Design, Fabrication and Characterization of Biodegradable Composites Containing Closo-Borates as Potential Materials for Boron Neutron Capture Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
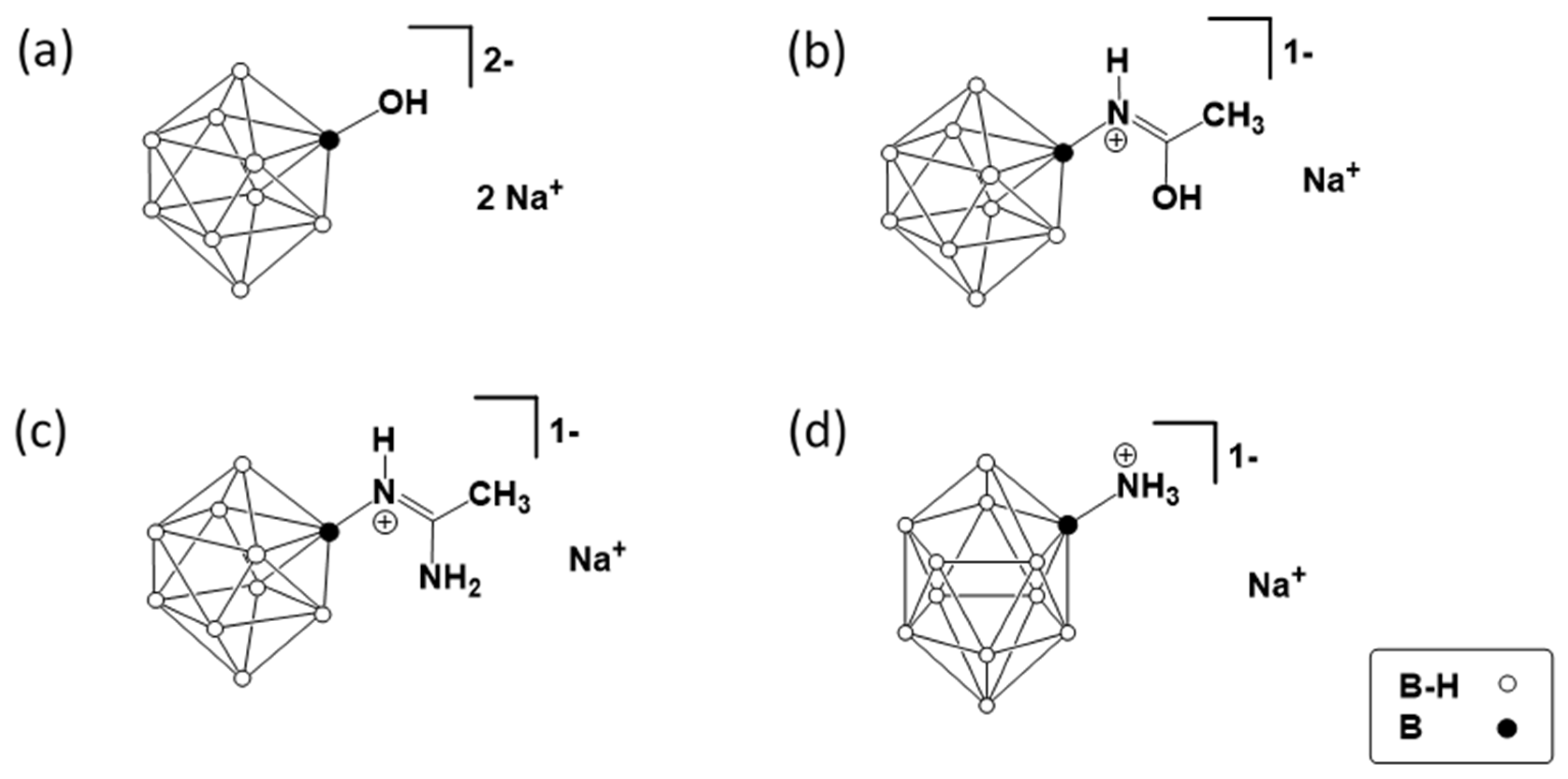
2.2. Synthesis of Closo-Borates
2.3. Synthesis and Characterization of Polymers
2.4. Manufacturing of Films
2.5. Modification of PDLLA- and PCL-Films Surface
2.6. Study of Adsorption/Desorption Processes of Closo-Borates
2.7. Manufacturing of 3D-Printed Macroporous Matrices
2.8. Fabrication of 3D-Printed Matrices with Closo-Borate-Containing Hydrogel
2.9. Characterization of Materials
2.10. Investigation of Closo-Borate Release and Material Degradation
2.11. Biological Evaluation
3. Results and Discussion
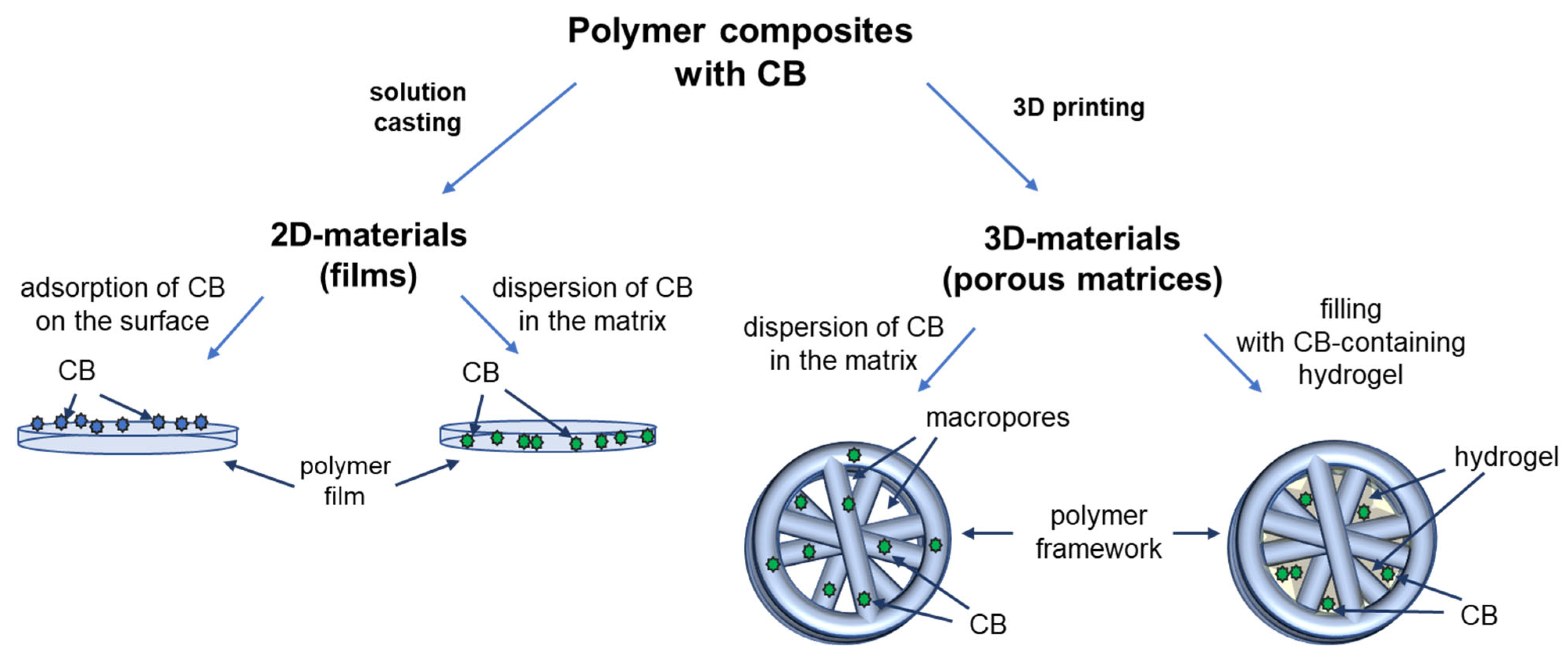
3.1. Design of Composite Materials
3.2. Polymer Films with Adsorbed Closo-Borates
3.2.1. Fabrication of Films with Different Surface Functionality
3.2.2. Adsorption of Closo-Borates
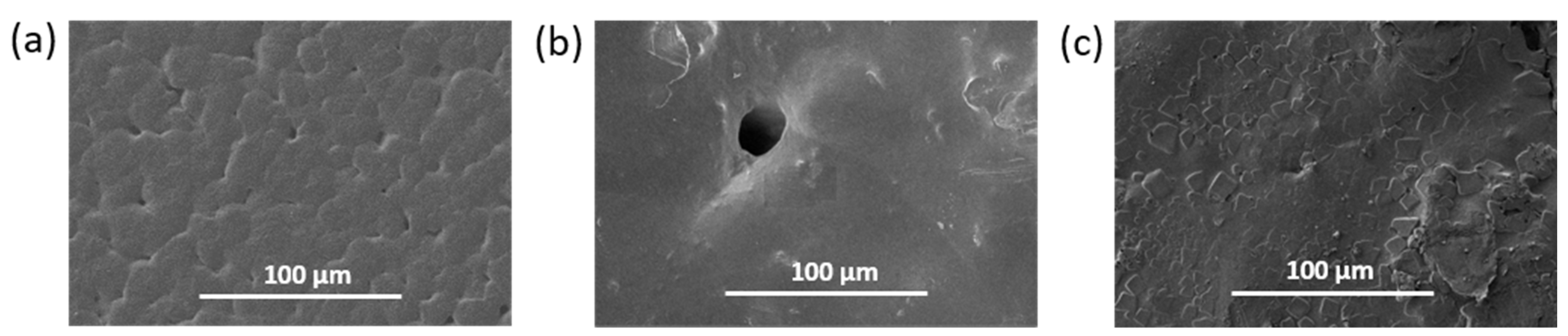
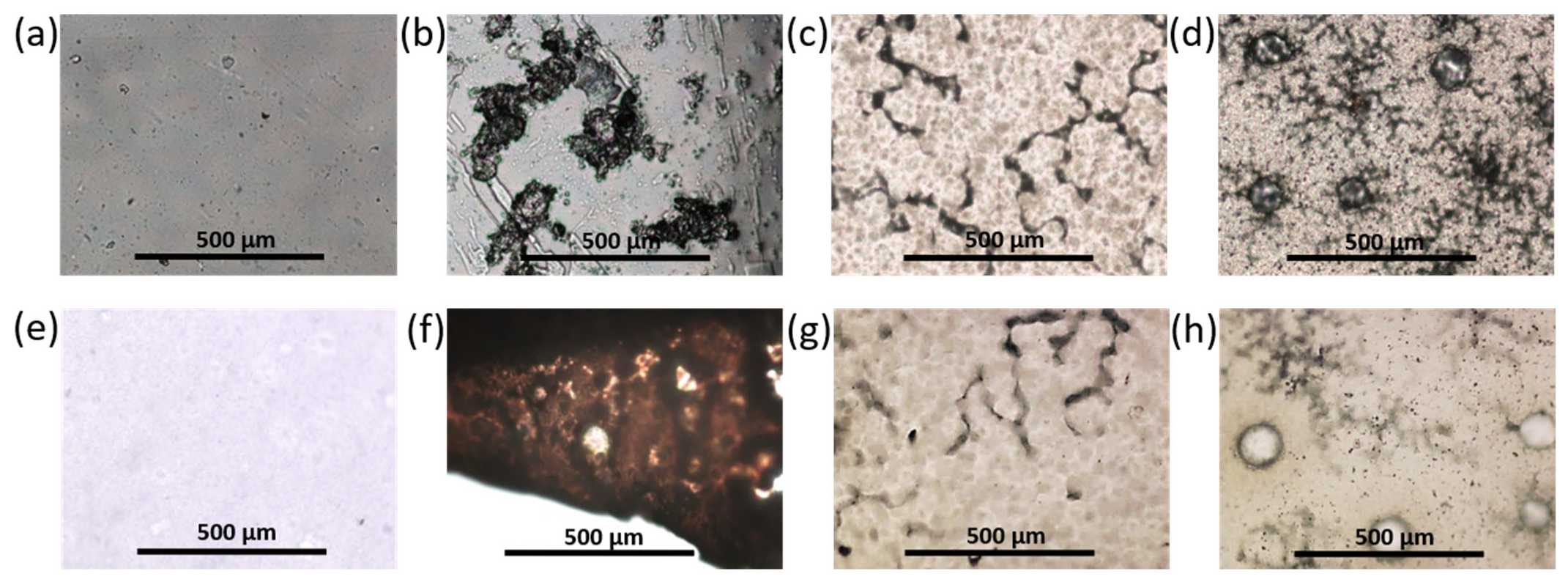
3.2.3. Morphology of Prepared Materials
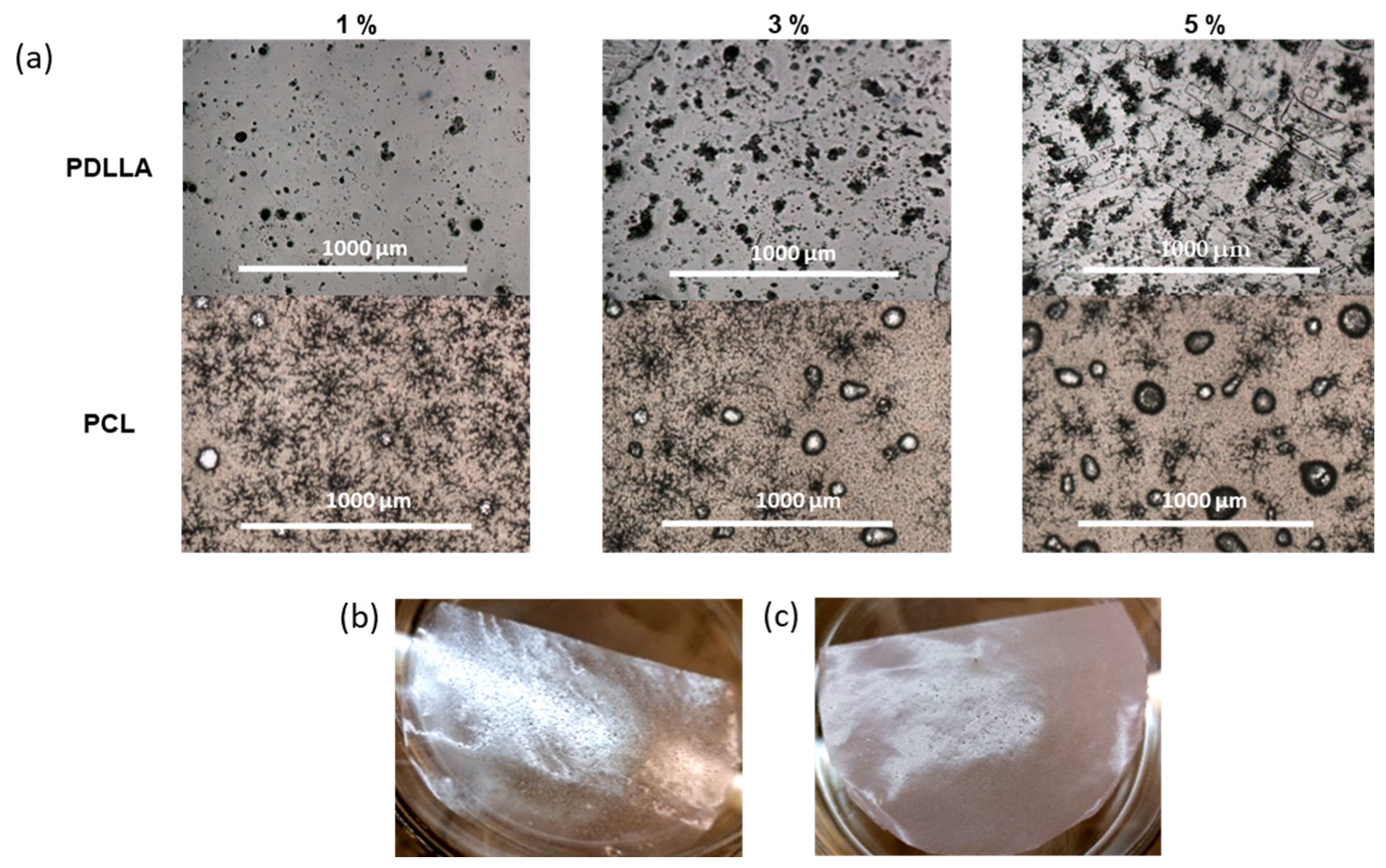
3.3. Composite Films with Closo-Borates Dispersed in the Polymer Matrix
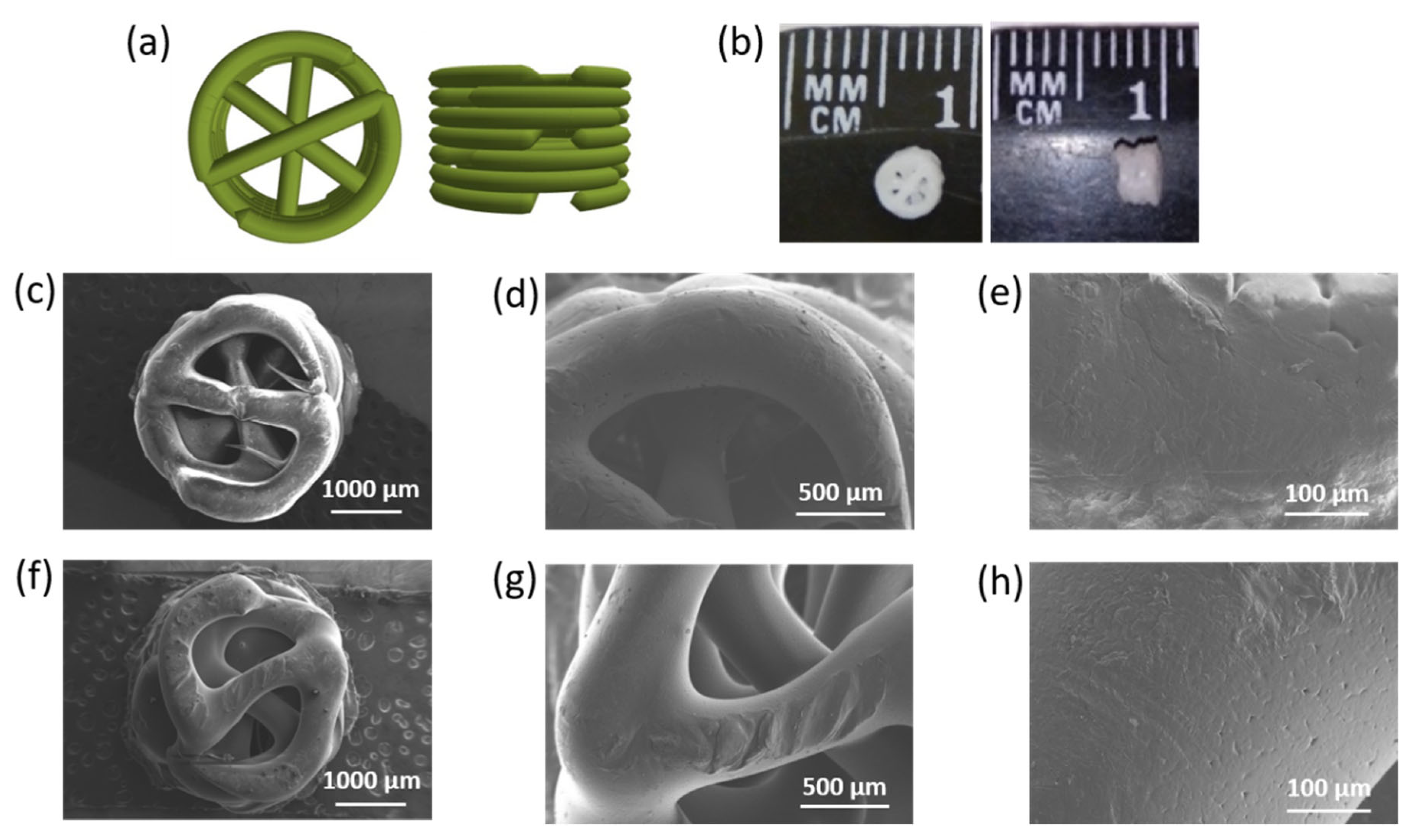
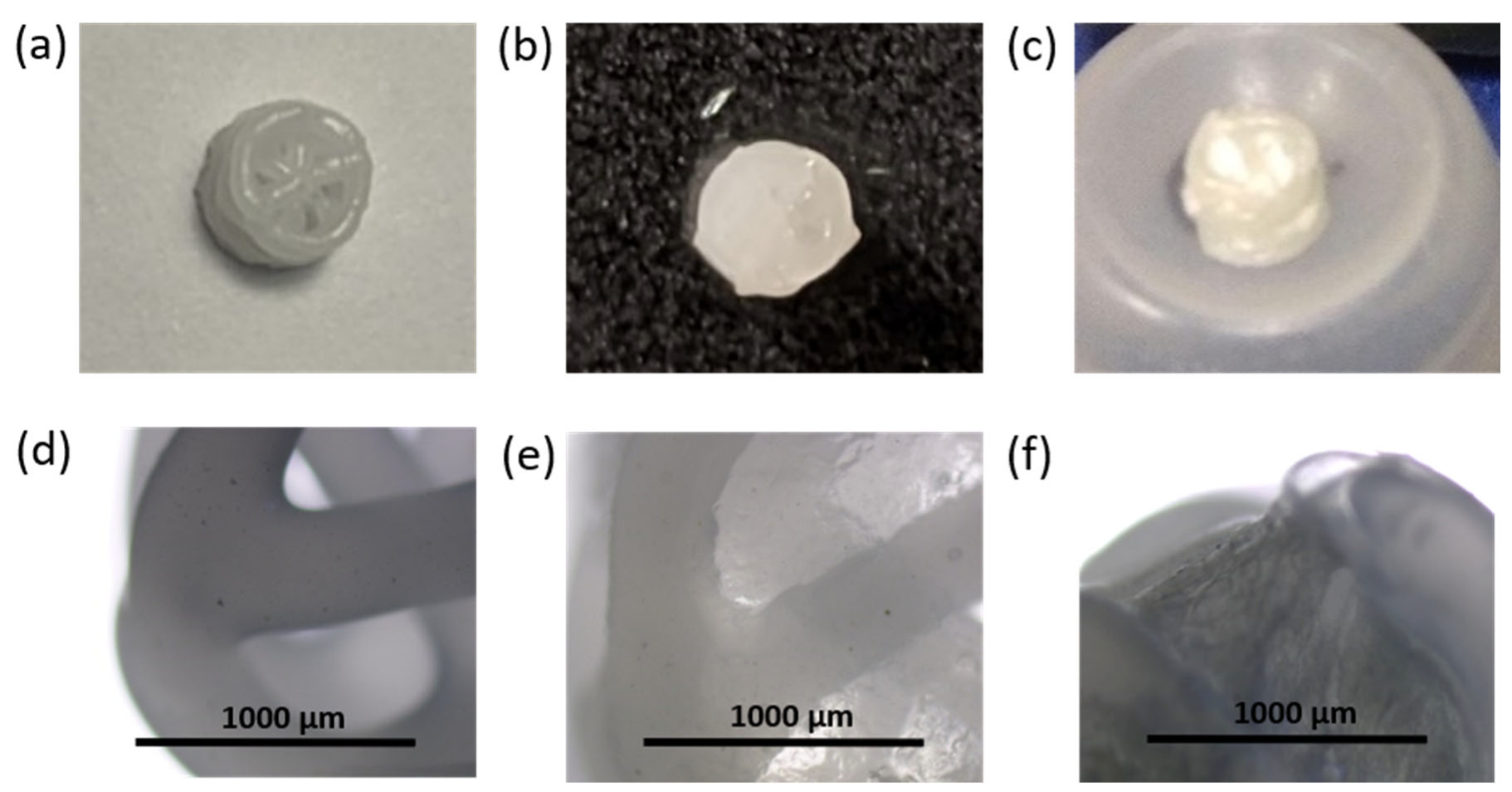
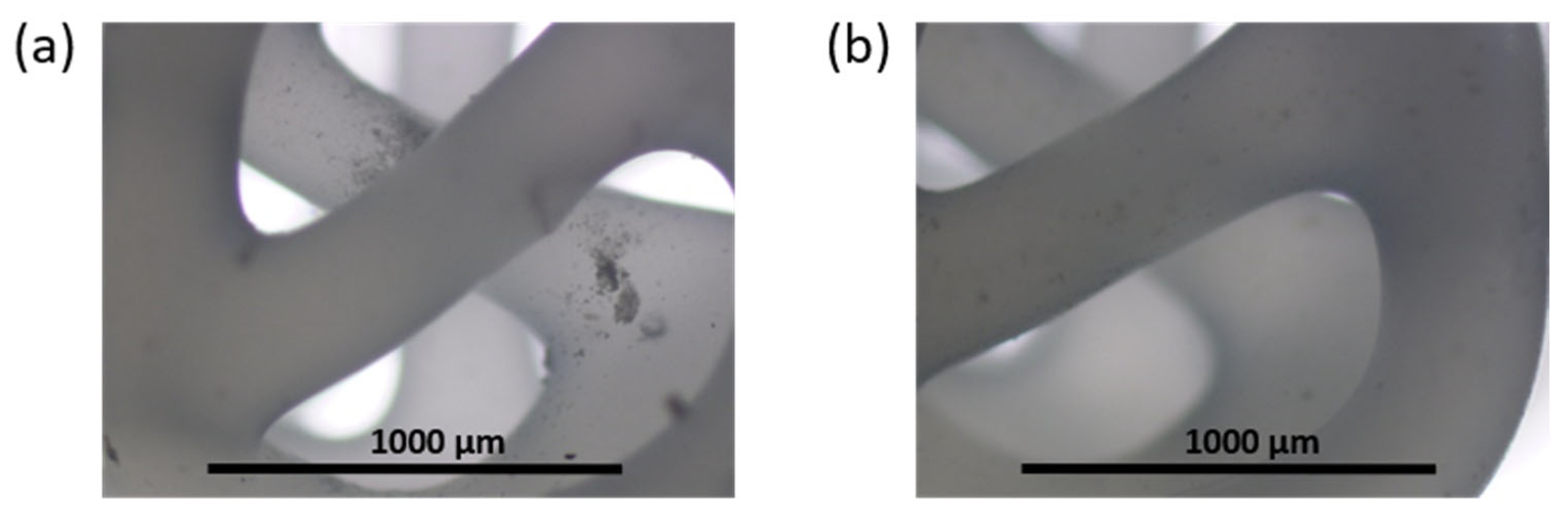
3.4. 3D-Composite Materials Containing Closo-Borates
3.5. 3D-Printed Composites Filled with Hydrogel
3.6. Mechanical Properties of Composite Materials
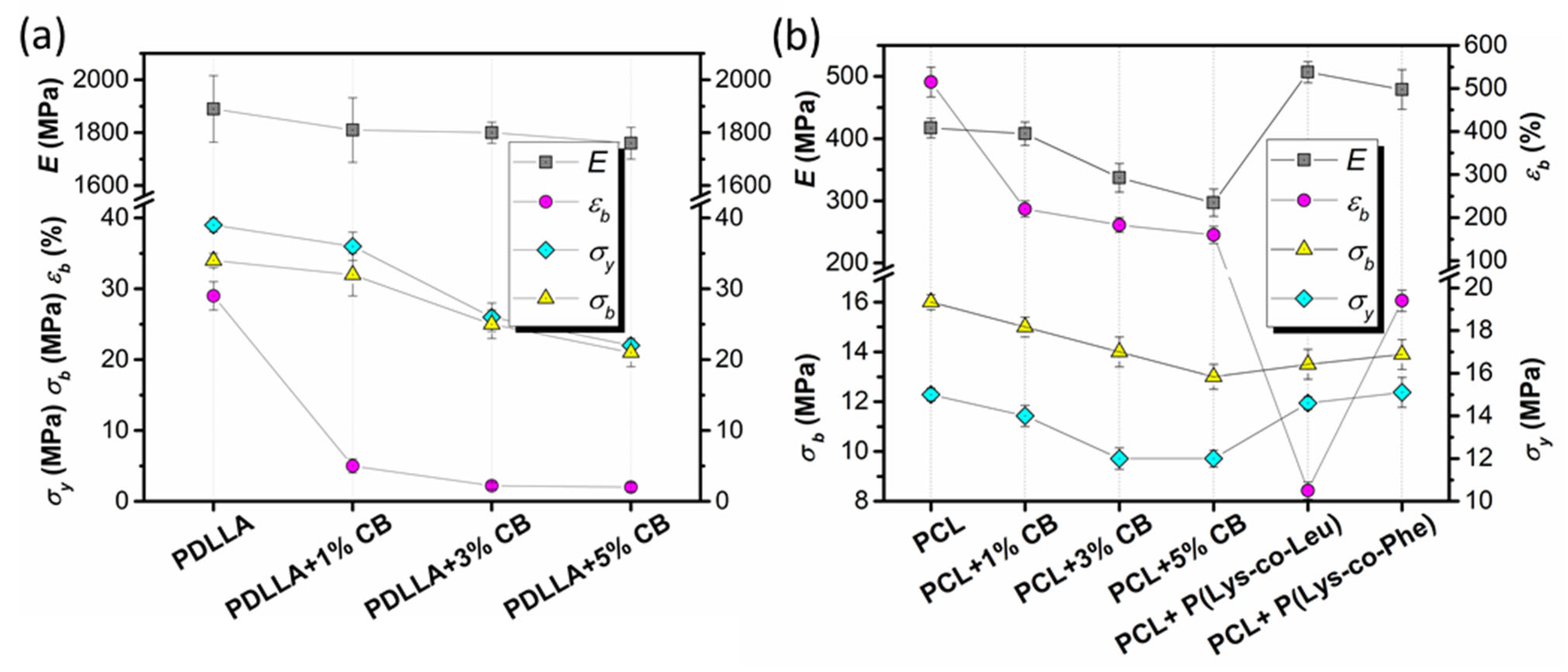
3.6.1. Composite Films
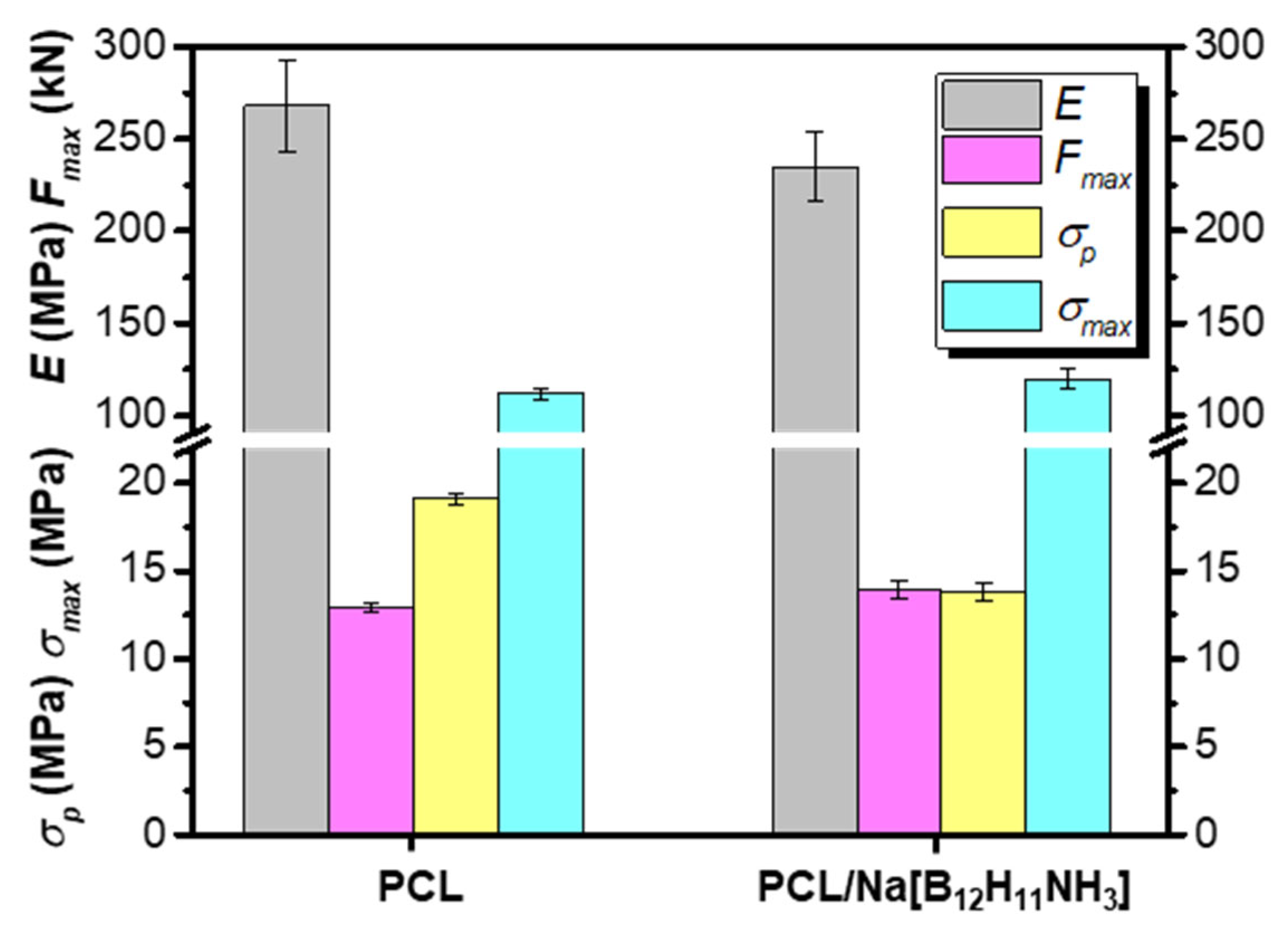
3.6.2. 3D-Composites
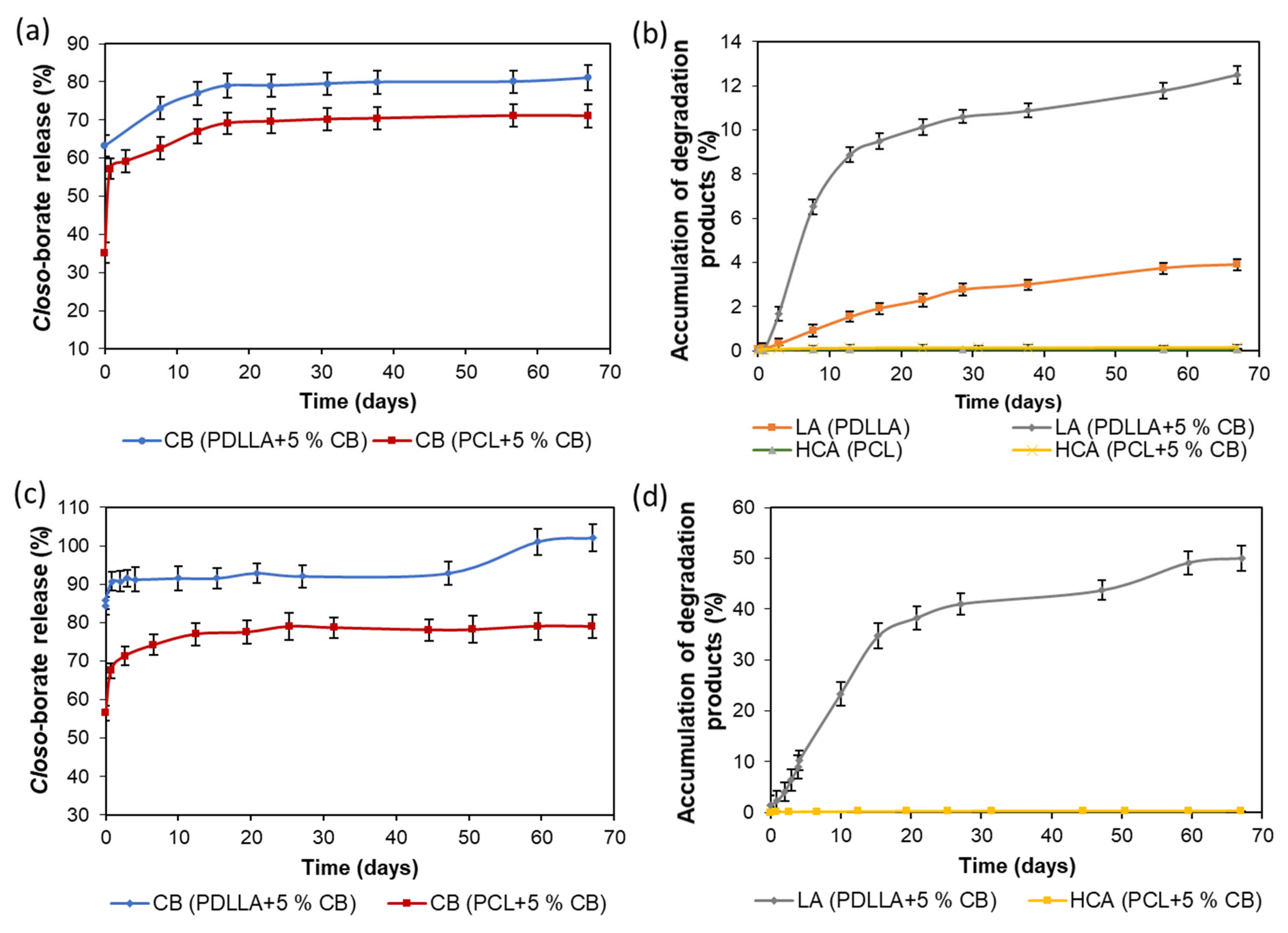
3.7. Release of Closo-Borates and Matrix Degradation
3.7.1. Composite Films
3.7.2. 3D-Printed Matrices
3.7.3. Comparative Analysis of Closo-Borate Release from Composites
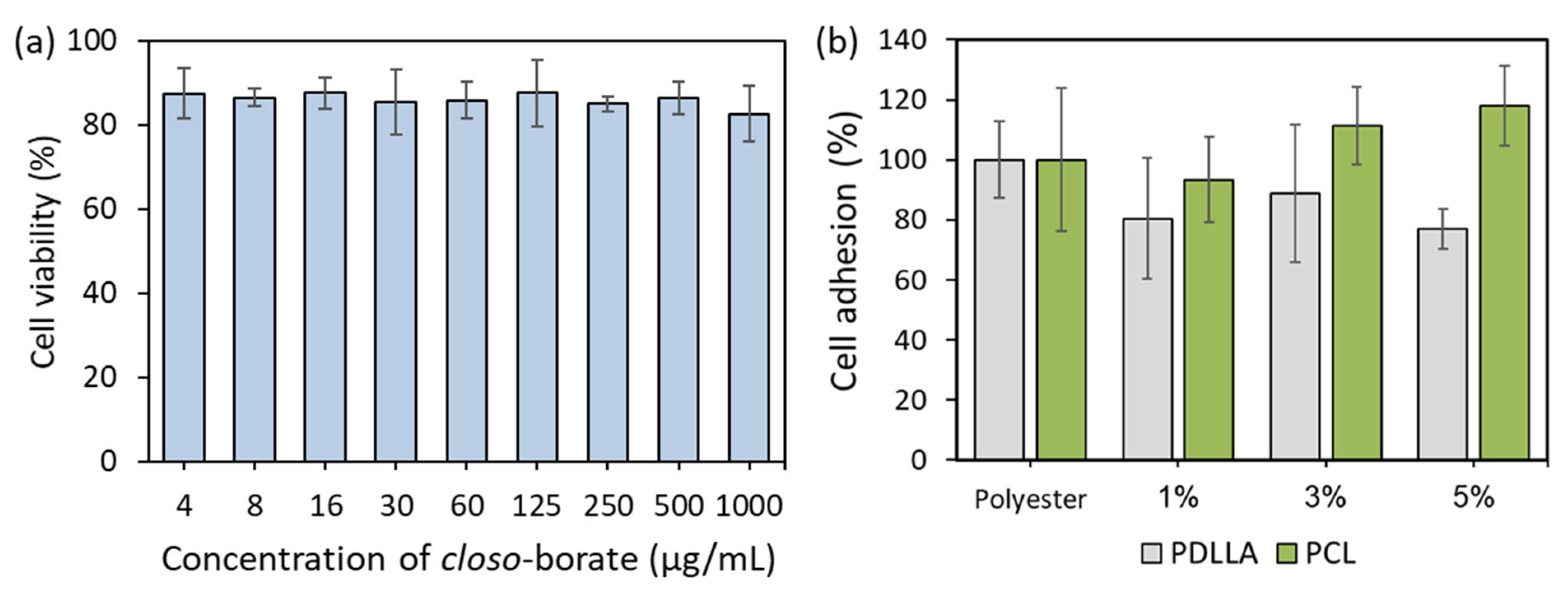
3.8. In Vitro Biological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raijada, D.; Wac, K.; Greisen, E.; Rantanen, J.; Genina, N. Integration of personalized drug delivery systems into digital health. Adv. Drug Deliv. Rev. 2021, 176, 113857. [Google Scholar] [CrossRef] [PubMed]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Jain, S.; Mahajan, S.C. Nanomedicines based drug delivery systems for anti-cancer targeting and treatment. Curr. Drug Deliv. 2015, 12, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.N.; Celia, C.; Petrikaite, V. Challenges towards targeted drug delivery in nanomedicines. Processes 2021, 9, 1527. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal antibodies in cancer therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Levit, M.; Vdovchenko, A.; Dzhuzha, A.; Zashikhina, N.; Katernyuk, E.; Gostev, A.; Sivtsov, E.; Lavrentieva, A.; Tennikova, T.; Korzhikova-Vlakh, E. Self-Assembled Nanoparticles Based on Block-Copolymers of Poly(2-Deoxy-2-methacrylamido-d-glucose)/Poly(N-Vinyl Succinamic Acid) with Poly(O-Cholesteryl Methacry-late) for Delivery of Hydrophobic Drugs. Int. J. Mol. Sci. 2021, 22, 11457. [Google Scholar] [CrossRef]
- Suzuki, M. Boron neutron capture therapy (BNCT): A unique role in radiotherapy with a view to entering the accelerator-based BNCT era. Int. J. Clin. Oncol. 2020, 25, 43–50. [Google Scholar] [CrossRef]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef]
- Pitto-Barry, A. Polymers and boron neutron capture therapy (BNCT): A potent combination. Polym. Chem. 2021, 12, 2035–2044. [Google Scholar] [CrossRef]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron neutron capture therapy: A review of clinical applications. Front. Oncol. 2021, 11, 601820. [Google Scholar] [CrossRef] [PubMed]
- Zelenetskii, A.N.; Uspenskii, S.; Zaboronok, A.; Cherkaev, G.; Shchegolihin, A.; Mathis, B.J.; Selyanin, M.; Yamamoto, T.; Matsumura, A. Polycomplexes of hyaluronic acid and borates in a solid state and solution: Synthesis, characterization and perspectives of application in boron neutron capture therapy. Polymers 2018, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Yoneoka, S.; Park, K.C.; Nakagawa, Y.; Ebara, M.; Tsukahara, T. Synthesis and evaluation of thermoresponsive boron-containing poly(N-isopropylacrylamide) diblock copolymers for self-assembling nanomicellar boron carriers. Polymers 2019, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Liu, L.; Fu, L.; Xing, T.; Yan, L. An amphiphilic block copolymer conjugated with carborane and a NIR fluorescent probe for potential imaging-guided BNCT therapy. Polym. Chem. 2016, 7, 4411–4418. [Google Scholar] [CrossRef]
- Sumitani, S.; Oishi, M.; Yaguchi, T.; Murotani, H.; Horiguchi, Y.; Suzuki, M.; Ono, K.; Yanagie, H.; Nagasaki, Y. Pharmacokinetics of core-polymerized, boron-conjugated micelles designed for boron neutron capture therapy for cancer. Biomaterials 2012, 33, 3568–3577. [Google Scholar] [CrossRef]
- Wu, G.; Barth, R.F.; Yang, W.; Chatterjee, M.; Tjarks, W.; Ciesielski, M.J.; Fenstermaker, R.A. Site-specific conjugation of boron-containing dendrimers to anti-EGF receptor monoclonal antibody cetuximab (IMC-C225) and its evaluation as a potential delivery agent for neutron capture therapy. Bioconjug. Chem. 2004, 15, 185–194. [Google Scholar] [CrossRef]
- Alberti, D.; Michelotti, A.; Lanfranco, A.; Protti, N.; Altieri, S.; Deagostino, A.; Geninatti Crich, S. In vitro and in vivo BNCT investigations using a carborane containing sulfonamide targeting CAIX epitopes on malignant pleural mesothelioma and breast cancer cells. Sci. Rep. 2020, 10, 19274. [Google Scholar] [CrossRef]
- Ueno, M.; Ban, H.S.; Nakai, K.; Inomata, R.; Kaneda, Y.; Matsumura, A.; Nakamura, H. Dodecaborate lipid liposomes as new vehicles for boron delivery system of neutron capture therapy. Bioorg. Med. Chem. 2010, 18, 3059–3065. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Matsumoto, K.; Murakami, S.; Kanesaki, T.; Matsumoto, A.; Kishino, M.; Furukawa, S. MRI in a case of osteosarcoma in the temporomandibular joint. Dentomaxillofac. Radiol. 2014, 43, 20130280. [Google Scholar] [CrossRef]
- Futamura, G.; Kawabata, S.; Siba, H.; Kuroiwa, T.; Suzuki, M.; Kondo, N.; Ono, K.; Sakura, Y.; Tanaka, M.; Todo, T.; et al. A case of radiation-induced osteosarcoma treated effectively by boron neutron capture therapy. Radiat. Oncol. 2014, 9, 237. [Google Scholar] [CrossRef]
- Bortolussi, S.; Postuma, I.; Protti, N.; Provenzano, L.; Ferrari, C.; Cansolino, L.; Dionigi, P.; Galasso, O.; Gasparini, G.; Altieri, S.; et al. Understanding the potentiality of accelerator based-boron neutron capture therapy for osteosarcoma: Dosimetry assessment based on the reported clinical experience. Radiat. Oncol. 2017, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Saito, N. Biodegradable polymers as drug delivery systems for bone regeneration. Pharmaceutics 2020, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Eremin, A.; Averianov, I.; Gofman, I.; Lavrentieva, A.; Korzhikov-Vlakh, V.; Korzhikova-Vlakh, E. Comparison of supermacroporous polyester matrices fabricated by thermally induced phase separation and 3D printing techniques. Key Eng. Mater. 2019, 822, 277–283. [Google Scholar]
- Wang, Y.; Cui, W.; Zhao, X.; Wen, S.; Sun, Y.; Han, J.; Zhang, H. Bone remodeling-inspired dual delivery electrospun nanofibers for promoting bone regeneration. Nanoscale 2018, 11, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Averianov, I.V.; Stepanova, M.A.; Gofman, I.V.; Nikolaeva, A.L.; Korzhikov-Vlakh, V.A.; Karttunen, M.; Korzhikova-Vlakh, E.G. Chemical modification of nanocrystalline cellulose for improved interfacial compatibility with poly(lactic acid). Mendeleev Commun. 2019, 29, 220–222. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, X.; Huang, Q.; Zhang, R.; Feng, Q. Incorporation of silica nanoparticles to PLGA electrospun fibers for osteogenic differentiation of human osteoblast-like cells. Regen. Biomater. 2018, 5, 229–238. [Google Scholar] [CrossRef]
- Stepanova, M.; Averianov, I.; Solomakha, O.; Zabolotnykh, N.; Gofman, I.; Serdobintsev, M.; Vinogradova, T.; Korzhikov-Vlakh, V.; Korzhikova-Vlakh, E. Composite biomaterials based on poly(L-lactic acid) and functionalized cellulose nanocrystals. J. Renew. Mater. 2020, 8, 383–395. [Google Scholar] [CrossRef]
- Enayati, M.S.; Behzad, T.; Sajkiewicz, P.; Rafienia, M.; Bagheri, R.; Ghasemi-Mobarakeh, L.; Kolbuk, D.; Pahlevanneshan, Z.; Bonakdar, S.H. Development of electrospun poly (vinyl alcohol)-based bionanocomposite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 1111–1120. [Google Scholar] [CrossRef]
- Averianov, I.; Stepanova, M.; Solomakha, O.; Gofman, I.; Serdobintsev, M.; Blum, N.; Kaftuirev, A.; Baulin, I.; Nashchekina, J.; Lavrentieva, A.; et al. 3D-Printed composite scaffolds based on poly(ε-caprolactone) filled with poly(glutamic acid)-modified cellulose nanocrystals for improved bone tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2022; in press. [Google Scholar] [CrossRef]
- Stepanova, M.; Averianov, I.; Gofman, I.; Solomakha, O.; Nashchekina, Y.; Korzhikov-Vlakh, V.; Korzhikova-Vlakh, E. Poly(ε-caprolactone)-based biocomposites reinforced with nanocrystalline cellulose grafted with poly(L-lactic acid). IOP Conf. Ser. Mater. Sci. Eng. 2019, 500, 012021. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Adhikari, J.; Saha, P. Facile fabrication of electrospun regenerated cellulose nanofiber scaffold for potential bone-tissue engineering application. Int. J. Biol. Macromol. 2019, 122, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Peng, H.; Wu, Y.; Zhang, C.; Cai, Y.; Xu, G.; Li, Q.; Chen, X.; Ji, J.; Zhang, Y.; et al. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials 2013, 34, 4404–4417. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.J.; Casalini, T.; Hulsart-Billström, G.; Wang, S.; Oommen, P.; Salvalaglio, M.; Larsson, S.; Hilborn, J.; Varghese, O.P. Synthetic design of growth factor sequestering extracellular matrix mimetic hydrogel for promoting in vivo bone formation. Biomaterials 2018, 161, e190–e202. [Google Scholar] [CrossRef] [PubMed]
- Hertler, W.R.; Raasch, M.S. Chemistry of Boranes. XIV. Amination of B10H10−2 and B12H12−2 with Hydroxylamine-O-sulfonic Acid. J. Am. Chem. Soc. 1964, 86, 3661–3668. [Google Scholar] [CrossRef]
- Klyukin, I.N.; Zhdanov, A.P.; Razgonyaeva, G.A.; Zhizhin, K.Y.; Kuznetsov, N.T. New methods of preparation of hydroxy-closo-decaborates [B10H10 − n (OH) n ]2− (n = 1, 2). Russ. J. Inorg. Chem. 2013, 58, 1395–1399. [Google Scholar] [CrossRef]
- Zhdanov, A.P.; Bykov, A.Y.; Kubasov, A.S.; Polyakova, I.N.; Razgonyaeva, G.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Hydrolysis of nitrilium derivatives of the closo-decaborate anion [2-B10H9(N≡CR)]− (R = CH3, C2H5, C(CH3)3, or C6H5). Russ. J. Inorg. Chem. 2017, 62, 468–475. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Selivanov, N.A.; Bykov, A.Y.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Karpechenko, N.Y.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Primary amine nucleophilic addition to nitrilium closo-dodecaborate [B12H11NCCH3]−: A simple and effective route to the new BNCT drug design. Int. J. Mol. Sci. 2021, 22, 13391. [Google Scholar] [CrossRef]
- Zashikhina, N.; Sharoyko, V.; Antipchik, M.; Tarasenko, I.; Anufrikov, Y.; Lavrentieva, A.; Tennikova, T.; Korzhikova-Vlakh, E. Novel formulations of C-peptide with long-acting therapeutic potential for treatment of diabetic complications. Pharmaceutics 2019, 11, 27. [Google Scholar] [CrossRef]
- Pilipenko, I.; Korovkina, O.; Gubina, N.; Ekimova, V.; Ishutinova, A.; Korzhikova-Vlakh, E.; Tennikova, T.; Korzhikov-Vlakh, V. Random copolymers of lysine and isoleucine for efficient mRNA delivery. Int. J. Mol. Sci. 2022, 23, 5363. [Google Scholar] [CrossRef]
- Stepanova, M.; Averianov, I.; Serdobintsev, M.; Gofman, I.; Blum, N.; Semenova, N.; Nashchekina, Y.; Vinogradova, T.; Korzhikov-Vlakh, V.; Karttunen, M.; et al. PGlu-modified nanocrystalline cellulose improves mechanical properties, biocompatibility, and mineralization of polyester-based composites. Materials 2019, 12, 3435. [Google Scholar] [CrossRef] [PubMed]
- Korzhikov-Vlakh, V.; Krylova, M.; Sinitsyna, E.; Ivankova, E.; Averianov, I.; Tennikova, T.B. Hydrogel layers on the surface of polyester-based materials for improvement of their biointeractions and controlled release of proteins. Polymers 2016, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Korzhikov-Vlakh, V.; Averianov, I.; Sinitsyna, E.; Nashchekina, Y.; Polyakov, D.; Guryanov, I.; Lavrentieva, A.; Raddatz, L.; Korzhikova-Vlakh, E.; Scheper, T.; et al. Novel pathway for efficient covalent modification of polyester materials of different design to prepare biomimetic surfaces. Polymers 2018, 10, 1299. [Google Scholar] [CrossRef]
- Stepanova, M.; Solomakha, O.; Rabchinskii, M.; Averianov, I.; Gofman, I.; Nashchekina, Y.; Antonov, G.; Smirnov, A.; Ber, B.; Nashchekin, A.; et al. Aminated graphene-graft-oligo(glutamic acid)/poly(ε-caprolactone) composites: Preparation, characterization and biological evaluation. Polymers 2021, 13, 2628. [Google Scholar] [CrossRef]
- Shubina, E.S.; Bakhmutova, E.V.; Filin, A.M.; Sivaev, I.B.; Teplitskaya, L.N.; Chistyakov, A.L.; Stankevich, I.V.; Bakhmutov, V.I.; Bregadze, V.I.; Epstein, L.M. Dihydrogen bonding of decahydro-closo-decaborate(2−) and dodecahydro-closo-dodecaborate(2−) anions with proton donors: Experimental and theoretical investigation. J. Organomet. Chem. 2002, 657, 155–162. [Google Scholar] [CrossRef]
- Filippov, O.A.; Filin, A.M.; Teplitskaya, L.N.; Belkova, N.V.; Shmyrova, Y.V.; Sivaev, I.B.; Bregadze, V.I.; Epstein, L.M.; Shubina, E.S. Hydrogen bonding of the undecahydro-thiocyanato-closo-dodecaborate anion with proton donors. Main Group Chem. 2005, 4, 97–110. [Google Scholar] [CrossRef]
- Stepanova, M.; Korzhikova-Vlakh, E. Modification of cellulose micro- and nanomaterials to improve properties of aliphatic polyesters/cellulose composites: A review. Polymers 2022, 14, 1477. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Dominguez-Candela, I.; Castello-Palacios, S.; Vallés-Lluch, A.; Fombuena, V. Development and characterization of polyester and acrylate-based composites with hydroxyapatite and halloysite nanotubes for medical applications. Polymers 2020, 12, 1703. [Google Scholar] [CrossRef]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E. Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties. e-Polymers 2020, 20, 571–599. [Google Scholar] [CrossRef]
- Fernandes, C.; Moura, C.; Ascenso, R.M.T.; Amado, S.; Alves, N.; Pascoal-Faria, P. Comprehensive review on full bone regeneration through 3D printing approaches. In Design and Manufacturing; Yasa, E., Mhadhbi, M., Santecchia, E., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S. Alginates in Drug Delivery; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Echave, M.C.; Sánchez, P.; Pedraz, J.L.; Orive, G. Progress of gelatin-based 3D approaches for bone regeneration. J. Drug Deliv. Sci. Technol. 2017, 42, 63–74. [Google Scholar] [CrossRef]
- Wu, S.-C.; Chang, W.-H.; Dong, G.-C.; Chen, K.-Y.; Chen, Y.-S.; Yao, C.-H. Cell adhesion and proliferation enhancement by gelatin nanofiber scaffolds. J. Bioact. Compat. Polym. 2011, 26, 565–577. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, S.S.; Sivashanmugam, A.; Kwon, J.; Kim, S.H.L.; Noh, M.Y.; Kwon, S.K.; Jayakumar, R.; Hwang, N.S. Bioglass-incorporated methacrylated gelatin cryogel for regeneration of bone defects. Polymers 2018, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Cerqueira, A.; Romero-Gavilán, F.; García-Arnáez, I.; Martinez-Ramos, C.; Ozturan, S.; Azkargorta, M.; Elortza, F.; Gurruchaga, M.; Goñi, I.; et al. Influence of calcium ion-modified implant surfaces in protein adsorption and implant integration. Int. J. Implant Dent. 2021, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Griensven, M.; Diederichs, S.; Roeker, S.; Boehm, S.; Peterbauer, A.; Wolbank, S.; Riechers, D.; Stahl, F.; Kasper, C. Mechanical strain using 2D and 3D bioreactors induces osteogenesis: Implications for bone tissue engineering. Adv. Biochem. Eng. Biotechnol. 2009, 112, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.A. The mechanical properties of trabecular bone: Dependence on anatomic location and function. J. Biomech. 1987, 20, 1055–1061. [Google Scholar] [CrossRef]
- Li, X.; Deng, L.; Li, Y.; Li, K. Preparation of microcrystalline cellulose from bagasse bleached pulp reinforced polylactic acid composite films. Sugar Tech 2020, 22, 1138–1147. [Google Scholar] [CrossRef]
- Vilela, C.; Engstrom, J.; Valente, B.F.A.; Jawerth, M.; Carlmark, A.; Freire, C.S.R. Exploiting poly(ε-caprolactone) and cellulose nanofibrils modified with latex nanoparticles for the development of biodegradable nanocomposites. Polym. Compos. 2019, 40, 1342–1353. [Google Scholar] [CrossRef]
- Da Silva Gois, G.; de Andrade, M.F.; Garcia, S.M.S.; Vinhas, G.M.; Santos, A.S.F.; Medeiros, E.S.; Oliveira, J.E.; De Almeida, Y.M.B. Soil biodegradation of PLA/CNW nanocomposites modified with ethylene oxide derivatives. Mater. Res. 2018, 20, 899–904. [Google Scholar] [CrossRef]





| Polymer Film | Closo-Borate | Adsorption Capacity ** | AE, % | |||
|---|---|---|---|---|---|---|
| Polymer | Modification | Functional Groups | µg/cm2 | µmol/cm2 | ||
| PCL | 10 wt% P(Lys-co-Leu) | -NH2 | Na2[B10H9OH] | 1.8 | 0.010 | 3.6 |
| PCL | Surface hydrolysis | -COOH and -OH | Na2[B10H9OH] | 1.6 | 0.009 | 3.1 |
| PDLLA | Surface hydrolysis | -COOH and -OH | Na2[B10H9OH] | 3.2 | 0.018 | 6.2 |
| PDLLA | Surface hydrolysis | -COOH and -OH | Na[B12H11NH3] | 1.3 | 0.007 | 2.6 |
| PDLLA | Surface hydrolysis | -COOH and -OH | Na[B10H9NH=C(OH)CH3] * | 2.8 | 0.014 | 4.8 |
| PDLLA | Surface hydrolysis | -COOH and -OH | Na[B10H9NH=C(NH2)CH3] * | 0.4 | 0.002 | 0.6 |
| Type of Composite | Material Containing CB | Amount of CB Released | |||||
|---|---|---|---|---|---|---|---|
| 1st Day | From 2 to 17 Days | From 47 to 67 Days | |||||
| % | μg/cm2 b/μg/mg c | % | μg/mg | % | μg/mg | ||
| Films with adsorbed CB a | PCL/ P(Lys-co-Leu) | 33–35 | 13–14 | 0 | 0 | Not studied | |
| Films with dispersed CB | PDLLA | to 91 | to 45 | 0 | 0 | 9 | 4 |
| PCL | to 67 | to 33 | 11 | 6 | 3 | 1 | |
| 3D-printed porous matrices with dispersed CB | PCL | to 81 | to 41 | 12 | 6 | 4 | 2 |
| 3D-printed porous matrices filled with CB containing hydrogel | Alginate/ gelatin hydrogel | to 100 | to 50 d | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, M.; Dobrodumov, A.; Averianov, I.; Gofman, I.; Nashchekina, J.; Guryanov, I.; Klyukin, I.; Zhdanov, A.; Korzhikova-Vlakh, E.; Zhizhin, K. Design, Fabrication and Characterization of Biodegradable Composites Containing Closo-Borates as Potential Materials for Boron Neutron Capture Therapy. Polymers 2022, 14, 3864. https://doi.org/10.3390/polym14183864
Stepanova M, Dobrodumov A, Averianov I, Gofman I, Nashchekina J, Guryanov I, Klyukin I, Zhdanov A, Korzhikova-Vlakh E, Zhizhin K. Design, Fabrication and Characterization of Biodegradable Composites Containing Closo-Borates as Potential Materials for Boron Neutron Capture Therapy. Polymers. 2022; 14(18):3864. https://doi.org/10.3390/polym14183864
Chicago/Turabian StyleStepanova, Mariia, Anatoliy Dobrodumov, Ilia Averianov, Iosif Gofman, Juliya Nashchekina, Ivan Guryanov, Ilya Klyukin, Andrey Zhdanov, Evgenia Korzhikova-Vlakh, and Konstantin Zhizhin. 2022. "Design, Fabrication and Characterization of Biodegradable Composites Containing Closo-Borates as Potential Materials for Boron Neutron Capture Therapy" Polymers 14, no. 18: 3864. https://doi.org/10.3390/polym14183864
APA StyleStepanova, M., Dobrodumov, A., Averianov, I., Gofman, I., Nashchekina, J., Guryanov, I., Klyukin, I., Zhdanov, A., Korzhikova-Vlakh, E., & Zhizhin, K. (2022). Design, Fabrication and Characterization of Biodegradable Composites Containing Closo-Borates as Potential Materials for Boron Neutron Capture Therapy. Polymers, 14(18), 3864. https://doi.org/10.3390/polym14183864











