu, S.J. Synthesis and properties of novel poly(phthalazinone ether ketone ketone). J. Appl. Polym. Sci. 2001, 82, 823–826. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Liu, C.; Zhou, H.; Jian, X. Synthesis of poly (arylene ether nitrile ketone) s bearing phthalazinone moiety and their properties. Polym. Bull. 2013, 70, 1467–1481. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Wang, H.; Zhou, G. Carbazole-based poly(aryl ether ketone) containing crosslinkable allyl groups on the side chains: Synthesis, characterization and properties. Polym. Int. 2015, 64, 858–866. [Google Scholar] [CrossRef]
- Berard, N.; Hay, A.S. Polymers derived from phenolphthaleins. Polym. Prepr. (Am. Chem. Soc. Div. Pol. Chem.) 1993, 34, 148–149. [Google Scholar]
- Wang, S.J.; Meng, Y.Z.; Hlil, A.R.; Hay, A.S. Synthesis and characterization of phthalazinone containing poly(arylene ether)s via a novel N-C coupling reaction. Macromolecules 2012, 37, 60–65. [Google Scholar] [CrossRef]
- Wang, G.; Yang, M.; Li, Z.; Lin, K.; Jin, Q.; Xing, C.; Hu, Z.; Wang, D. Synthesis and characterization of Zn-doped MgAl-layered double hydroxide nanoparticles as PVC heat stabilizer. J. Nanoparticle Res. 2013, 15, 1882. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, C.; Xing, D.; Yang, D.; Jian, X.J. Chemical and mechanical degradation of sulfonated poly(sulfone) membranes in vanadium redox flow batteries. J. Appl. Electrochem. 2011, 41, 1201–1213. [Google Scholar]
- Xing, D.; Zhang, S.; Yin, C.; Zhang, B.; Jian, X. Effect of amination agent on the properties of quaternized poly(phthalazinone ether sulfone) anion exchange membrane for vanadium redox flow battery application. J. Membr. Sci. 2010, 354, 68–73. [Google Scholar] [CrossRef]
- Pan, J.; Li, K.; Chuayprakong, S.; Hsu, T.; Wang, Q. High-temperature poly(phthalazinone ether ketone) thin films for dielectric energy storage. ACS Appl. Mater. Interfaces 2010, 2, 1286. [Google Scholar] [CrossRef]
- Pan, H.; Zhu, X.; Chen, J.; Jian, X. Synthesis of fluoro-containing sulfonated poly(phthalazinone ether ketone ketone)s and their properties as PEM in PEMFC and DMFC. J. Membr. Sci. 2009, 326, 453–459. [Google Scholar] [CrossRef]
- Chen, C.M.; Zhang, Q.; Huang, J.Q.; Zhang, W.; Zhao, X.C.; Huang, C.H.; Wei, F.; Yang, Y.G.; Wang, M.Z.; Su, D.S. Chemically derived graphene–metal oxide hybrids as electrodes for electrochemical energy storage: Pre-graphenization or post-graphenization? J. Mater. Chem. 2012, 19, 3724–3729. [Google Scholar] [CrossRef]
- Zhang, F.F.; Zhou, C.h.H.; Yan, J.P. New advances in the research of carbazole compounds. Org. Chem. 2010, 30, 783–796. [Google Scholar]
- Fortney, A.; Fossum, E. Soluble semi-crystalline PEEK analogs based on 3,5-difluorobenzophenone: Synthesis and characterization. Polymer 2012, 53, 2327–2333. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Wang, H.; Zhou, G. Properties of Poly(arylene ether ketone)s Containing N-Alkylcarbazole in Main Chains. Appl. Chem. 2015, 32, 379–385. [Google Scholar]
- Zhang, Z.Y.; Sheng, S.R.; Zhang, X.L.; Pan, Y.; Liu, X.L. New poly(aryl ether ketone)s containing 2,6-diphenylpyridyl units and diphenylphosphinophenyl pendant groups. High Perform. Polym. 2020, 32, 753–769. [Google Scholar] [CrossRef]
- Bao, F.; Zhang, F.; Wang, C.; Song, Y.; Li, N.; Wang, J.; Jian, X. Study of the Effects of the Structure of Phthalazinone’s Side-Group on the Properties of the Poly(phthalazinone ether ketone)s Resins. Polymers 2019, 11, 803. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Xu, Y.; Lei, H.; Lei, Y.; Huo, J.; Zhang, L. Novel benzimidazole and pyridine-containing poly(ether ether ketone) s with good solubility, thermal properties and fluorescence. J. Polym. Res. 2019, 26, 158–169. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, C.; Wang, J.; Jian, X. Synthesis and characterization of phthalazinone-based poly(aryl ether ketone) derived from 4,4′-dichlorobenzophenone. Polym. Adv. Technol. 2012, 23, 742–750. [Google Scholar] [CrossRef]
- Li, X.; Gao, Y.; Long, Q.; Hay, A.S. Synthesis and characterization of highly fluorinated poly(phthalazinone ether)s based on AB-type monomers. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1761–1770. [Google Scholar] [CrossRef]
- Li, X.; Hay, A.S. Synthesis of High Molecular Weight Fluorinated Poly(phthalazinone ether)s by Self-Condensation of an AB-Type Monomer and by Condensation of AA Monomers with Decafluorobiphenyl. J. Macromol. Sci. Part A Chem. 2007, 44, 249–258. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, S.; Wang, Z.; Chen, Y.; Jian, X. Effect of pendent phenyl and bis-phthalazinone moieties on the properties of N-heterocyclic poly(aryl ether ketone ketone)s. Polymer 2020, 198, 122525–122535. [Google Scholar] [CrossRef]
- Ouellette, E.S.; Gilbert, J.L. Production and characterization of melt-spun Poly(Ether Ether Ketone) fibers for biomedical applications. Polymer 2015, 63, 10–18. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Li, W.M.; Hlil, A.R.; Meng, Y.Z.; Hay, A.S. Synthesis and Properties of Carbazole-containing Copolymers from Bis [3-(4-fluorobenzoyl)carbazole] Methane. J. Macromol. Sci. Part A 2010, 47, 1051–1054. [Google Scholar] [CrossRef]
- Jin, W.; Wang, L.; Zhang, H.; Liang, J.; Cao, S.; Zhang, Q.; Wang, J.; Ye, Z. Crosslinkable polyaryletherketone ultrafiltration membranes with solvent-resistant improvement. Mater. Today Commun. 2019, 21, 100696. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, J.; Huo, Y.; Zhao, J. Heat resistant microporous membranes based on soluble poly (aryl ether ketone) copolymers for lithium ion battery separator. J. Appl. Polym. Sci. 2021, 138, 50895. [Google Scholar] [CrossRef]
- Devaux, J.; Delimoy, D.; Daoust, D.; Legras, R.; Mercier, J.P.; Strazielle, C.; Nield, E. On the molecular weight determination of a poly(aryl-ether-ether-ketone) (PEEK). Polymer 1985, 26, 1994–2000. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, S.; Wang, Z.; Li, N.; Chen, Y.; Xu, P.; Jian, X. Poly(aryl ether ketone ketone)s containing diphenyl-biphthalazin-dione moieties with excellent thermo-mechanical performance and solubility. Eur. Polym. J. 2020, 143, 110205. [Google Scholar] [CrossRef]





| Polymer | Mn a/kg/mol | Mw a/kg/mol | Mw/Mn a | Yield/% |
|---|---|---|---|---|
| PCDD | 4.181 × 103 | 4.244 × 103 | 1.015 | 90.5 |
| PBCD | 72.793 × 103 | 169.894 × 103 | 2.334 | 88.1 |
| PBD | 75.313 × 103 | 162.799 × 103 | 2.162 | 93.8 |
| Polymer | CH2Cl2 | CHCl3 | THF | NMP | DMSO | DMAC | DMF |
|---|---|---|---|---|---|---|---|
| PCDD | + | ++ | ++ | + | + | ++ | ++ |
| PBCD | + | ++ | + | + | + | ++ | ++ |
| PBD | − | − | + | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, X.; Jiang, J.; Jia, H.; Jian, X.; Wang, J. Synthesis and Characterization of a Self-Polycondensation Diazaphthalanone Monomer and Its Polymers from Polycondensation Reactions. Polymers 2022, 14, 3904. https://doi.org/10.3390/polym14183904
Liu X, Zhang X, Jiang J, Jia H, Jian X, Wang J. Synthesis and Characterization of a Self-Polycondensation Diazaphthalanone Monomer and Its Polymers from Polycondensation Reactions. Polymers. 2022; 14(18):3904. https://doi.org/10.3390/polym14183904
Chicago/Turabian StyleLiu, Xin, Xiaozhou Zhang, Jiawei Jiang, Hongge Jia, Xigao Jian, and Jinyan Wang. 2022. "Synthesis and Characterization of a Self-Polycondensation Diazaphthalanone Monomer and Its Polymers from Polycondensation Reactions" Polymers 14, no. 18: 3904. https://doi.org/10.3390/polym14183904
APA StyleLiu, X., Zhang, X., Jiang, J., Jia, H., Jian, X., & Wang, J. (2022). Synthesis and Characterization of a Self-Polycondensation Diazaphthalanone Monomer and Its Polymers from Polycondensation Reactions. Polymers, 14(18), 3904. https://doi.org/10.3390/polym14183904







