A Molecular Dynamics Simulation of Polymers’ Interactions with Kaolinite (010) Surfaces in Saline Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymers
2.2. Kaolinite Surface
2.3. Force Field
2.4. Initial Setup
- •
- The kaolinite surface with the (010) side exposed to the liquid was placed at one end of the simulation box at z = 0. As such, the simulation box was adjusted to the x and y dimensions while the z direction was extended to obtain a total of 12 nm. This finally generated the system dimension of (Lx, Ly, Lz) = (11.325, 10.01, 12.00) nm3 and (α,β,γ) = (90, 90, 72.2).
- •
- The polymer was placed in the void space at an average distance between the surface and its periodic image in the z-direction. The initial configurations of the polymers were previously obtained in simulations in water. The length of the polymers was 48 monomers for the PAM, HPAM, PAMPS, and PAA polymers, 32 monomers for the PEO polymer, and 12 monomers for the GUAR polymer. These values were chosen to generate a similar length between the polymers. The concentration of these polymers was 0.001 M and approximately 5 g/L, which was necessary in the simulation to model the adsorption process.
- •
- The ions needed to neutralize, and the salts were added randomly in the system’s free space, leaving 0.3 nm between the other molecules present. Two concentrations of NaCl were studied: 0.006 M and 0.6 M, to emulate fresh and saline water systems, respectively.
- •
- Water was added from an equilibrated 30 × 30 × 30 nm3 configuration at 300 K, which was inserted as blocks over the simulation box. Water molecules overlapping with other atoms in the system were removed. The criterion for overlap is a distance of less than 0.2 nm.
2.5. Molecular Simulation
- 1.
- Force minimization steps to relax the atoms from their initial positions until a tolerance of less than 10 kJ/mol/nm was achieved. The surface was constrained in its position where only the hydrogen atoms had free movement.
- 2.
- A NVT equilibration simulation for a time of 0.1 ns at a temperature of 300 K where only the water molecules moved.
- 3.
- A NVT annealing simulation was carried out with the free molecules and ions, and the temperature was increased rapidly from 300 to 450 K in a time of 0.001 ns, then held for 0.5 ns, and then cooled slowly from 450 to 300 K for 0.5 ns.
- 4.
- A NPT equilibration simulation was performed for 2 ns at 300 K and 1 bar, where the system matched its velocity and case size for the set temperature and pressure.
- 5.
- A NVT production simulation was carried out for 100 ns at 300 K.
2.6. Results Processing
3. Results
3.1. Total Polymer Interactions with the Surface
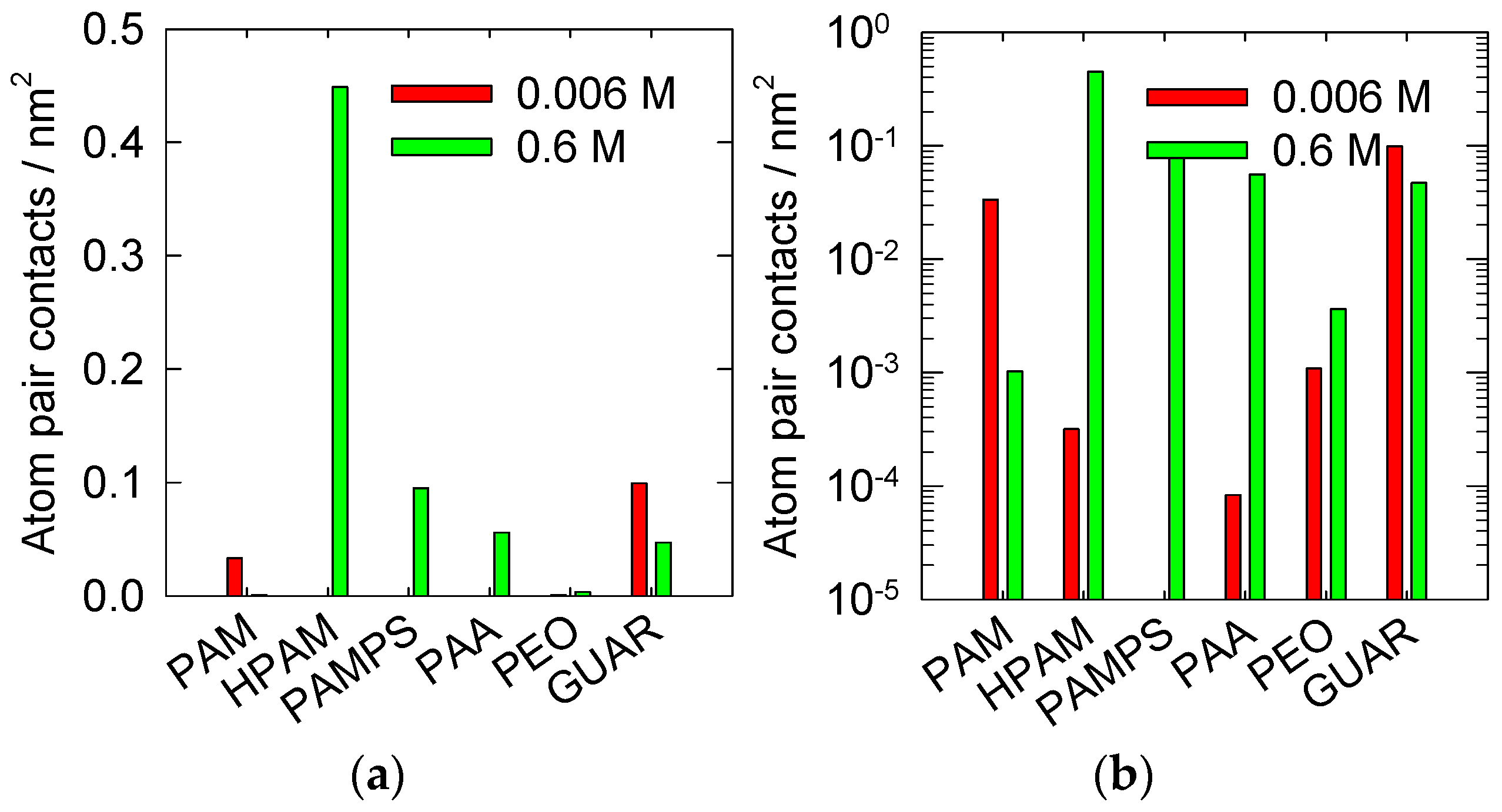
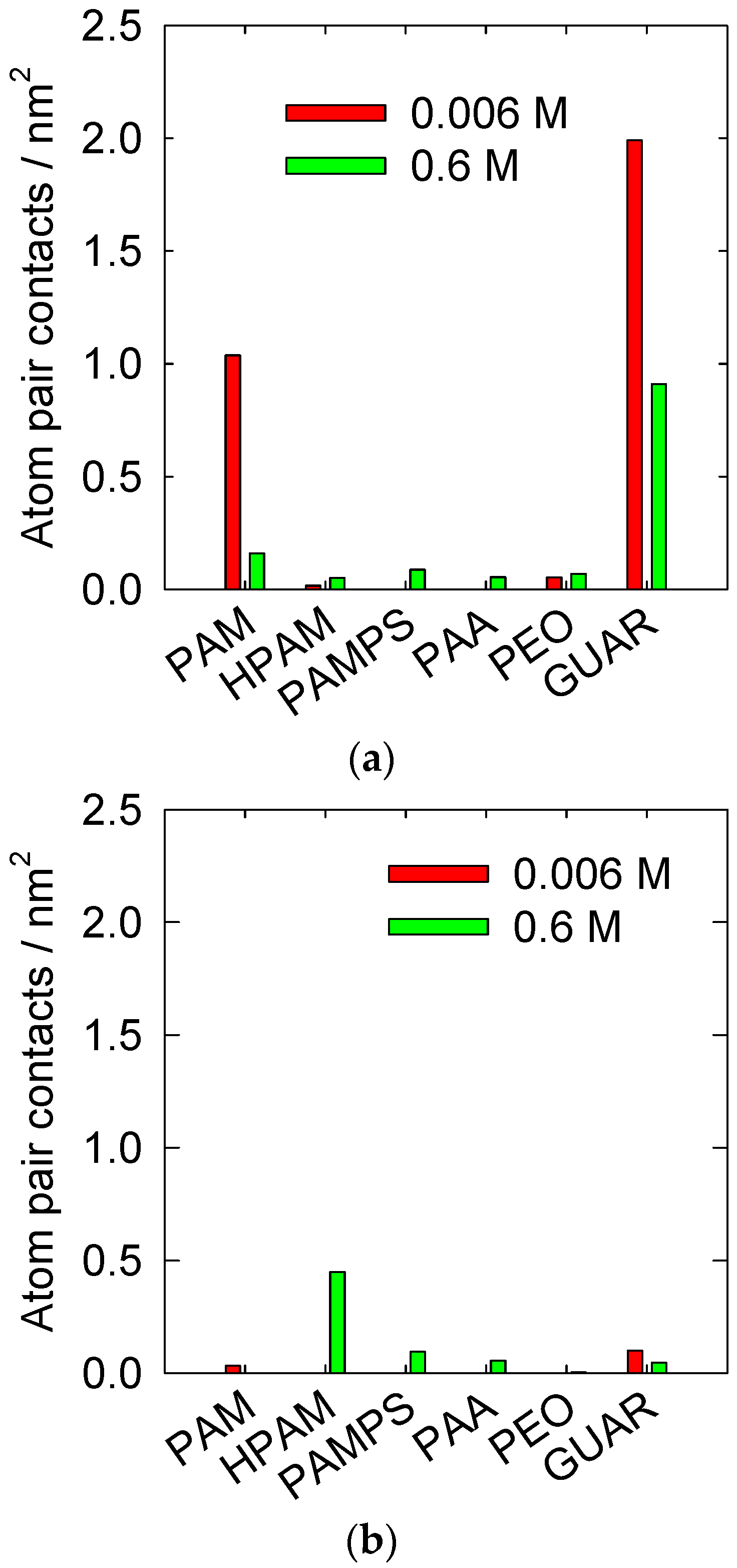
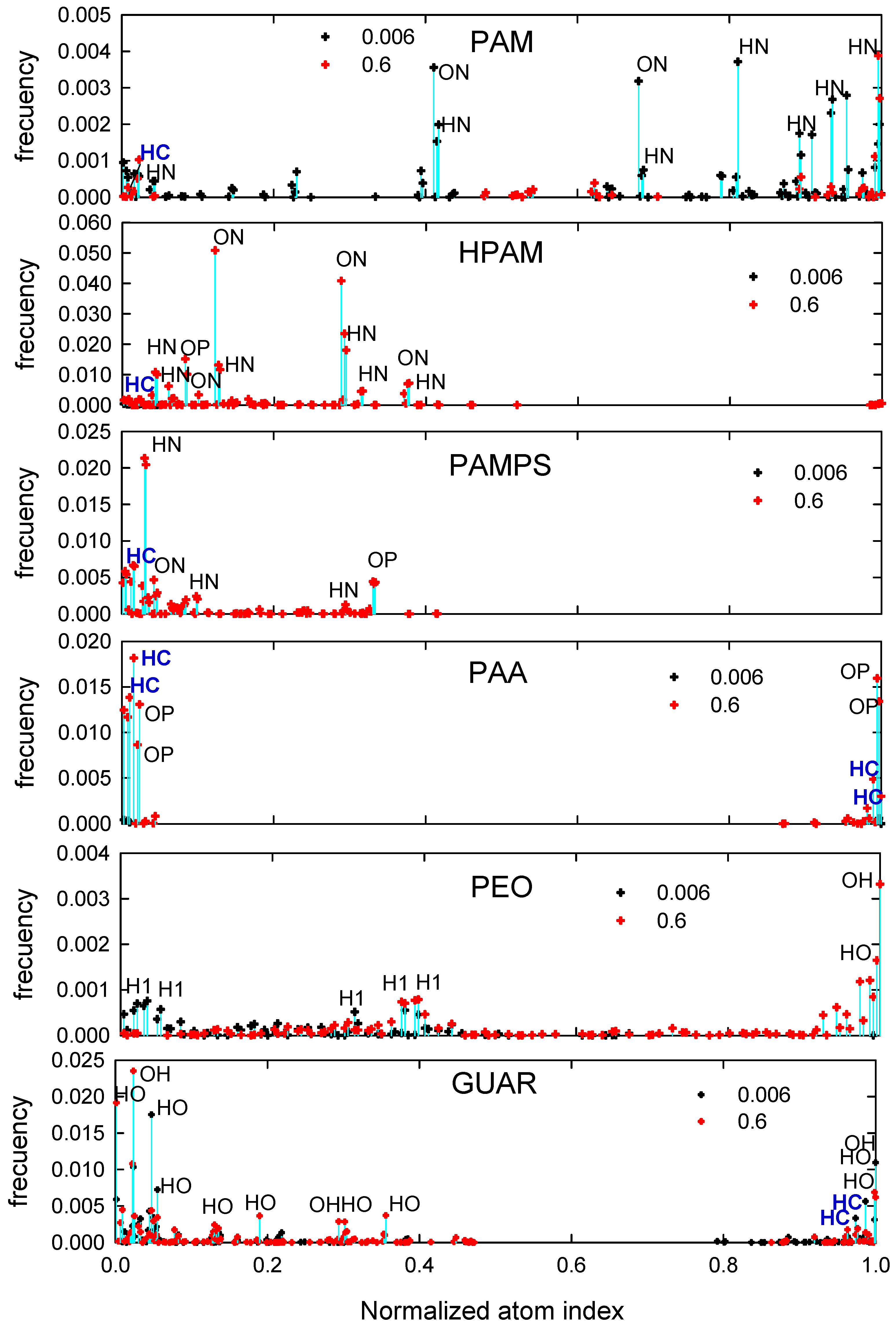
3.2. Atomic Interactions between the Surface and Polymers
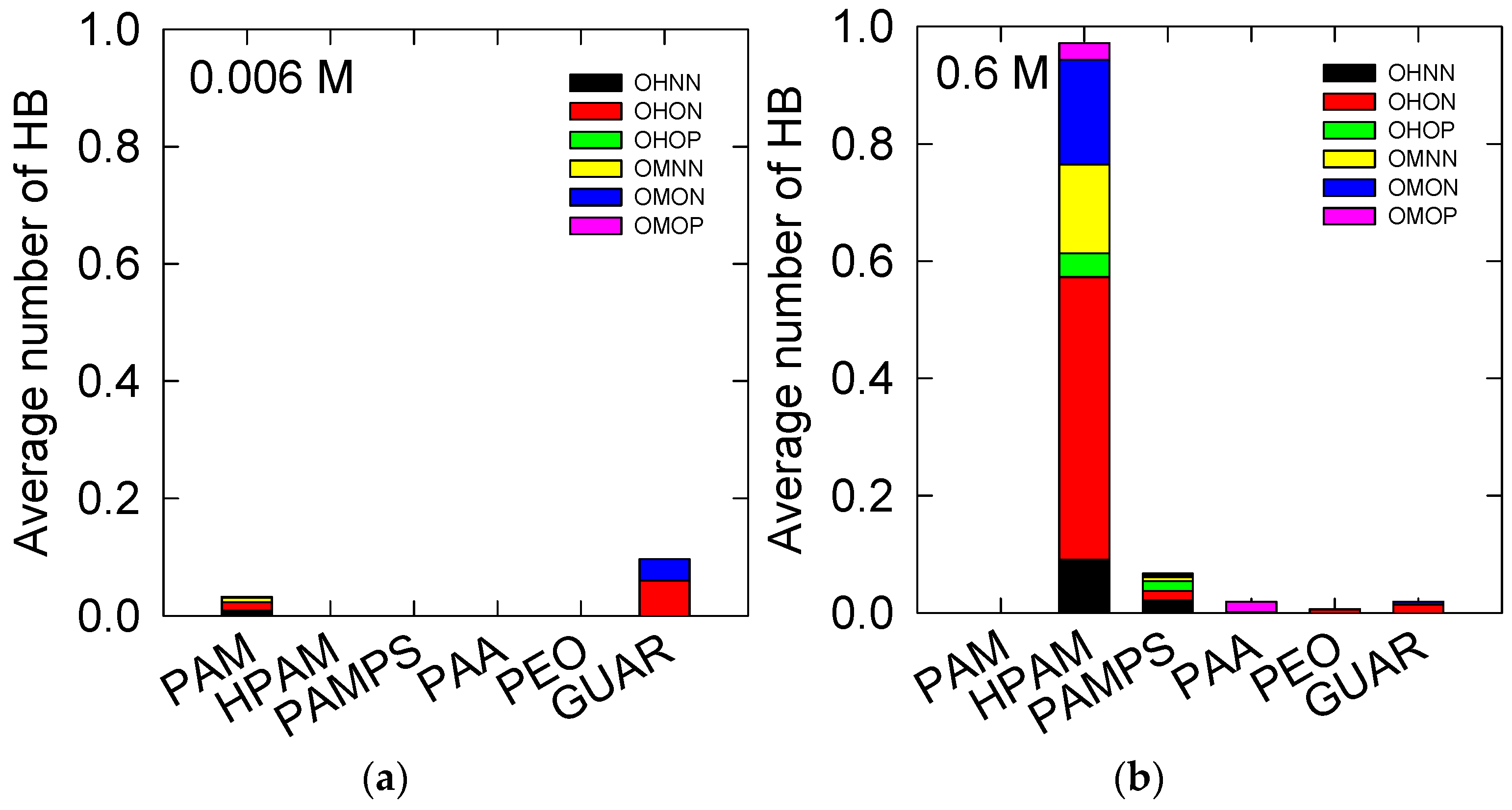
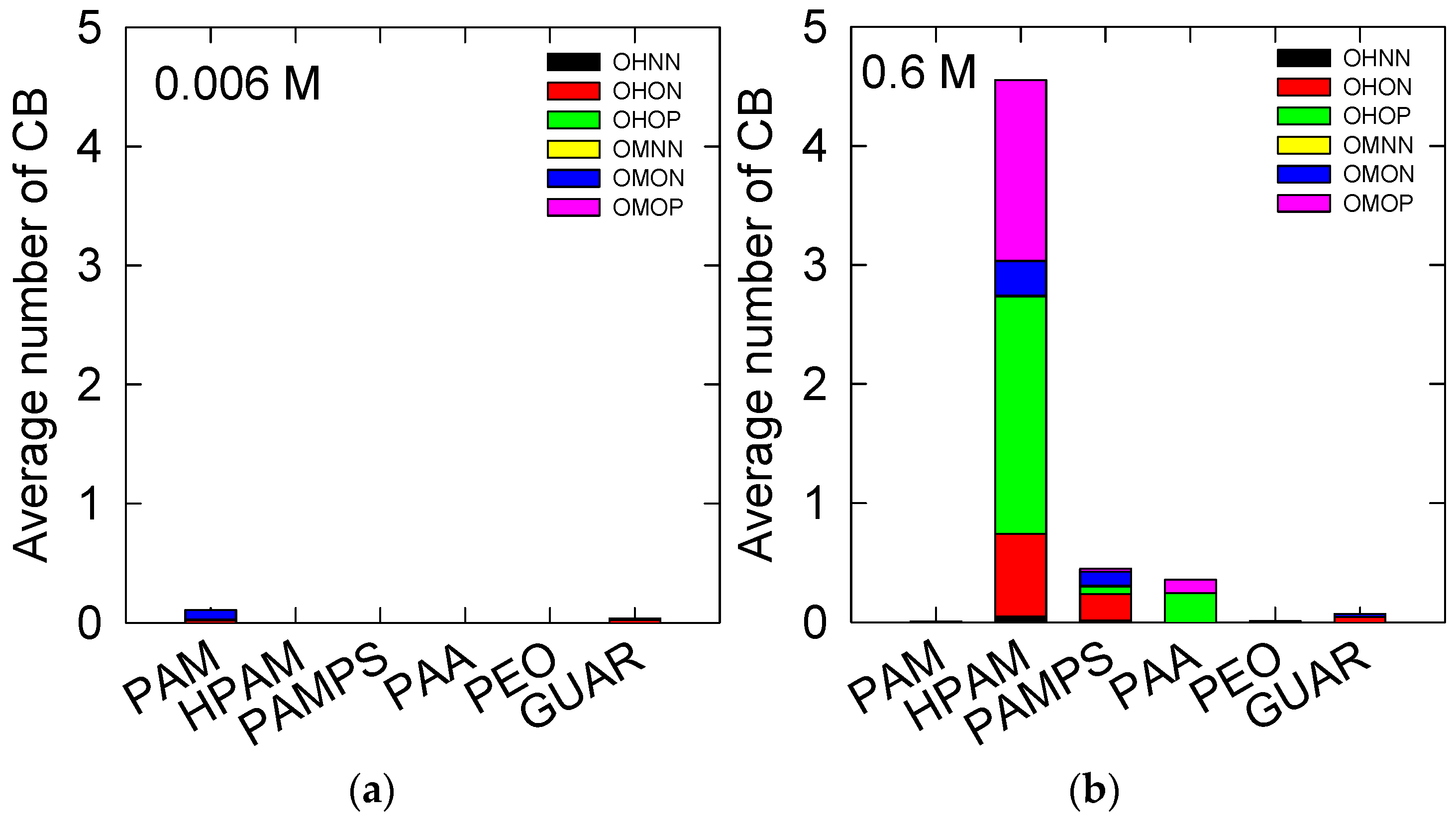
3.3. Insight of the Adsorption
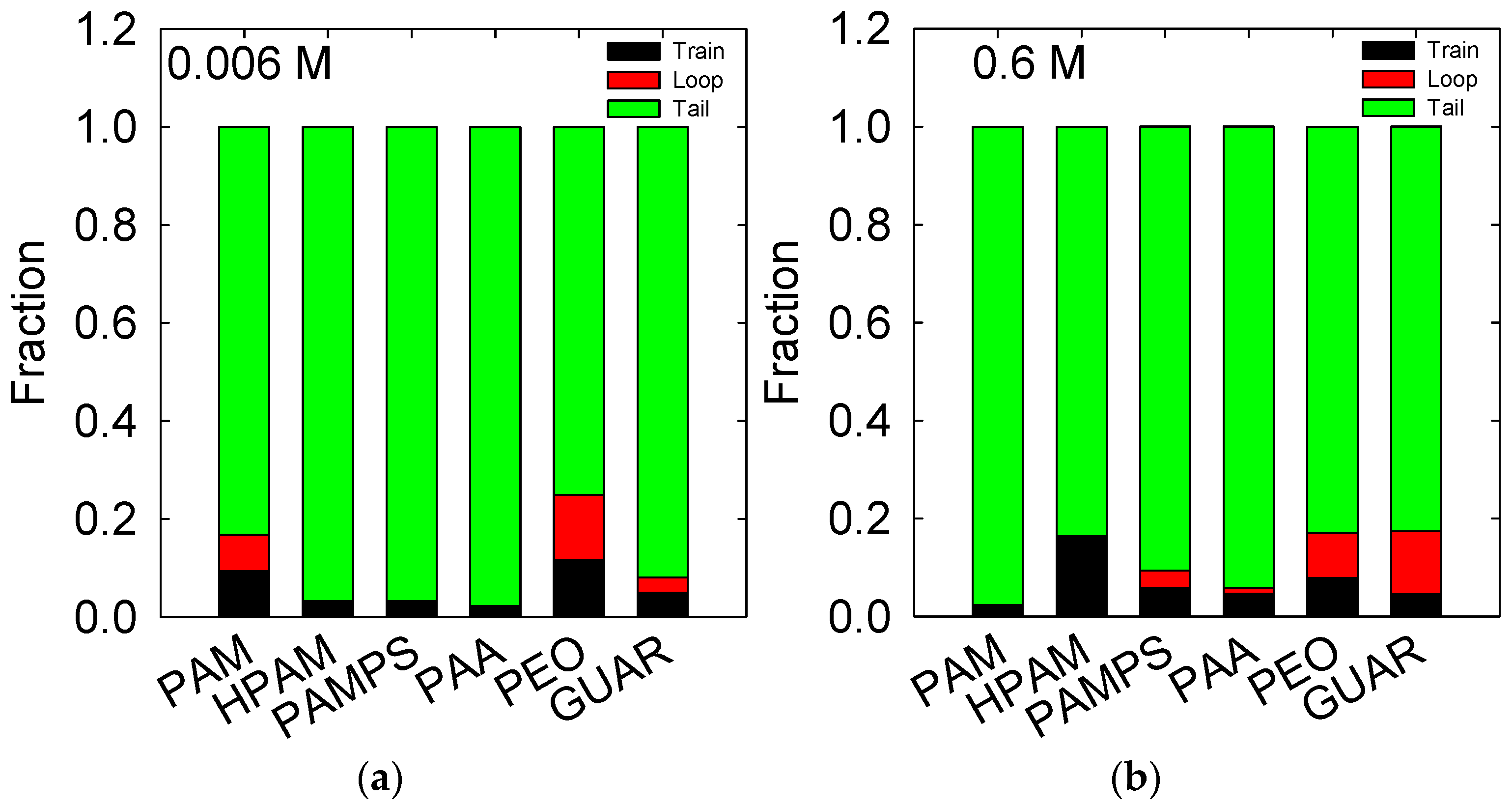
3.4. Conformation of the Polymer on the Quartz Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Z.; Gunter, W.D. The nature of the surface charge of kaolinite. Clays Clay Miner. 1992, 40, 365–368. [Google Scholar] [CrossRef]
- Bergaya, F.; Theng, B.; Lagaly, G. Surface and Interface Chemistry of Clay Minerals. In Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; pp. 139–172. [Google Scholar]
- Gupta, V.; Hampton, M.A.; Stokes, J.R.; Nguyen, A.V.; Miller, J.D. Particle interactions in kaolinite suspensions and corresponding aggregate structures. J. Colloid Interface Sci. 2011, 359, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Pulgar, F.; Ayala, L.; Jeldres, M.; Robles, P.; Toledo, P.G.; Salazar, I.; Jeldres, R.I. Lime/sodium carbonate treated seawater to improve flocculation and sedimentation of clay-based tailings. Polymers 2021, 13, 4108. [Google Scholar] [CrossRef] [PubMed]
- Jeldres, R.I.; Piceros, E.C.; Leiva, W.H.; Toledo, P.G.; Herrera, N. Viscoelasticity and yielding properties of flocculated kaolinite sediments in saline water. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 1009–1015. [Google Scholar] [CrossRef]
- Cisternas, L.A.; Gálvez, E.D. The use of seawater in mining. Miner. Process. Extr. Metall. Rev. 2018, 39, 18–33. [Google Scholar] [CrossRef]
- Costine, A.; Cox, J.; Travaglini, S.; Lubansky, A.; Fawell, P.; Misslitz, H. Variations in the molecular weight response of anionic polyacrylamides under different flocculation conditions. Chem. Eng. Sci. 2018, 176, 127–138. [Google Scholar] [CrossRef]
- Johnson, S.B.; Franks, G.V.; Scales, P.J.; Boger, D.V.; Healy, T.W. Surface chemistry–rheology relationships in concentrated mineral suspensions. Int. J. Miner. Process. 2000, 58, 267–304. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Jeldres, M. Rheological perspectives of clay-based tailings in the mining industry. In Clay Science and Technology; IntechOpen: London, UK, 2020. [Google Scholar]
- Grabsch, A.F.; Fawell, P.D.; Adkins, S.J.; Beveridge, A. The impact of achieving a higher aggregate density on polymer-bridging flocculation. Int. J. Miner. Process. 2013, 124, 83–94. [Google Scholar] [CrossRef]
- Tanguay, M.; Fawell, P.; Adkins, S. Modelling the impact of two different flocculants on the performance of a thickener feedwell. Appl. Math. Model. 2014, 38, 4262–4276. [Google Scholar] [CrossRef]
- Li, J.; Jiao, S.; Zhong, L.; Pan, J.; Ma, Q. Optimizing coagulation and flocculation process for kaolinite suspension with chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2013, 428, 100–110. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Yan, B.; Xu, H.; Liu, Q.; Zeng, H. Molecular and surface interactions between polymer flocculant chitosan-g-polyacrylamide and kaolinite particles: Impact of salinity. J. Phys. Chem. C 2015, 119, 7327–7339. [Google Scholar] [CrossRef]
- Ma, X.; Pawlik, M. Adsorption of guar gum onto quartz from dilute mixed electrolyte solutions. J. Colloid Interface Sci. 2006, 298, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Golitsyn, Y.; Pulst, M.; Kressler, J.; Reichert, D. Molecular dynamics in the crystalline regions of poly(ethylene oxide) containing a well-defined point defect in the middle of the polymer chain. J. Phys. Chem. B 2017, 121, 4620–4630. [Google Scholar] [CrossRef] [PubMed]
- Mpofu, P.; Addai-Mensah, J.; Ralston, J. Investigation of the effect of polymer structure type on flocculation, rheology and dewatering behaviour of kaolinite dispersions. Int. J. Miner. Process. 2003, 71, 247–268. [Google Scholar] [CrossRef]
- McFarlane, A.J.; Bremmell, K.E.; Addai-Mensah, J. Optimising the dewatering behaviour of clay tailings through interfacial chemistry, orthokinetic flocculation and controlled shear. Powder Technol. 2005, 160, 27–34. [Google Scholar] [CrossRef]
- Zhao, N.; Al Bitar, H.; Zhu, Y.; Xu, Y.; Shi, Z. Synthesis of polymer grafted starches and their flocculation properties in clay suspension. Minerals 2020, 10, 1054. [Google Scholar] [CrossRef]
- Lee, B.J.; Schlautman, M.A. Effects of Polymer Molecular Weight on Adsorption and Flocculation in Aqueous Kaolinite Suspensions Dosed with Nonionic Polyacrylamides. Water 2015, 7, 5896–5909. [Google Scholar] [CrossRef]
- Besra, L.; Sengupta, D.K.; Roy, S.K.; Ay, P. Influence of polymer adsorption and conformation on flocculation and dewatering of kaolin suspension. Sep. Purif. Technol. 2004, 37, 231–246. [Google Scholar] [CrossRef]
- Fijałkowska, G.; Szewczuk-Karpisz, K.; Wiśniewska, M. Anionic polyacrylamide as a substance strengthening the Pb(II) immobilization on the kaolinite surface. Int. J. Environ. Sci. Technol. 2020, 17, 1101–1112. [Google Scholar] [CrossRef]
- Quezada, G.R.; Rozas, R.E.; Toledo, P.G. Polyacrylamide adsorption on (1 0 1) quartz surfaces in saltwater for a range of pH values by molecular dynamics simulations. Miner. Eng. 2021, 162, 106741. [Google Scholar] [CrossRef]
- Liu, D.; Edraki, M.; Fawell, P.; Berry, L. Improved water recovery: A review of clay-rich tailings and saline water interactions. Powder Technol. 2020, 364, 604–621. [Google Scholar] [CrossRef]
- Murgich, J.; Jesus Rodríguez, M.; Izquierdo, A.; Carbognani, L.; Rogel, E. Interatomic interactions in the adsorption of asphaltenes and resins on kaolinite calculated by molecular dynamics. Energy Fuels 1998, 12, 339–343. [Google Scholar] [CrossRef]
- Chang, Z.; Sun, C.; Kou, J.; Fu, G.; Qi, X. Experimental and molecular dynamics simulation study on the effect of polyacrylamide on bauxite flotation. Miner. Eng. 2021, 164, 106810. [Google Scholar] [CrossRef]
- Ren, B.; Lv, K.; Min, F.; Chen, J.; Liu, C. A new insight into the adsorption behavior of NPAM on kaolinite/water interface: Experimental and theoretical approach. Fuel 2021, 303, 121299. [Google Scholar] [CrossRef]
- Jacquet, A.; Geatches, D.L.; Clark, S.J.; Greenwell, H.C. Understanding Cationic Polymer Adsorption on Mineral Surfaces: Kaolinite in Cement Aggregates. Minerals 2018, 8, 130. [Google Scholar] [CrossRef]
- Sun, W.; Zeng, H.; Tang, T. Synergetic adsorption of polymers on montmorillonite: Insights from molecular dynamics simulations. Appl. Clay Sci. 2020, 193, 105654. [Google Scholar] [CrossRef]
- Quezada, G.R.; Jeldres, M.; Toro, N.; Robles, P.; Toledo, P.G.; Jeldres, R.I. Understanding the flocculation mechanism of quartz and kaolinite with polyacrylamide in seawater: A molecular dynamics approach. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125576. [Google Scholar] [CrossRef]
- Quezada, G.R.; Toro, N.; Saavedra, J.; Robles, P.; Salazar, I.; Navarra, A.; Jeldres, R.I. Molecular dynamics study of the conformation, ion adsorption, diffusion, and water structure of soluble polymers in saline solutions. Polymers 2021, 13, 3550. [Google Scholar] [CrossRef]
- Quezada, G.R.; Piceros, E.; Saavedra, J.H.; Robles, P.; Jeldres, R.I. Polymer affinity with quartz (1 0 1) surface in saline solutions: A molecular dynamics study. Miner. Eng. 2022, 186, 107750. [Google Scholar] [CrossRef]
- Pouvreau, M.; Greathouse, J.A.; Cygan, R.T.; Kalinichev, A.G. Structure of hydrated kaolinite edge surfaces: DFT results and further development of the clayFF classical force field with metal-O-H angle bending terms. J. Phys. Chem. C 2019, 123, 11628–11638. [Google Scholar] [CrossRef]
- Jiang, M.-Q.; Jin, X.-Y.; Lu, X.-Q.; Chen, Z.-L. liang Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- Yavuz, Ö.; Altunkaynak, Y.; Güzel, F. Removal of copper, nickel, cobalt and manganese from aqueous solution by kaolinite. Water Res. 2003, 37, 948–952. [Google Scholar] [CrossRef]
- Srivastava, P.; Singh, B.; Angove, M. Competitive adsorption behavior of heavy metals on kaolinite. J. Colloid Interface Sci. 2005, 290, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Quezada, G.R.; Rozas, R.E.; Toledo, P.G. Ab initio calculations of partial charges at kaolinite edge sites and molecular dynamics simulations of cation adsorption in saline solutions at and above the pH of zero charge. J. Phys. Chem. C 2019, 123, 22971–22980. [Google Scholar] [CrossRef]
- Mintis, D.G.; Mavrantzas, V.G. Effect of pH and molecular length on the structure and dynamics of short poly(acrylic acid) in dilute solution: Detailed molecular dynamics study. J. Phys. Chem. B 2019, 123, 4204–4219. [Google Scholar] [CrossRef]
- Dupradeau, F.-Y.; Pigache, A.; Zaffran, T.; Savineau, C.; Lelong, R.; Grivel, N.; Lelong, D.; Rosanski, W.; Cieplak, P. The R.E.D. Tools: Advances in RESP and ESP charge derivation and force field library building. Phys. Chem. Chem. Phys. 2010, 12, 7821–7839. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian. Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Kroutil, O.; Chval, Z.; Skelton, A.A.; Předota, M. Computer simulations of quartz (101)-water interface over a range of pH values. J. Phys. Chem. C 2015, 119, 9274–9286. [Google Scholar] [CrossRef]
- Li, P.; Song, L.F.; Merz, K.M. Systematic parameterization of monovalent ions employing the nonbonded model. J. Chem. Theory Comput. 2015, 11, 1645–1657. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- da Silva, A.W.S.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser interfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Bekker, H.; Berendsen, H.J.C.; Fraaije, J.G.E.M. LINCS: A Linear Constraint Solver for molecular simulations. J. Comput. Chem. 1997, 18, 1463–1472. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Donets, S.; Sommer, J.-U. Molecular dynamics simulations of strain-induced phase transition of poly(ethylene oxide) in water. J. Phys. Chem. B 2018, 122, 392–397. [Google Scholar] [CrossRef]
- Zeitler, T.R.; Greathouse, J.A.; Cygan, R.T.; Fredrich, J.T.; Jerauld, G.R. Molecular dynamics simulation of resin adsorption at kaolinite edge sites: Effect of surface deprotonation on interfacial structure. J. Phys. Chem. C 2017, 121, 22787–22796. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quezada, G.R.; Leiva, W.; Saavedra, J.H.; Robles, P.; Gálvez, E.; Jeldres, R.I. A Molecular Dynamics Simulation of Polymers’ Interactions with Kaolinite (010) Surfaces in Saline Solutions. Polymers 2022, 14, 3851. https://doi.org/10.3390/polym14183851
Quezada GR, Leiva W, Saavedra JH, Robles P, Gálvez E, Jeldres RI. A Molecular Dynamics Simulation of Polymers’ Interactions with Kaolinite (010) Surfaces in Saline Solutions. Polymers. 2022; 14(18):3851. https://doi.org/10.3390/polym14183851
Chicago/Turabian StyleQuezada, Gonzalo R., Williams Leiva, Jorge H. Saavedra, Pedro Robles, Edelmira Gálvez, and Ricardo I. Jeldres. 2022. "A Molecular Dynamics Simulation of Polymers’ Interactions with Kaolinite (010) Surfaces in Saline Solutions" Polymers 14, no. 18: 3851. https://doi.org/10.3390/polym14183851
APA StyleQuezada, G. R., Leiva, W., Saavedra, J. H., Robles, P., Gálvez, E., & Jeldres, R. I. (2022). A Molecular Dynamics Simulation of Polymers’ Interactions with Kaolinite (010) Surfaces in Saline Solutions. Polymers, 14(18), 3851. https://doi.org/10.3390/polym14183851









