Design and Preparation of a Biobased Colorimetric pH Indicator from Cellulose and Pigments of Bacterial Origin, for Potential Application as Smart Food Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Resources: Production and Recovery
2.1.1. Bacterial Pigments
2.1.2. Bacterial Cellulose
2.2. BC Functionalization with Prodigiosin, Violacein and Flexirubin-Type Pigment
2.3. Factorial Experimental Design and Optimization of the Variables
2.4. Color Evaluation
2.5. Preparation of pH Indicator
2.6. Response to pH Buffer Solutions
2.7. Sensitivity of pH Indicators to Acid and Base Vapors
2.8. Leaching Assessment
2.9. Color Stability of pH Indicators Produced
2.10. Statistical Analysis
3. Results and Discussion
3.1. Full Factorial Design for Optimization of BC Functionalization
3.2. Colorimetric Parameters Evaluation
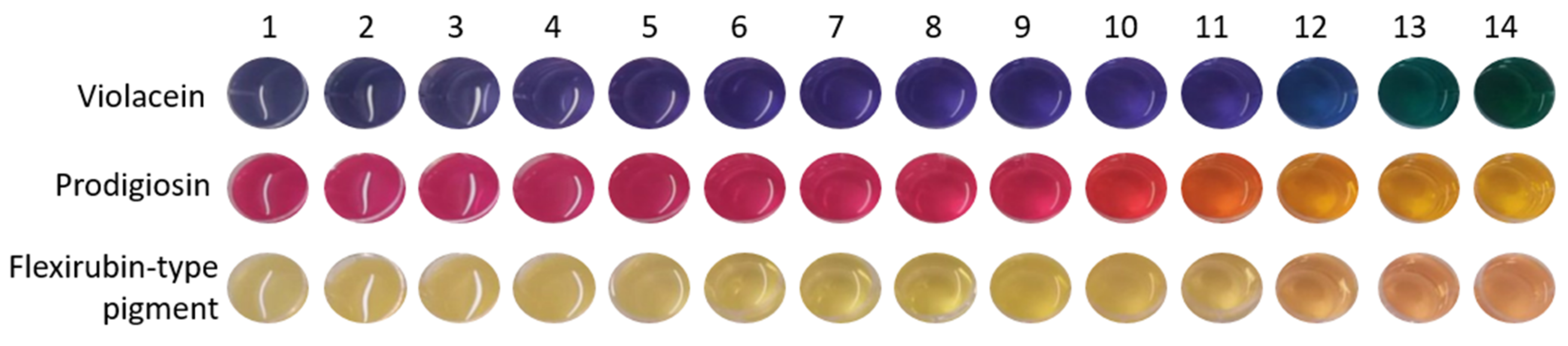
3.3. pH Responsive Properties of BC Functionalized with the Bacterial Pigments
3.4. Sensor Response to Ammonia and Acetic Acid Vapors
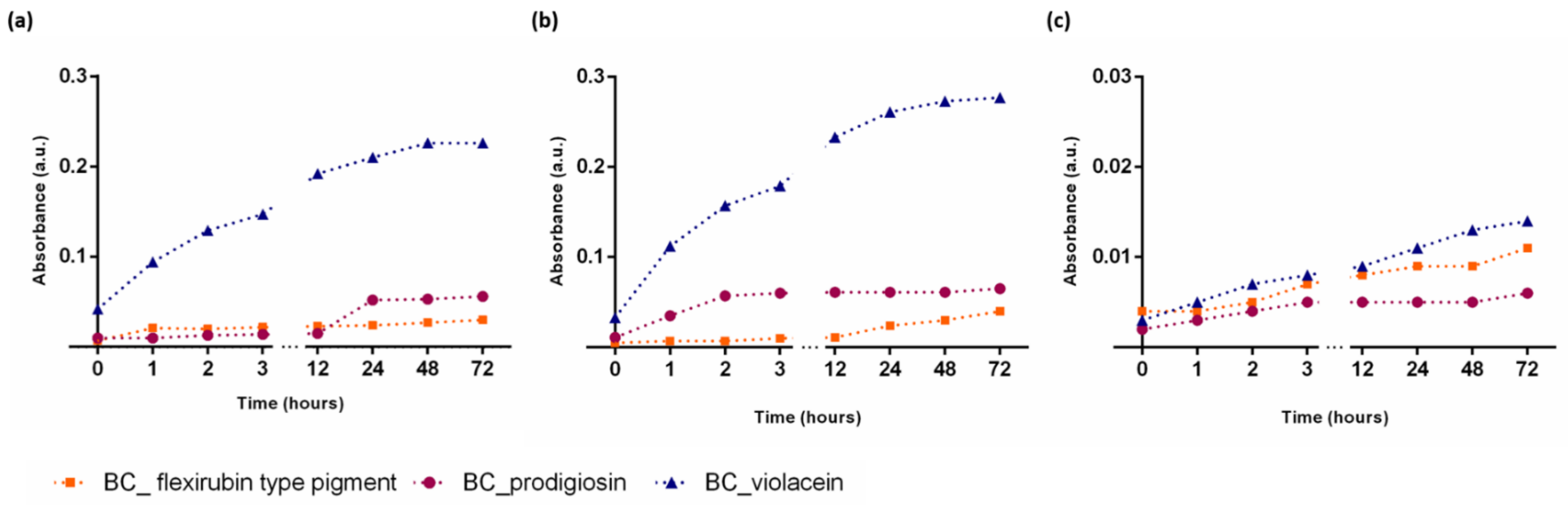
3.5. Release of Violacein, Prodigiosin, and Flexirubin-Type Pigment into Food Simulant Solutions
3.6. Color Stability of the pH Indicators Produced
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, M.; Ingle, A.P.; Gupta, I.; Pandit, R.; Paralikar, P.; Gade, A.; Chaud, M.V.; dos Santos, C.A. Smart Nanopackaging for the Enhancement of Food Shelf Life. Environ. Chem. Lett. 2019, 17, 277–290. [Google Scholar] [CrossRef]
- Balbinot-Alfaro, E.; Craveiro, D.V.; Lima, K.O.; Costa, H.L.G.; Lopes, D.R.; Prentice, C. Intelligent Packaging with PH Indicator Potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-Loaded Nanocarriers for Food Packaging Applications. Adv. Colloid. Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef]
- Tran, T. Active Antioxidant Additives in Sustainable Food Packaging. In Sustainable Food Packaging Technology; Wiley: Hoboken, NJ, USA, 2021; pp. 349–367. [Google Scholar]
- Almasi, H.; Jahanbakhsh Oskouie, M.; Saleh, A. A Review on Techniques Utilized for Design of Controlled Release Food Active Packaging. Crit. Rev. Food Sci. Nutr. 2020, 61, 2601–2621. [Google Scholar] [CrossRef] [PubMed]
- Terra, A.L.M.; Moreira, J.B.; Costa, J.A.V.; de Morais, M.G. Development of Time-PH Indicator Nanofibers from Natural Pigments: An Emerging Processing Technology to Monitor the Quality of Foods. LWT 2021, 142, 111020. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; Mohammadian, E.; Ehsani, A.; Khaniki, G.J.; Priyadarshi, R.; Rhim, J.-W. PH-Responsive Color Indicator Films Based on Methylcellulose/Chitosan Nanofiber and Barberry Anthocyanins for Real-Time Monitoring of Meat Freshness. Int. J. Biol. Macromol. 2021, 166, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Tavassoli, M.; McClements, D.J.; Hamishehkar, H. Multifunctional Halochromic Packaging Materials: Saffron Petal Anthocyanin Loaded-Chitosan Nanofiber/Methyl Cellulose Matrices. Food Hydrocoll. 2021, 111, 106237. [Google Scholar] [CrossRef]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in Smart Packaging Concepts for Food: An Extensive Review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef]
- Koxmak, S.; Yimamumaimaiti, T.; Abdukeremu, H.; Nizamidin, P.; Yimit, A. Detection of Amines in Lamb Spoilage by Optical Waveguide Sensor Based on Bromophenol Blue-Silicon Composite Film. Chem. Res. Chin. Univ. 2019, 35, 193–199. [Google Scholar] [CrossRef]
- Morsy, M.K.; Zór, K.; Kostesha, N.; Alstrøm, T.S.; Heiskanen, A.; El-Tanahi, H.; Sharoba, A.; Papkovsky, D.; Larsen, J.; Khalaf, H.; et al. Development and Validation of a Colorimetric Sensor Array for Fish Spoilage Monitoring. Food Control 2016, 60, 346–352. [Google Scholar] [CrossRef]
- Pacquit, A.; Lau, K.T.; McLaughlin, H.; Frisby, J.; Quilty, B.; Diamond, D. Development of a Volatile Amine Sensor for the Monitoring of Fish Spoilage. Talanta 2006, 69, 515–520. [Google Scholar] [CrossRef]
- Kuswandi, B.; Jayus; Restyana, A.; Abdullah, A.; Heng, L.Y.; Ahmad, M. A Novel Colorimetric Food Package Label for Fish Spoilage Based on Polyaniline Film. Food Control 2012, 25, 184–189. [Google Scholar] [CrossRef]
- Yusuf, M.; Shabbir, M.; Mohammad, F. Natural Colorants: Historical, Processing and Sustainable Prospects. Nat. Prod. Bioprospect. 2017, 7, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, F.; Zhan, J. Introduction of Natural Pigments from Microorganisms. In Bio-Pigmentation and Biotechnological Implementations; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–22. [Google Scholar]
- Ebrahimi Tirtashi, F.; Moradi, M.; Tajik, H.; Forough, M.; Ezati, P.; Kuswandi, B. Cellulose/Chitosan PH-Responsive Indicator Incorporated with Carrot Anthocyanins for Intelligent Food Packaging. Int. J. Biol. Macromol. 2019, 136, 920–926. [Google Scholar] [CrossRef]
- Pourjavaher, S.; Almasi, H.; Meshkini, S.; Pirsa, S.; Parandi, E. Development of a Colorimetric PH Indicator Based on Bacterial Cellulose Nanofibers and Red Cabbage (Brassica oleraceae) Extract. Carbohydr. Polym. 2017, 156, 193–201. [Google Scholar] [CrossRef]
- Ezati, P.; Bang, Y.-J.; Rhim, J.-W. Preparation of a Shikonin-Based PH-Sensitive Color Indicator for Monitoring the Freshness of Fish and Pork. Food Chem. 2021, 337, 127995. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, X.; Zhang, J.; Yang, Z.; Sun, Y.; Li, Z.; Huang, X.; Holmes, M.; Gong, Y.; Povey, M.; et al. Extruded Low Density Polyethylene-Curcumin Film: A Hydrophobic Ammonia Sensor for Intelligent Food Packaging. Food Packag. Shelf Life 2020, 26, 100595. [Google Scholar] [CrossRef]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an Alternate Biofactory for Carotenoid Production: A Review of Its Applications, Opportunities and Challenges. J. Funct. Foods 2020, 67, 103867. [Google Scholar] [CrossRef]
- Nambela, L.; Haule, L.V.; Mgani, Q. A Review on Source, Chemistry, Green Synthesis and Application of Textile Colorants. J. Clean Prod. 2020, 246, 119036. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial Cellulose as a Biodegradable Food Packaging Material: A Review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- De Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and Bacterial Nanocellulose: Production, Properties and Applications in Medicine, Food, Cosmetics, Electronics and Engineering. A Review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in Bio-Based Food Packaging Applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Zhai, X.; Lin, D.; Liu, D.; Yang, X. Emulsions Stabilized by Nanofibers from Bacterial Cellulose: New Potential Food-Grade Pickering Emulsions. Food Res. Int. 2018, 103, 12–20. [Google Scholar] [CrossRef]
- Paximada, P.; Koutinas, A.A.; Scholten, E.; Mandala, I.G. Effect of Bacterial Cellulose Addition on Physical Properties of WPI Emulsions. Comparison with Common Thickeners. Food Hydrocoll. 2016, 54, 245–254. [Google Scholar] [CrossRef]
- Giacomelli, F.O. Avaliação Das Condições de Crescimento Da Serratia Plymuthica Para Produção de Prodigiosina; University of Beira Interior: Covilhã, Portugal, 2018. [Google Scholar]
- Elkenawy, N.M.; Yassin, A.S.; Elhifnawy, H.N.; Amin, M.A. Optimization of Prodigiosin Production by Serratia Marcescens Using Crude Glycerol and Enhancing Production Using Gamma Radiation. Biotechnol. Rep. 2017, 14, 47–53. [Google Scholar] [CrossRef]
- Ahmad, W.A.; Yusof, N.Z.; Nordin, N.; Zakaria, Z.A.; Rezali, M.F. Production and Characterization of Violacein by Locally Isolated Chromobacterium Violaceum Grown in Agricultural Wastes. Appl. Biochem. Biotechnol. 2012, 167, 1220–1234. [Google Scholar] [CrossRef]
- Rettori, D.; Durán, N. Production, Extraction and Purificationof Violacein: An Antibiotic Pigment Producedby Chromobacterium Violaceum. World J. Microbiol. Biotechnol. 1998, 14, 685–688. [Google Scholar] [CrossRef]
- Mendes, A.; de Carvalho, J.; Duarte, M. Factorial Design and Response Surface Optimization of Crude Violacein for Chromobacterium violaceum Production. Biotechnol. Lett. 2001, 23, 1963–1969. [Google Scholar] [CrossRef]
- Venil, C.K.; Zakaria, Z.A.; Usha, R.; Ahmad, W.A. Isolation and Characterization of Flexirubin Type Pigment from Chryseobacterium sp. UTM-3T. Biocatal. Agric. Biotechnol. 2014, 3, 103–107. [Google Scholar] [CrossRef]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-Based Analysis of the Bacterial and Fungal Compositions of Multiple Kombucha (Tea Fungus) Samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Hwang, J.W.; Yang, Y.K.; Hwang, J.K.; Pyun, Y.R.; Kim, Y.S. Effects of PH and Dissolved Oxygen on Cellulose Production by Acetobacter Xylinum BRC5 in Agitated Culture. J. Biosci. Bioeng. 1999, 88, 183–188. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Catchmark, J.M.; Demirci, A. Effect of Different Additives on Bacterial Cellulose Production by Acetobacter Xylinum and Analysis of Material Property. Cellulose 2009, 16, 1033–1045. [Google Scholar] [CrossRef]
- Amorim, L.F.A.; Fangueiro, R.; Gouveia, I.C. Characterization of Bioactive Colored Materials Produced from Bacterial Cellulose and Bacterial Pigments. Materials 2022, 15, 2069. [Google Scholar] [CrossRef]
- Pandiyarajan, S.; Premasudha, P.; Kadirvelu, K. Bio-Production of Novel Water-Soluble Yellow Pigment from Aspergillus sp. and Exploring Its Sustainable Textile Applications. 3 Biotech 2018, 8, 398. [Google Scholar] [CrossRef]
- Jiménez, M.E.P.; Pinilla, C.M.B.; Rodrigues, E.; Brandelli, A. Extraction and Partial Characterisation of Antioxidant Pigment Produced by Chryseobacterium sp. Kr6. Nat. Prod. Res. 2019, 33, 1541–1549. [Google Scholar] [CrossRef]
- Razavi, R.; Molaei, R.; Moradi, M.; Tajik, H.; Ezati, P.; Shafipour Yordshahi, A. Biosynthesis of Metallic Nanoparticles Using Mulberry Fruit (Morus alba L.) Extract for the Preparation of Antimicrobial Nanocellulose Film. Appl. Nanosci. 2020, 10, 465–476. [Google Scholar] [CrossRef]
- Antony, J. Full Factorial Designs. In Design of Experiments for Engineers and Scientists; Elsevier: Amsterdam, The Netherlands, 2014; pp. 63–85. [Google Scholar]
- Kazachenko, A.S.; Malyar, Y.N.; Vasilyeva, N.Y.; Borovkova, V.S.; Issaoui, N. Optimization of Guar Gum Galactomannan Sulfation Process with Sulfamic Acid. Biomass Convers. Biorefin. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Emmanuel, E.; Yong, L.L.; Asadi, A.; Anggraini, V. Full-Factorial Two-Level Design in Optimizing the Contents of Olivine and Coir Fiber for Improving the Strength Property of a Soft Marine Clay. J. Nat. Fibers 2022, 19, 546–561. [Google Scholar] [CrossRef]
- Teja, S.P.S.; Damodharan, N. 23 Full Factorial Model for Particle Size Optimization of Methotrexate Loaded Chitosan Nanocarriers: A Design of Experiments (DoE) Approach. Biomed. Res. Int. 2018, 2018, 7834159. [Google Scholar] [CrossRef]
- Akar Sen, G. Application of Full Factorial Experimental Design and Response Surface Methodology for Chromite Beneficiation by Knelson Concentrator. Minerals 2016, 6, 5. [Google Scholar] [CrossRef]
- Dos Santos Silva, P.M.; Fiaschitello, T.R.; de Queiroz, R.S.; Freeman, H.S.; da Costa, S.A.; Leo, P.; Montemor, A.F.; da Costa, S.M. Natural Dye from Croton Urucurana Baill. Bark: Extraction, Physicochemical Characterization, Textile Dyeing and Color Fastness Properties. Dye. Pigment. 2020, 173, 107953. [Google Scholar] [CrossRef]
- Ren, Y.; Gong, J.; Fu, R.; Li, Z.; Yu, Z.; Lou, J.; Wang, F.; Zhang, J. Dyeing and Functional Properties of Polyester Fabric Dyed with Prodigiosins Nanomicelles Produced by Microbial Fermentation. J. Clean Prod. 2017, 148, 375–385. [Google Scholar] [CrossRef]
- Luo, M.R. CIELAB. In Encyclopedia of Color Science and Technology; Shamey, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–7. ISBN 978-3-642-27851-8. [Google Scholar]
- Hernández, B.; Sáenz, C.; Alberdi, C.; Diñeiro, J.M. CIELAB Color Coordinates versus Relative Proportions of Myoglobin Redox Forms in the Description of Fresh Meat Appearance. J. Food Sci. Technol. 2016, 53, 4159–4167. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour Difference ∆E—A Survey Mokrzycki. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial Nanocellulose—A Biobased Polymer for Active and Intelligent Food Packaging Applications: Recent Advances and Developments. Polymers 2020, 12, 2209. [Google Scholar] [CrossRef]
- Genovese, M.E.; Abraham, S.; Caputo, G.; Nanni, G.; Kumaran, S.K.; Montemagno, C.D.; Athanassiou, A.; Fragouli, D. Photochromic Paper Indicators for Acidic Food Spoilage Detection. ACS Omega 2018, 3, 13484–13493. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Asih, N.P.N.; Pratoko, D.K.; Kristiningrum, N.; Moradi, M. Edible PH Sensor Based on Immobilized Red Cabbage Anthocyanins into Bacterial Cellulose Membrane for Intelligent Food Packaging. Packag. Technol. Sci. 2020, 33, 321–332. [Google Scholar] [CrossRef]
- Mohammadalinejhad, S.; Almasi, H.; Moradi, M. Immobilization of Echium Amoenum Anthocyanins into Bacterial Cellulose Film: A Novel Colorimetric PH Indicator for Freshness/Spoilage Monitoring of Shrimp. Food Control 2020, 113, 107169. [Google Scholar] [CrossRef]
- Durán, N.; Justo, G.Z.; Ferreira, C.V.; Melo, P.S.; Cordi, L.; Martins, D. Violacein: Properties and Biological Activities. Biotechnol. Appl. Biochem. 2007, 48, 127. [Google Scholar]
- Williams, R.P.; Hearn, W.R. Prodigiosin. In Biosynthesis; Springer: Berlin/Heidelberg, Germany, 1967; pp. 410–432. [Google Scholar]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry—Challenges and the Way Forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef]

| Factors | Units | Code | Low Level (−1) | High Level (+1) |
|---|---|---|---|---|
| Functionalization temperature | °C | A | 40 | 90 |
| Functionalization duration | min | B | 20 | 60 |
| Pigment concentration | % | C | 5 | 30 |
| Pigment | Run | Independent Variables | Response: Color Strength (K/S) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Replicates | Mean | SD | |||||||
| A | B | C | I | II | III | ||||
| Violacein | 1 | −1 | −1 | −1 | 1.55 | 1.22 | 1.55 | 1.44 | 0.190526 |
| 2 | −1 | −1 | 1 | 3.61 | 3.59 | 4.05 | 3.75 | 0.260000 | |
| 3 | −1 | 1 | −1 | 1.63 | 1.61 | 1.62 | 1.62 | 0.010000 | |
| 4 | −1 | 1 | 1 | 4.35 | 4.07 | 4.42 | 4.28 | 0.185203 | |
| 5 | 1 | −1 | −1 | 1.36 | 1.49 | 1.71 | 1.52 | 0.176918 | |
| 6 | 1 | −1 | 1 | 6.40 | 5.79 | 5.71 | 5.97 | 0.377403 | |
| 7 | 1 | 1 | −1 | 2.15 | 2.05 | 2.30 | 2.17 | 0.125831 | |
| 8 | 1 | 1 | 1 | 6.47 | 6.46 | 6.62 | 6.52 | 0.089629 | |
| 9 | 0 | 0 | 0 | 3.35 | 3.41 | 3.53 | 3.51 | 0.210898 | |
| 10 | 0 | 0 | 0 | 3.36 | 3.34 | 3.96 | |||
| 11 | 0 | 0 | 0 | 3.33 | 3.67 | 3.60 | |||
| Prodigiosin | 1 | −1 | −1 | −1 | 0.30 | 0.29 | 0.48 | 0.36 | 0.106927 |
| 2 | −1 | −1 | 1 | 1.15 | 1.69 | 1.34 | 1.39 | 0.273922 | |
| 3 | −1 | 1 | −1 | 0.45 | 0.49 | 0.44 | 0.46 | 0.026458 | |
| 4 | −1 | 1 | 1 | 1.83 | 1.62 | 1.96 | 1.80 | 0.171561 | |
| 5 | 1 | −1 | −1 | 0.70 | 0.78 | 0.86 | 0.78 | 0.080000 | |
| 6 | 1 | −1 | 1 | 2.74 | 2.61 | 2.76 | 2.70 | 0.081445 | |
| 7 | 1 | 1 | −1 | 0.67 | 0.94 | 0.87 | 0.83 | 0.140119 | |
| 8 | 1 | 1 | 1 | 2.94 | 2.98 | 2.97 | 2.96 | 0.020817 | |
| 9 | 0 | 0 | 0 | 1.97 | 1.61 | 1.45 | 1.55 | 0.243641 | |
| 10 | 0 | 0 | 0 | 1.78 | 1.64 | 1.48 | |||
| 11 | 0 | 0 | 0 | 1.57 | 1.24 | 1.20 | |||
| Flexirubin-type pigment | 1 | −1 | −1 | −1 | 0.10 | 0.11 | 0.11 | 0.11 | 0.005774 |
| 2 | −1 | −1 | 1 | 0.24 | 0.21 | 0.20 | 0.22 | 0.020817 | |
| 3 | −1 | 1 | −1 | 0.13 | 0.12 | 0.13 | 0.13 | 0.005774 | |
| 4 | −1 | 1 | 1 | 0.23 | 0.24 | 0.20 | 0.22 | 0.020817 | |
| 5 | 1 | −1 | −1 | 0.56 | 0.56 | 0.47 | 0.53 | 0.051962 | |
| 6 | 1 | −1 | 1 | 1.50 | 1.06 | 1.43 | 1.33 | 0.236432 | |
| 7 | 1 | 1 | −1 | 0.51 | 0.45 | 0.60 | 0.52 | 0.075498 | |
| 8 | 1 | 1 | 1 | 1.40 | 1.13 | 1.02 | 1.18 | 0.195533 | |
| 9 | 0 | 0 | 0 | 0.28 | 0.25 | 0.27 | 0.36 | 0.072648 | |
| 10 | 0 | 0 | 0 | 0.40 | 0.38 | 0.35 | |||
| 11 | 0 | 0 | 0 | 0.44 | 0.39 | 0.44 | |||
| Pigment | Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Remarks |
|---|---|---|---|---|---|---|---|
| Violacein | Model | 87.60 | 4 | 21.90 | 574.15 | <0.0001 | Significant |
| Temperature | 9.68 | 1 | 9.68 | 253.71 | <0.0001 | Significant | |
| Duration | 1.36 | 1 | 1.36 | 35.74 | <0.0001 | Significant | |
| Concentration | 71.07 | 1 | 71.07 | 1863.20 | <0.0001 | Significant | |
| Temperature/Concentration | 5.49 | 1 | 5.49 | 143.96 | <0.0001 | Significant | |
| Curvature | 0.063 | 1 | 0.063 | 1.65 | 0.2107 | Not significant | |
| Residual | 0.95 | 25 | 0.038 | - | - | - | |
| Lack of Fit | 0.79 | 19 | 0.041 | 1.48 | 0.3281 | Not significant | |
| Pure Error | 0.17 | 6 | 0.028 | - | - | - | |
| Prodigiosin | Model | 20.85 | 4 | 5.21 | 146.26 | <0.0001 | Significant |
| Temperature | 3.99 | 1 | 3.99 | 111.84 | <0.0001 | Significant | |
| Duration | 0.25 | 1 | 0.25 | 7.08 | 0.0134 | Significant | |
| Concentration | 15.55 | 1 | 15.55 | 436.43 | <0.0001 | Significant | |
| Temperature/Concentration | 1.06 | 1 | 1.06 | 29.70 | <0.0001 | Significant | |
| Curvature | 0.12 | 1 | 0.12 | 3.50 | 0.0731 | Not significant | |
| Residual | 0.89 | 25 | 0.036 | - | - | - | |
| Lack of Fit | 0.66 | 19 | 0.035 | 0.93 | 0.5913 | Not significant | |
| Pure Error | 0.23 | 6 | 0.038 | - | - | - | |
| Flexirubin-type pigment | Model | 19.09 | 2 | 9.54 | 369.86 | <0.0001 | Significant |
| Temperature | 15.69 | 1 | 15.69 | 608.07 | <0.0001 | Significant | |
| Concentration | 3.40 | 1 | 3.40 | 131.65 | <0.0001 | Significant | |
| Curvature | 0.005 | 1 | 0.005 | 0.19 | 0.6654 | Not significant | |
| Residual | 0.70 | 27 | 0.026 | - | - | - | |
| Lack of Fit | 0.34 | 21 | 0.016 | 0.27 | 0.9884 | Not significant | |
| Pure Error | 0.36 | 6 | 0.060 | - | - | - |
| BC_Violacein | BC_Prodigiosin | BC_Flexirubin-Type Pigment | ||
|---|---|---|---|---|
| Apparent color |  |  |  | |
| Reflectance (%R) | 8.37 ± 0.05 | 16.12 ± 0.40 | 25.88 ± 0.70 | |
| Color strength (K/S) | 5.02 ± 0.03 | 2.18 ± 0.07 | 1.06 ± 0.05 | |
| Color strength (K/S) predicted | 4.98 95% CI [4.85, 5.12] | 2.24 95% CI [2.10, 2.37] | 1.05 95% CI [0.95, 1.17] | |
| CIELAB system colorimetric parameters | L* | 40.33 ± 0.24 | 62.73 ± 1.00 | 81.23 ± 0.42 |
| a* | 6.13 ± 0.26 | 29.30 ± 1.28 | 5.83 ± 0.98 | |
| b* | −25.98 ± 0.78 | −5.22 ± 0.61 | 36.71 ± 3.31 | |
| Chroma (C*) | 26.69 ± 0.82 | 29.75 ± 1.37 | 34.17 ± 4.00 | |
| Hue angle (°) | 276.6 ± 11.67 | 349.92 ± 0.78 | 81.02 ± 0.74 | |
| pH | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | |||||||||||||||
| Initial |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| L* | 40.33 ± 0.24 | 49.99 ± 1.34 | 47.83 ± 0.88 | 47.86 ± 0.28 | 48.21 ± 0.06 | 48.25 ± 1.18 | 46.76 ± 2.10 | 46.91 ± 1.30 | 45.88 ± 1.91 | 45.47 ± 1.94 | 39.50 ± 1.45 | 44.21 ± 0.21 | 48.08 ± 0.82 | 46.61 ± 0.59 | 54.11 ± 3.13 |
| a* | 6.13 ± 0.26 | 2.96 ± 0.22 | 1.48 ± 0.06 | 3.48 ± 0.11 | 6.02 ± 0.13 | 6.75 ± 0.02 | 6.81 ± 0.70 | 6.41 ± 0.40 | 5.53 ± 0.21 | 6.28 ± 0.17 | 7.03 ± 0.43 | 7.31 ± 0.18 | 3.78 ± 0.99 | −14.28 ± 0.27 | −17.55 ± 2.41 |
| b* | −25.98 ± 0.78 | −14.02 ± 1.33 | −14.65 ± 0.97 | −20.33 ± 0.20 | −22.11 ± 0.09 | −23.44 ± 0.14 | −22.27 ± 0.37 | −22.85 ± 0.86 | −21.32 ± 0.60 | −21.24 ± 0.13 | −23.73 ± 1.22 | −24.76 ± 0.40 | −24.53 ± 0.18 | −17.55 ± 0.09 | 3.06 ± 1.79 |
| ΔE | 0 | 15.70 ± 1.88 | 14.39 ± 0.32 | 9.78 ± 0.30 | 8.78 ± 0.09 | 8.34 ± 1.08 | 7.53 ± 1.67 | 7.30 ± 1.52 | 7.30 ± 1.82 | 7.05 ± 1.51 | 2.76 ± 1.29 | 4.24 ± 0.25 | 8.24 ± 1.09 | 22.96 ± 0.44 | 40.03 ± 0.95 |
| After light exposure | Control | ||||||||||||||
 |  |  |  |  |  |  |  |  |  |  |  |  |  |  | |
| L* | 44.59 ± 0.13 | 45.02 ± 1.29 | 47.18 ± 2.02 | 48.56 ± 1.14 | 49.48 ± 0.28 | 37.16 ± 1.95 | 37.92 ± 1.27 | 38.32 ± 0.88 | 38.49 ± 0.39 | 40.14 ± 1.05 | 39.84 ± 0.92 | 42.68 ± 1.69 | 42.71 ± 0.71 | 44.38 ± 1.82 | 49.00 ± 0.25 |
| a* | 3.32 ± 0.11 | 2.78 ± 0.25 | 2.54 ± 0.25 | 2.73 ± 0.01 | 3.51 ± 0.04 | 5.43 ± 0.26 | 5.65 ± 0.16 | 5.58 ± 0.54 | 4.86 ± 0.05 | 5.04 ± 0.06 | 2.68 ± 0.05 | 6.78 ± 0.11 | 0.01 ± 1.88 | −11.95 ± 1.48 | −16.66 ± 0.81 |
| b* | −21.79 ± 0.42 | −13.64 ± 1.06 | −14.09 ± 1.20 | −14.64 ± 0.41 | −16.15 ± 0.37 | −21.89 ± 0.21 | −22.19 ± 0.03 | −24.21 ± 0.79 | −19.78 ± 0.45 | −19.78 ± 0.45 | −20.11 ± 0.40 | −25. 81 ± 0.20 | −22.60 ± 0.22 | −11.20 ± 0.15 | 1.53 ± 0.76 |
| ΔE | 6.61 ± 0.24 | 8.23 ± 1.14 | 8.24 ± 1.78 | 8.22 ± 0.91 | 7.47 ± 0.46 | 7.74 ± 1.80 | 7.07 ± 1.25 | 7.09 ± 1.22 | 6.57 ± 0.30 | 5.21 ± 0.70 | 5.08 ± 0.99 | 5.75 ± 0.63 | 3.93 ± 1.88 | 18.64 ± 1.27 | 31.03 ± 0.09 |
| After storage at 4 °C | Control | ||||||||||||||
 |  |  |  |  |  |  |  |  |  |  |  |  |  |  | |
| L* | 42.61 ± 0.07 | 54.62 ± 0.82 | 51.35 ± 0.90 | 39.89 ± 1.56 | 39.10 ± 1.42 | 48.20 ± 0.86 | 48.61 ± 1.76 | 49.21 ± 0.49 | 47.03 ± 0.47 | 45.32 ± 0.46 | 41.19 ± 2.33 | 42.36 ± 1.32 | 48.74 ± 0.12 | 45.38 ± 0.25 | 53.39 ± 2.35 |
| a* | 3.39 ± 0.40 | 3.21 ± 0.16 | 4.89 ± 0.11 | 1.33 ± 0.11 | 3.29 ± 0.06 | 5.19 ± 0.33 | 6.34 ± 0.44 | 5.96 ± 0.35 | 5.98 ± 0.07 | 6.38 ± 0.10 | 7.25 ± 0.28 | 6.30 ± 0.35 | 0.52 ± 1.28 | −13.59 ± 0.33 | −18.57 ± 1.49 |
| b* | −22.44 ± 1.17 | −13.80 ± 0.52 | −14.96 ± 0.82 | −13.93 ± 0.76 | −15.32 ± 0.76 | −18.01 ± 0.82 | −18.66 ± 0.29 | −18.85 ± 0.02 | −19.01 ± 0.78 | −19.46 ± 0.37 | −20.64 ± 0.18 | −20.54 ± 0.41 | −20.30 ± 0.91 | −14.43 ± 1.63 | 3.35 ± 1.63 |
| ΔE | 5.04 ± 1.07 | 14.80 ± 0.97 | 11.60 ± 1.19 | 9.26 ± 0.26 | 8.03 ± 0.05 | 7.37 ± 1.06 | 7.73 ± 1.39 | 7.95 ± 0.29 | 6.17 ± 0.81 | 5.03 ± 0.41 | 4.75 ± 0.86 | 3.62 ± 0.16 | 7.17 ± 0.68 | 19.01 ± 0.43 | 35.61 ± 097 |
| pH | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | |||||||||||||||
| Initial |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| L* | 62.73 ± 1.00 | 65.97 ± 0.54 | 59.93 ± 0.30 | 64.08 ± 1.94 | 62.04 ± 1.34 | 62.00 ± 3.06 | 61.98 ± 0.24 | 63.89 ± 1.99 | 64.67 ± 0.88 | 64.75 ± 0.99 | 64.90 ± 0.63 | 71.19 ± 1.29 | 68.12 ± 0.49 | 67.47 ± 2.26 | 67.69 ± 0.66 |
| a* | 29.3 ± 1.28 | 27.23 ± 0.87 | 33.20 ± 0.29 | 33.26 ± 1.81 | 31.27 ± 1.70 | 31.42 ± 3.80 | 32.06 ± 1.07 | 24.72 ± 2.82 | 25.46 ± 2.68 | 26.10 ± 0.43 | 23.07 ± 0.45 | 17.89 ± 0.46 | 19.38 ± 1.73 | 14.44 ± 1.15 | 15.17 ± 1.41 |
| b* | −5.22 ± 0.61 | −0.20 ± 0.91 | −1.74 ± 0.92 | −2.74 ± 1.69 | −1.86 ± 0.30 | −2.18 ± 0.49 | 2.33 ± 0.03 | 4.71 ± 0.78 | 6.07 ± 1.62 | 8.82 ± 1.39 | 9.72 ± 0.50 | 11.96 ± 0.76 | 21. 62 ± 1.06 | 27.03 ± 0.18 | 29.47 ± 0.11 |
| ΔE | 0 | 6.33 ± 1.28 | 5.96 ± 0.49 | 5.35 ± 0.07 | 4.21 ± 0.77 | 4.98 ± 1.77 | 8.10 ± 0.36 | 11.18 ± 2.06 | 12.17 ± 2.49 | 14.57 ± 1.29 | 16.34 ± 0.55 | 22. 30 ± 1.31 | 29.13 ± 1.65 | 35.86 ± 0.93 | 37.80 ± 0.72 |
| After Light exposure | Control | ||||||||||||||
 |  |  |  |  |  |  |  |  |  |  |  |  |  |  | |
| L* | 61.36 ± 0.28 | 67.99 ± 0.92 | 66.79 ± 1.19 | 67.17 ± 0.07 | 62.50 ± 0.76 | 65.84 ± 0.18 | 61.37 ± 0.27 | 62.39 ± 0.28 | 66.23 ± 0.37 | 68.98 ± 0.20 | 71.61 ± 1.36 | 72.55 ± 0.79 | 68.18 ± 0.47 | 64.17 ± 0.01 | 69.12 ± 1.00 |
| a* | 20.48 ± 0.28 | 14.96 ± 0.24 | 17.25 ± 0.58 | 16.34 ± 0.83 | 17.06 ± 0.76 | 17.80 ± 0.66 | 20.27 ± 0.18 | 20.30 ± 0.60 | 15.28 ± 0.74 | 13.63 ± 0.35 | 13.33 ± 1.53 | 12.12 ± 0.16 | 13.66 ± 1.13 | 15.76 ± 0.23 | 14.11 ± 0.84 |
| b* | 1.1 ± 0.23 | 7.87 ± 0.69 | 5.06 ± 0.04 | 4.16 ± 0.62 | 2.46 ± 0.40 | 3.85 ± 1.41 | 7.87 ± 0.19 | 7.93 ± 0.54 | 9.99 ± 0.67 | 9.91 ± 1.15 | 10.10 ± 1.14 | 8.72 ± 0.04 | 16.26 ± 1.65 | 22.75 ± 0.88 | 21.84 ± 0.20 |
| ΔE | 10.94 ± 0.32 | 10.99 ± 0.25 | 7.48 ± 1.09 | 7.78 ± 0.74 | 3.87 ± 1.03 | 5.97 ± 0.81 | 6.77 ± 0.18 | 6.92± 0.59 | 11.39 ± 1.02 | 13.53 ± 0.81 | 15.40 ± 2.28 | 15.92 ± 0.62 | 17.97 ± 2.00 | 22.34 ± 0.90 | 23.06 ± 0.39 |
| After storage at 4 °C | Control | ||||||||||||||
 |  |  |  |  |  |  |  |  |  |  |  |  |  |  | |
| L* | 60.19 ± 0.16 | 59.81 ± 1.71 | 62.48 ± 2.06 | 63.70 ± 0.34 | 60.87 ± 0.44 | 63.83 ± 0.37 | 63.72 ± 0.57 | 62.88 ± 2.74 | 67.01 ± 2.34 | 63.46 ± 0.10 | 63.05 ± 0.83 | 68.38 ± 1.22 | 66.62 ± 1.63 | 68.93 ± 0.61 | 69.11 ± 0.78 |
| a* | 30.67 ± 1.14 | 33.07 ± 1.71 | 30.64 ± 1.73 | 29.62 ± 0.29 | 33.00 ± 1.70 | 33.34 ± 1.22 | 31.14 ± 0.06 | 35.23 ± 1.15 | 29.56 ± 2.74 | 27.44 ± 0.30 | 26.73 ± 0.81 | 21.65 ± 0.40 | 20.36 ± 0.34 | 17.59 ± 0.64 | 16.12 ± 1.07 |
| b* | −5.35 ± 0.54 | 0.59 ± 0.13 | −0.12 ± 1.13 | −1.08 ± 0.23 | −2.19 ± 0.01 | −2.14 ± 0.09 | 1.61 ± 0.34 | 0.72 ± 0.08 | 3.98 ± 0.33 | 7.29 ± 0.18 | 8.46 ± 0.16 | 11.47 ± 0.20 | 21.71 ± 1.20 | 27.40 ± 0.37 | 27.95 ± 1.48 |
| ΔE | 2.98 ± 0.68 | 6.63 ± 0.61 | 6.07 ± 0.19 | 5.63 ± 0.44 | 4.13 ± 0.88 | 4.30 ± 0.64 | 7.83 ± 0.05 | 8.28 ± 0.21 | 11.80 ± 1.87 | 13.45 ± 0.22 | 14.65 ± 0.53 | 20.78 ± 0.49 | 29.69 ± 0.86 | 36.33 ± 0.26 | 37.44 ± 0.72 |
| pH | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | |||||||||||||||
| Initial |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| L* | 81.23 ± 0.42 | 79.78 ± 1.06 | 80.83 ± 0.24 | 83.79 ± 1.34 | 80.24 ± 0.60 | 79.36 ± 0.23 | 82.96 ± 0.24 | 80.06 ± 0.58 | 81.12 ± 1.61 | 82.07 ± 0.13 | 82.13 ± 0.80 | 77.36 ± 0.00 | 79.18 ± 3.41 | 79.15 ±1.33 | 76.69 ± 0.35 |
| a* | 5.83 ± 0.98 | 5.15 ± 0.39 | 4.24 ± 0.40 | 4.24 ± 1.29 | 4.28 ± 0.91 | 5.37 ± 0.45 | 4.94 ± 0.37 | 6.47 ± 0.62 | 5.54 ± 0.84 | 4.93 ± 0.23 | 3.79 ± 0.41 | 6.51 ± 0.25 | 7.96 ± 2.84 | 9.06 ± 1.21 | 11.29 ± 0.54 |
| b* | 36.71 ± 3.31 | 29.91 ± 0.59 | 29.63 ± 0.26 | 29.58 ± 4.43 | 29.36 ±2.50 | 30.15 ± 1.14 | 33.23 ± 1.32 | 39.59 ± 1.04 | 30.45 ± 1.99 | 29.50 ± 1.85 | 27.00 ± 1.34 | 25.06 ± 1.12 | 25.77 ± 2.57 | 21.65 ± 0.70 | 19.31 ± 0.35 |
| ΔE | 0 | 7.04 ± 0.39 | 7.28 ± 0.33 | 7.75 ± 4.78 | 7.61 ± 2.52 | 6.86 ± 1.06 | 4.00 ± 1.34 | 3.18 ± 1.28 | 6.40 ± 1.96 | 7.32 ± 1.86 | 9.97 ± 1.46 | 12.30 ± 1.05 | 11.86 ± 1.27 | 15.60 ± 0.25 | 18.18 ± 0.25 |
| After light exposure | Control | ||||||||||||||
 |  |  |  |  |  |  |  |  |  |  |  |  |  |  | |
| L* | 81.19 ± 0.21 | 80.10 ± 0.53 | 79.68 ± 0.52 | 78.98 ± 0.01 | 76.29 ± 0.13 | 77.78 ± 0.36 | 83.75 ± 0.86 | 84.91 ± 0.40 | 84.60 ± 0.40 | 79.38 ± 0.38 | 75.79 ± 0.22 | 73.81 ± 0.91 | 72.62 ± 0.11 | 69.93 ± 0.95 | 69.15 ±0.06 |
| a* | 4.80 ± 0.13 | 7.02 ± 0.11 | 7.24 ± 0.16 | 7.20 ± 0.16 | 6.53 ± 0.13 | 6.54 ± 0.78 | 2.82 ± 0.64 | 2.89 ± 0.41 | 3.72 ± 0.17 | 8.17 ± 0.22 | 8.73 ± 0.17 | 9.85 ± 1.03 | 10.77 ± 0.27 | 13.53 ± 0.70 | 13.91 ± 0.10 |
| b* | 24.05 ± 0.12 | 31.10 ± 0.66 | 30.02 ± 0.74 | 29.99 ± 0.84 | 23.87 ± 1.45 | 22.89 ± 1.97 | 22.94 ± 1.70 | 25.09 ± 1.39 | 27.53 ± 0.47 | 29.80 ±2.06 | 30.56 ± 0.40 | 20.52 ± 1.27 | 21.38 ± 0.64 | 19.42 ± 0.57 | 20.37 ± 0.25 |
| ΔE | 12.71 ± 0.13 | 7.48 ± 0.74 | 6.65 ± 0.60 | 6.79 ± 0.69 | 5.30 ± 0.02 | 4.28 ± 0.08 | 3.55 ± 1.51 | 4.44 ± 0.18 | 5.02 ± 0.02 | 6.93 ± 1.91 | 9.33 ±0.23 | 9.63 ± 1.70 | 10.79 ± 0.10 | 14.99 ± 0.94 | 15.54 ± 0.07 |
| After storage at 4 °C | Control | ||||||||||||||
 |  |  |  |  |  |  |  |  |  |  |  |  |  |  | |
| L* | 79.75 ± 0.13 | 80.00 ± 0.37 | 79.95 ± 0.54 | 73.86 ± 1.36 | 74.00 ± 0.46 | 75.19 ± 0.43 | 80.90 ± 0.54 | 81.63 ± 0.21 | 74.57 ± 1.28 | 74.34 ± 0.23 | 73.92 ± 0.36 | 68.73 ± 0.37 | 67.94 ± 0.73 | 67.24 ± 0.16 | 66.68 ± 1.01 |
| a* | 6.62 ± 0.23 | 6.39 ± 0.09 | 6.77 ± 0.74 | 8.54 ± 0.61 | 8.61 ± 0.23 | 8.00 ± 0.18 | 4.33 ± 0.39 | 4.31 ± 0.05 | 9.53 ± 0.59 | 9.52 ± 0.27 | 9.83 ± 0.17 | 9.15 ± 0.33 | 11.15 ± 0.01 | 14.11 ± 0.45 | 14.29 ± 0.48 |
| b* | 30.19 ± 0.51 | 37.18 ± 0.55 | 36.22 ± 0.13 | 28.03 ± 0.25 | 28.41 ± 0.27 | 28.20 ± 0.31 | 27.43 ± 0.51 | 30.41 ± 0.83 | 32.17 ± 2.52 | 32.19 ± 0.61 | 33.31 ± 0.97 | 25.81 ± 1.55 | 24.57 ± 0.45 | 25.24 ± 1.57 | 22.36 ± 2.25 |
| ΔE | 6.74 ± 0.44 | 7.00 ± 0.56 | 6.07 ± 0.13 | 6.58 ± 1.31 | 6.35 ± 0.41 | 5.18 ± 0.31 | 3.77 ± 0.77 | 3.05 ± 0.15 | 6.44 ± 2.09 | 6.47 ± 0.50 | 7.37 ± 0.77 | 12.18 ± 0.16 | 13.85 ± 0.81 | 15.44 ± 0.59 | 17.16 ± 0.04 |
| Acetic Acid Vapors | Control | Ammonia Vapors | ||
|---|---|---|---|---|
| BC_violacein |  |  |  | |
| L* | 47.98 ± 0.86 | 40.33 ± 0.24 | 43.48 ± 0.28 | |
| a* | 0.88 ± 0.01 | 6.13 ± 0.26 | 2.85 ± 0.02 | |
| b* | −9.95 ± 0.22 | −25.98 ± 0.78 | −23.27 ± 0.13 | |
| ΔE | 18.53 ± 0.54 | 0 | 5.30 ± 0.12 | |
| BC_prodigiosin |  |  |  | |
| L* | 56.70 ± 1.08 | 62.73 ± 1.00 | 62.58 ± 1.25 | |
| a* | 36.68 ± 1.56 | 29.3 ± 1.28 | 23.42 ± 0.59 | |
| b* | −6.16 ± 0.93 | −5.22 ± 0.61 | 14.17 ± 0.81 | |
| ΔE | 9.59 ± 1.97 | 0 | 20.28 ± 0.61 | |
| BC_flexirubin-type pigment |  |  |  | |
| L* | 81.50 ± 0.13 | 81.23 ± 0.42 | 74.08 ± 0.40 | |
| a* | 4.70 ± 0.00 | 5.83 ± 0.98 | 10.47 ± 0.80 | |
| b* | 32.84 ± 0.16 | 36.71 ± 3.31 | 26.44 ± 0.19 | |
| ΔE | 4.05 ± 0.16 | 0 | 13.36 ± 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorim, L.F.A.; Gomes, A.P.; Gouveia, I.C. Design and Preparation of a Biobased Colorimetric pH Indicator from Cellulose and Pigments of Bacterial Origin, for Potential Application as Smart Food Packaging. Polymers 2022, 14, 3869. https://doi.org/10.3390/polym14183869
Amorim LFA, Gomes AP, Gouveia IC. Design and Preparation of a Biobased Colorimetric pH Indicator from Cellulose and Pigments of Bacterial Origin, for Potential Application as Smart Food Packaging. Polymers. 2022; 14(18):3869. https://doi.org/10.3390/polym14183869
Chicago/Turabian StyleAmorim, Lúcia F. A., Ana P. Gomes, and Isabel C. Gouveia. 2022. "Design and Preparation of a Biobased Colorimetric pH Indicator from Cellulose and Pigments of Bacterial Origin, for Potential Application as Smart Food Packaging" Polymers 14, no. 18: 3869. https://doi.org/10.3390/polym14183869
APA StyleAmorim, L. F. A., Gomes, A. P., & Gouveia, I. C. (2022). Design and Preparation of a Biobased Colorimetric pH Indicator from Cellulose and Pigments of Bacterial Origin, for Potential Application as Smart Food Packaging. Polymers, 14(18), 3869. https://doi.org/10.3390/polym14183869








