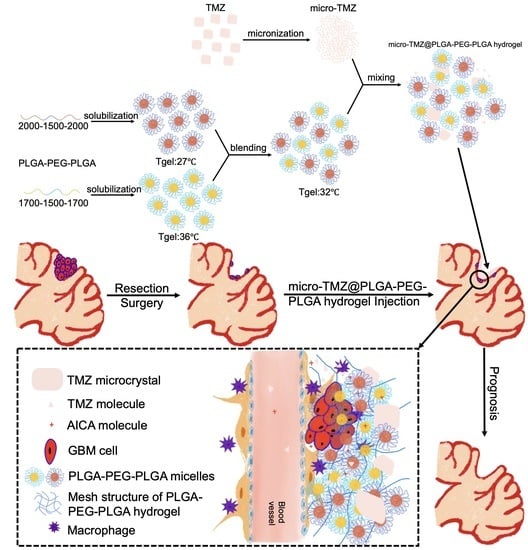
Intracranial In Situ Thermosensitive Hydrogel Delivery of Temozolomide Accomplished by PLGA–PEG–PLGA Triblock Copolymer Blending for GBM Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogel Preparation
2.3. Physicochemical Properties of PLGA–PEG–PLGA and Hydrogels
2.3.1. NMR Spectrum
2.3.2. GPC
2.3.3. Visual Inspection
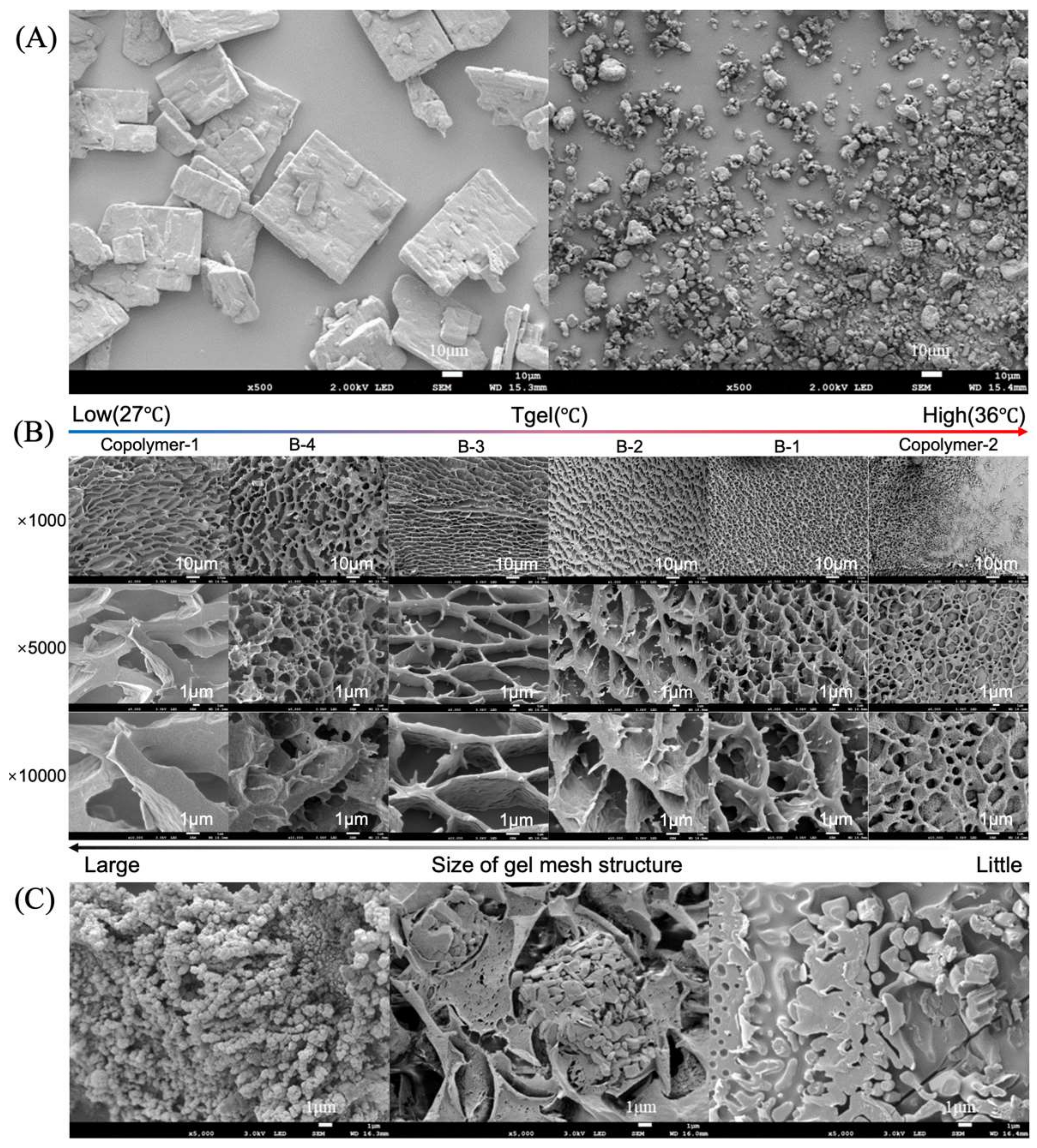
2.3.4. Microstructure of Hydrogel
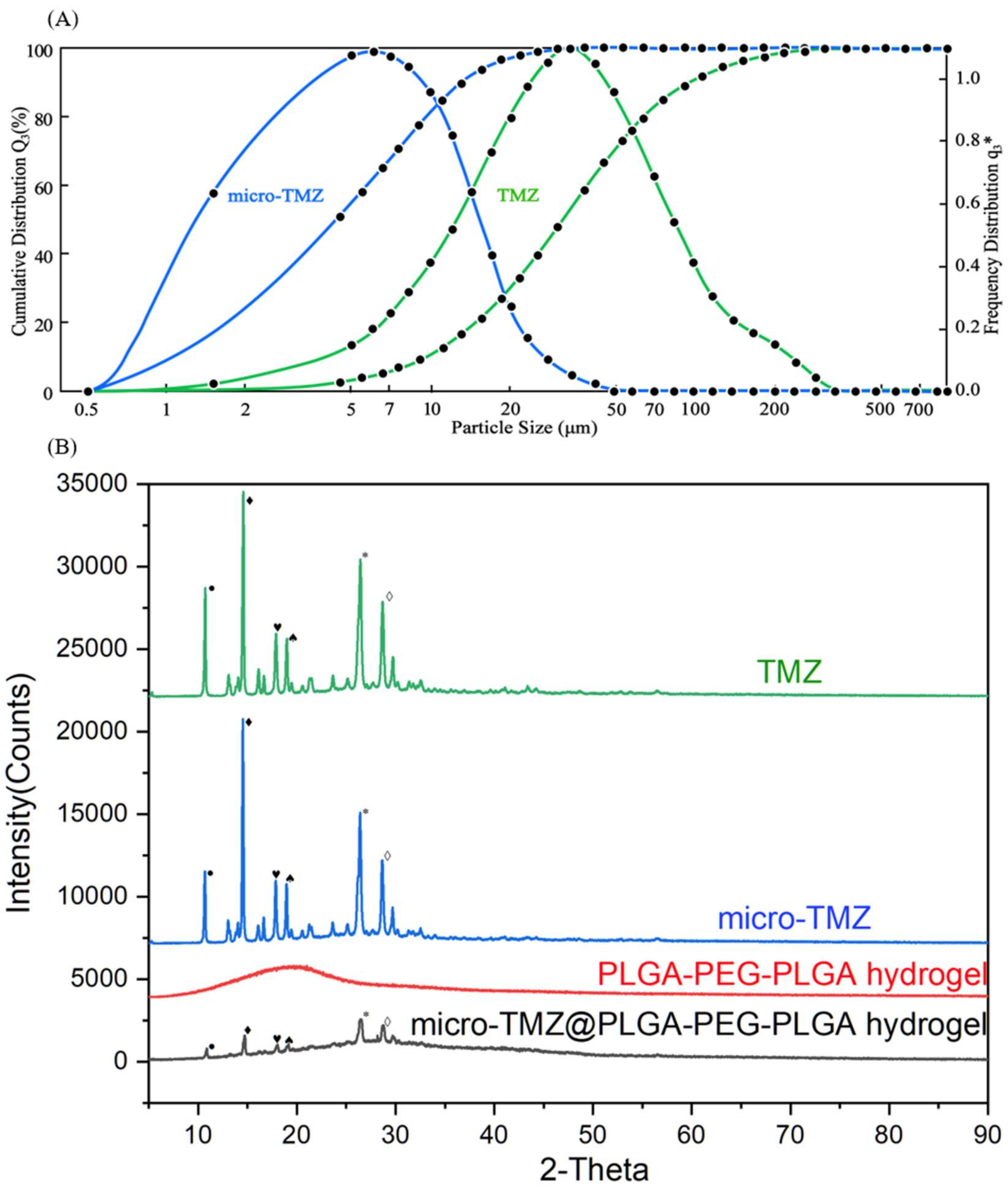
2.3.5. X-ray Diffraction Analysis
2.3.6. Particle Size Distribution
2.3.7. Rheological Tests
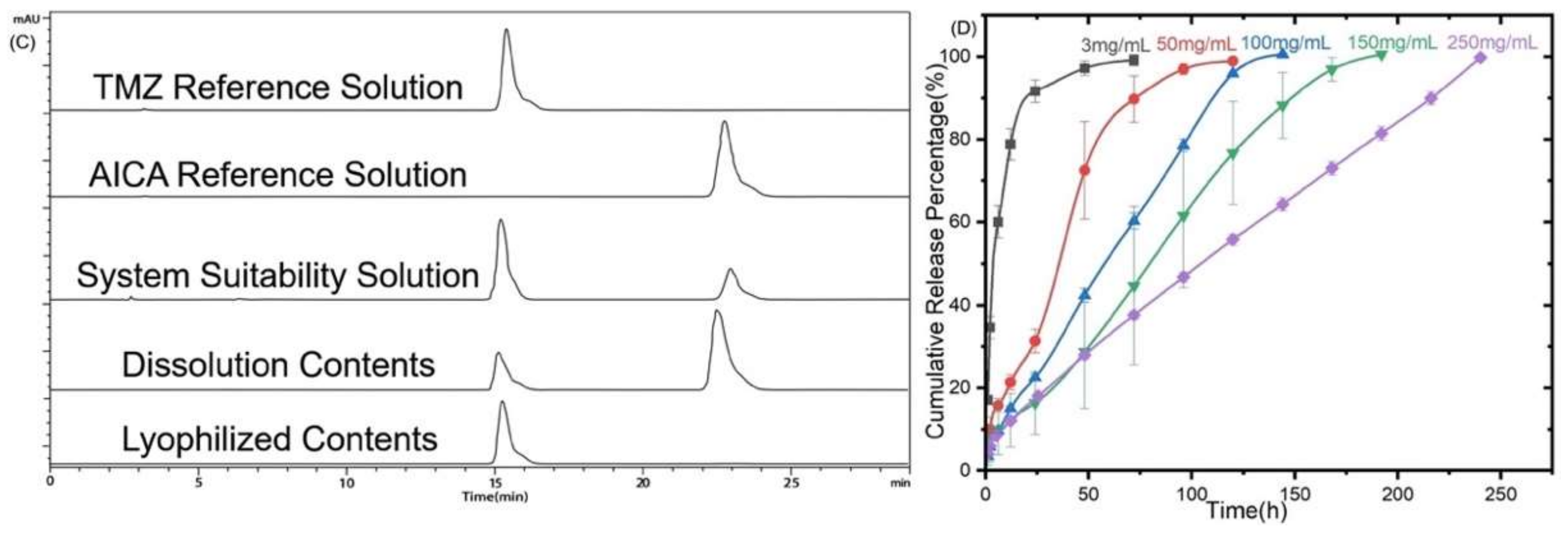
2.3.8. In Vitro Drug Release
2.3.9. Thermo–Reversibility Research
2.3.10. Statistical Analysis
2.4. Cell and Animal Experiments
2.4.1. Cell Culture
2.4.2. In Vitro Hydrogel Cytotoxicity Assay
2.4.3. Animal Experiments
2.4.4. Procedure of GBM Modeling in Balb/c Nude Mice
2.4.5. GBM Resection Surgery Process
2.4.6. Biosafety Evaluation
2.4.7. Suppression of GBM Relapse
3. Results and Discussion
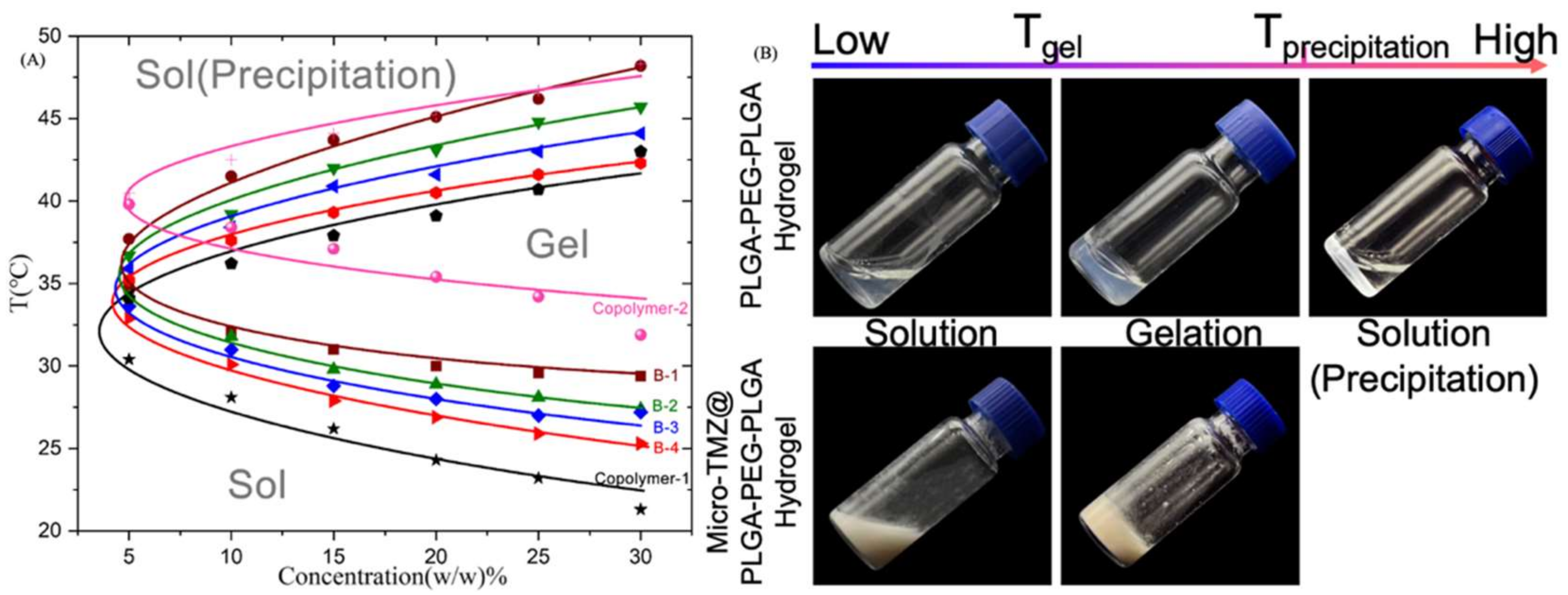
3.1. Macro Appearance
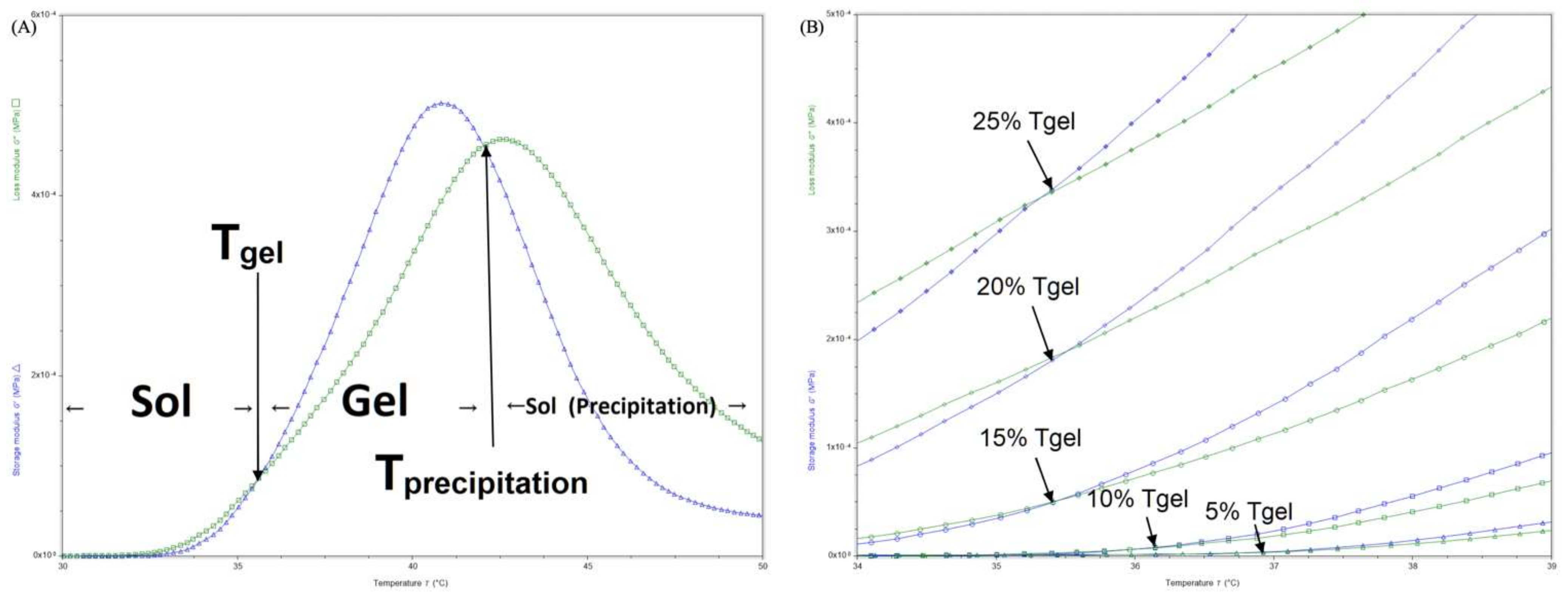
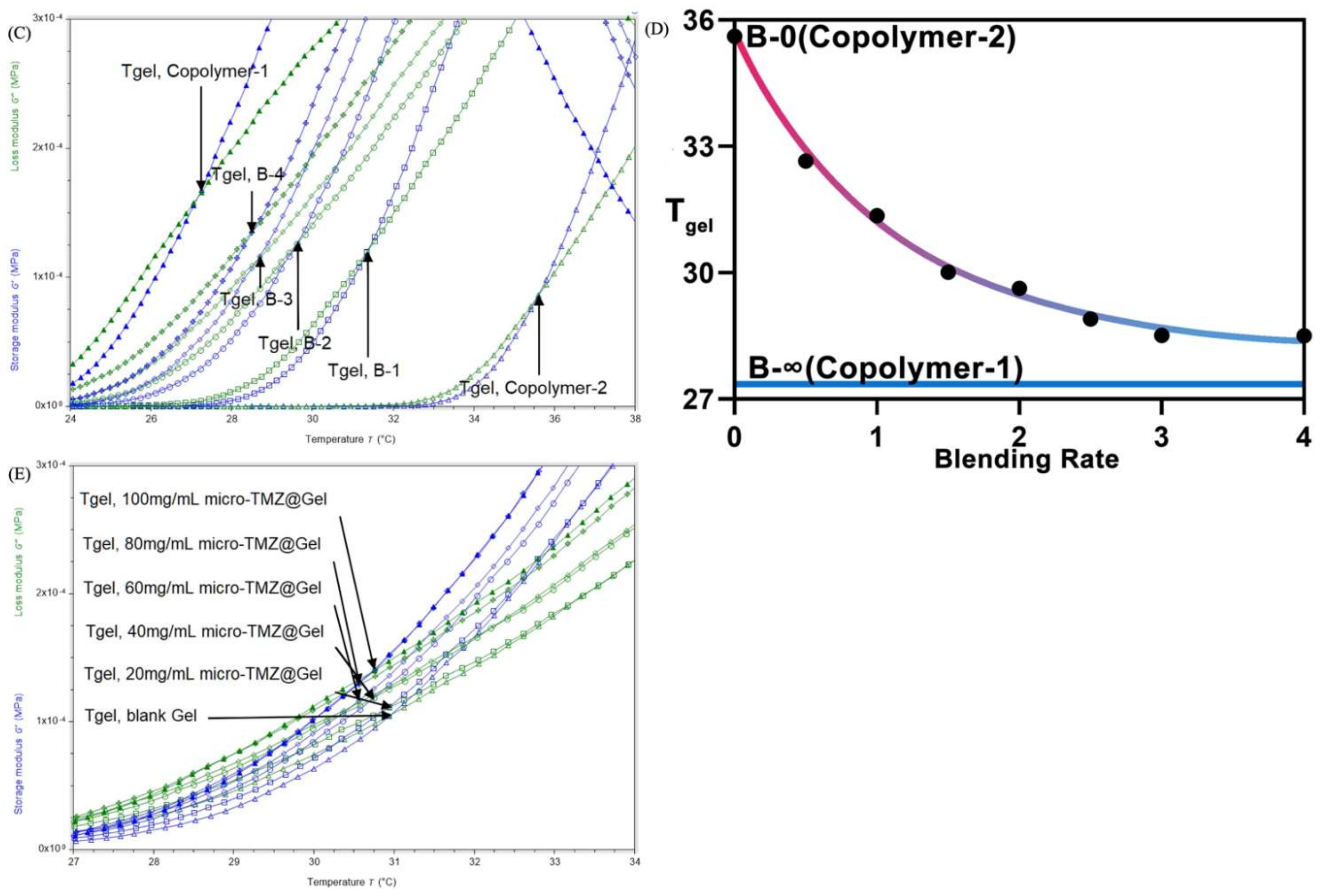
3.2. Rheology Analysis
3.3. Microstructure of the Hydrogel and Formulation
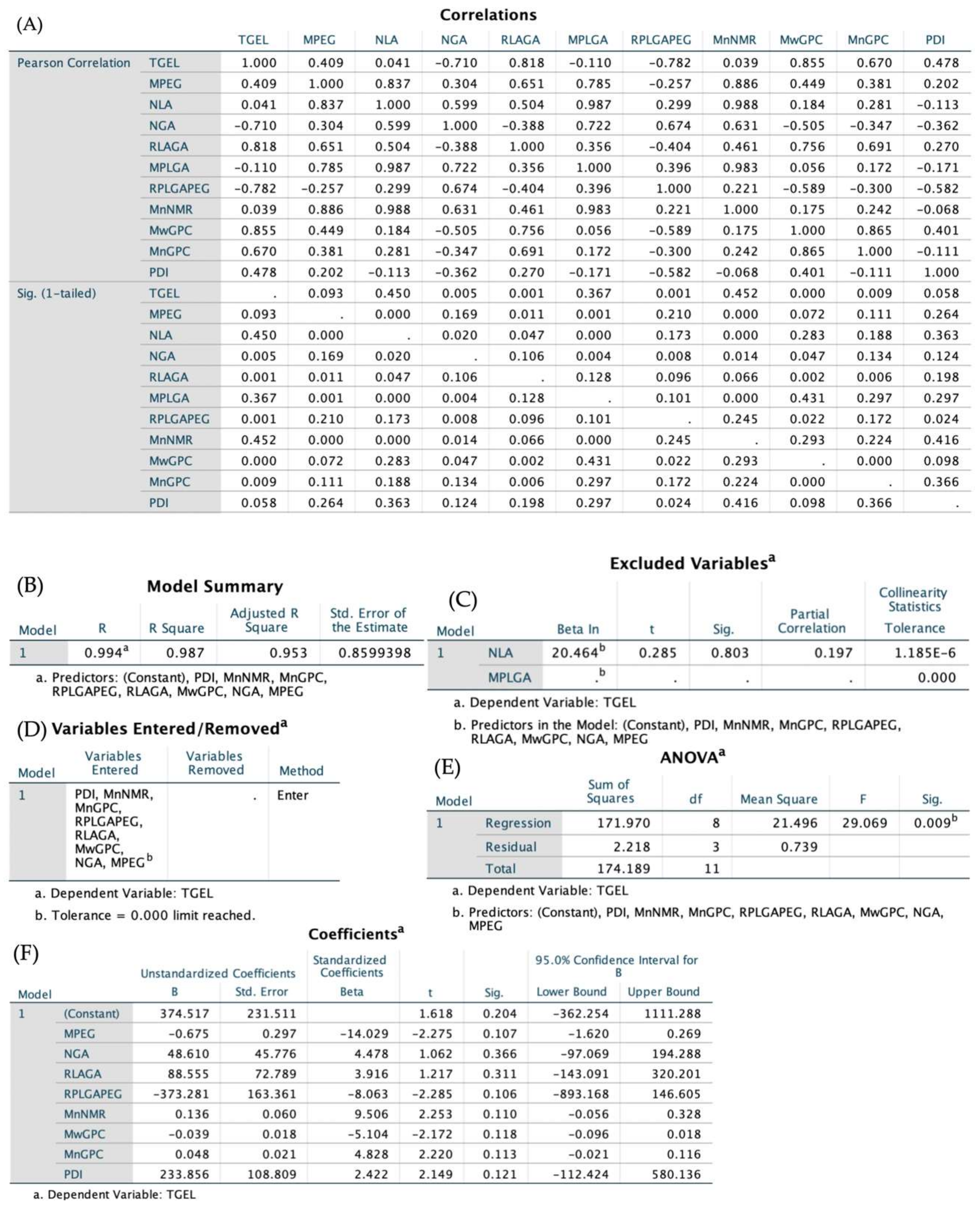
3.4. NMR Spectra and GPC Diagrams Revealing Molecular Properties and Statistic Analysis
3.5. Particle Size and XRD
3.6. In Vitro Drug Release
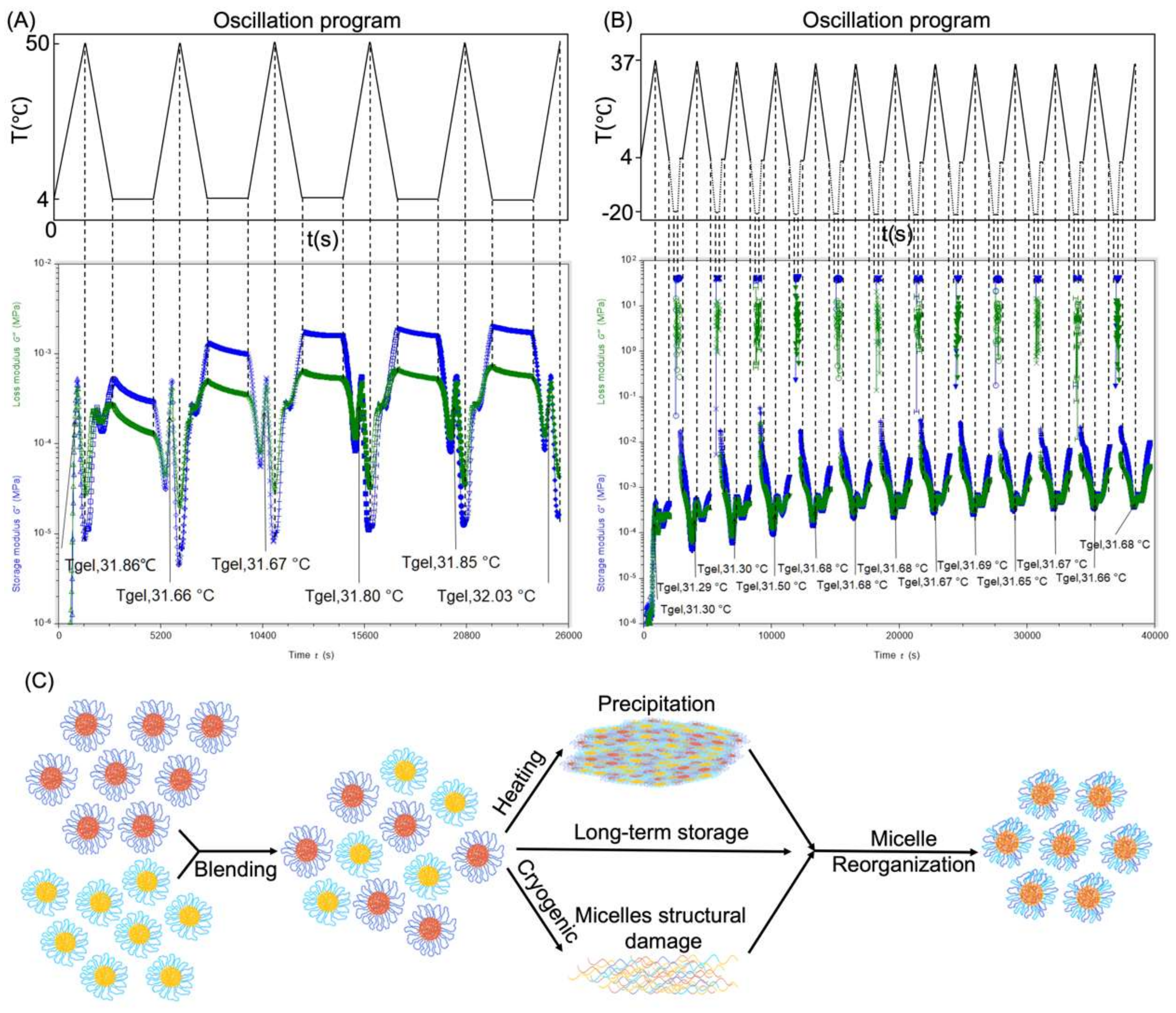
3.7. Thermo-Reversibility and Micelle Reorganization
3.8. MTT Assay
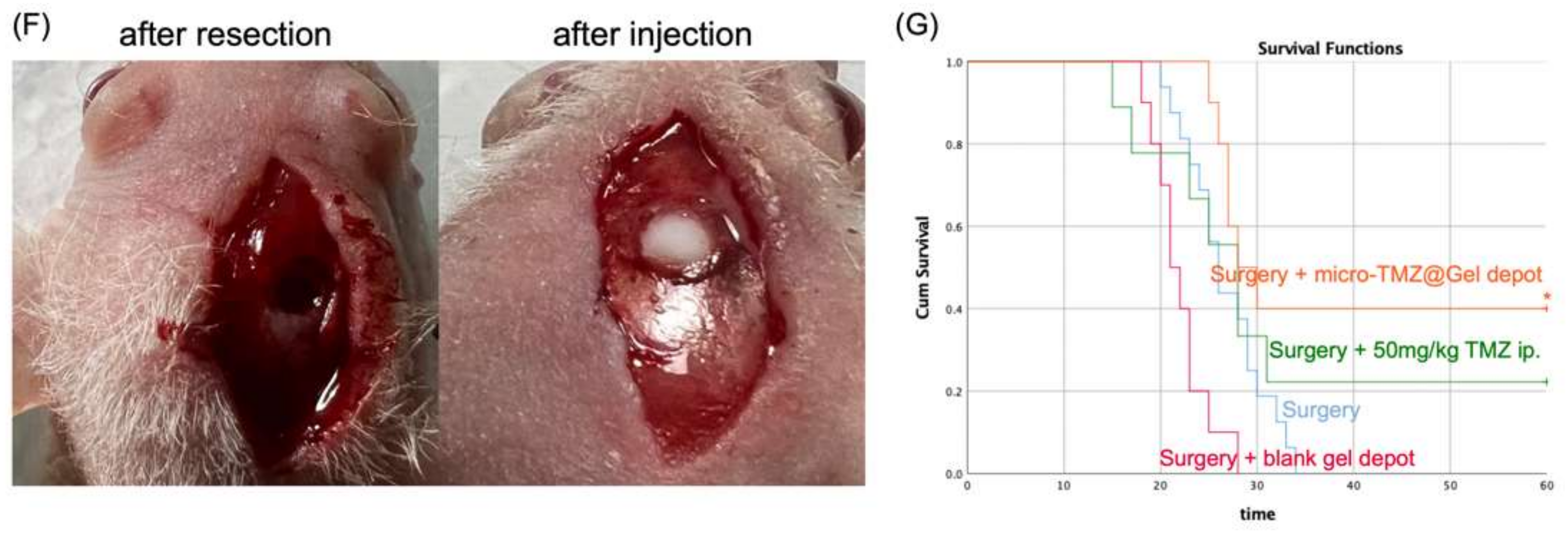
3.9. Biosafety and Efficacy of Micro-TMZ@PLGA–PEG–PLGA In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Mousa, A.; Al-Dwairy, S.; Dajani, N.; Sami, R.; Shtaya, A. Giant cell glioblastoma multiforme presents as acute pathological nontraumatic subdural haematoma. Br. J. Neurosurg. 2020. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22, 1–96. [Google Scholar] [CrossRef]
- Smith, S.J.; Tyler, B.M.; Gould, T.; Veal, G.J.; Gorelick, N.; Rowlinson, J.; Serra, R.; Ritchie, A.; Berry, P.; Otto, A.; et al. Overall Survival in Malignant Glioma Is Significantly Prolonged by Neurosurgical Delivery of Etoposide and Temozolomide from a Thermo-Responsive Biodegradable Paste. Clin. Cancer Res. 2019, 25, 5094–5106. [Google Scholar] [CrossRef]
- Vellimana, A.K.; Recinos, V.R.; Hwang, L.; Fowers, K.D.; Li, K.W.; Zhang, Y.; Okonma, S.; Eberhart, C.G.; Brem, H.; Tyler, B.M. Combination of paclitaxel thermal gel depot with temozolomide and radiotherapy significantly prolongs survival in an experimental rodent glioma model. J. Neurooncol. 2013, 111, 229–236. [Google Scholar] [CrossRef]
- Zhao, M.; Bozzato, E.; Joudiou, N.; Ghiassinejad, S.; Danhier, F.; Gallez, B.; Preat, V. Codelivery of paclitaxel and temozolomide through a photopolymerizable hydrogel prevents glioblastoma recurrence after surgical resection. J. Control. Release 2019, 309, 72–81. [Google Scholar] [CrossRef]
- Serris, I.; Serris, P.; Frey, K.M.; Cho, H. Development of 3D-Printed Layered PLGA Films for Drug Delivery and Evaluation of Drug Release Behaviors. AAPS Pharm. Sci. Tech. 2020, 21, 256. [Google Scholar] [CrossRef]
- Perry, J.; Chambers, A.; Spithoff, K.; Laperriere, N. Gliadel wafers in the treatment of malignant glioma: A systematic review. Curr. Oncol. 2007, 14, 189–194. [Google Scholar] [CrossRef]
- Kim, Y.Z.; Kim, C.Y.; Lim, J.; Sung, K.S.; Lee, J.; Oh, H.J.; Kang, S.G.; Kang, S.H.; Kong, D.S.; Kim, S.H.; et al. The Korean Society for Neuro-Oncology (KSNO) Guideline for Glioblastomas: Version 2018.01. Brain Tumor Res. Treat. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Andrade, S.; Coelho, M.A.N.; Loureiro, J.A.; Pereira, M.C. Biophysical interaction of temozolomide and its active metabolite with biomembrane models: The relevance of drug-membrane interaction for Glioblastoma Multiforme therapy. Eur. J. Pharm. Biopharm. 2019, 136, 156–163. [Google Scholar] [CrossRef]
- Masi, B.C.; Tyler, B.M.; Bow, H.; Wicks, R.T.; Xue, Y.; Brem, H.; Langer, R.; Cima, M.J. Intracranial MEMS based temozolomide delivery in a 9L rat gliosarcoma model. Biomaterials 2012, 33, 5768–5775. [Google Scholar] [CrossRef]
- Tseng, Y.Y.; Kau, Y.C.; Liu, S.J. Advanced interstitial chemotherapy for treating malignant glioma. Expert Opin. Drug Deliv. 2016, 13, 1533–1544. [Google Scholar] [CrossRef]
- Wang, P.; Zhuo, X.; Chu, W.; Tang, X. Exenatide-loaded microsphere/thermosensitive hydrogel long-acting delivery system with high drug bioactivity. Int. J. Pharm. 2017, 528, 62–75. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yang, X.; Li, Y.; Chen, Y.; Peng, X.; Yu, L.; Ding, J. Sustained Release Strategy Designed for Lixisenatide Delivery to Synchronously Treat Diabetes and Associated Complications. ACS Appl. Mater. Interfaces 2019, 11, 29604–29618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, W.; Luan, J.; Yang, D.; Wei, G.; Yu, L.; Lu, W.; Ding, J. Sustained intravitreal delivery of dexamethasone using an injectable and biodegradable thermogel. Acta Biomater. 2015, 23, 271–281. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Wu, K.; Wu, X.; Tang, J.; Cui, S.; Cao, D.; Liu, R.; Peng, C.; Yu, L.; et al. Visualizing the In Vivo Evolution of an Injectable and Thermosensitive Hydrogel Using Tri-Modal Bioimaging. Small Methods 2020, 4, 2000310. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, S.; Li, D.; Gong, Y.; Zang, J.; Liu, J.; Chen, X. The effect of PLGA-based hydrogel scaffold for improving the drug maximum-tolerated dose for in situ osteosarcoma treatment. Colloids Surf. B Biointerfaces 2018, 172, 387–394. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Wang, Y.; Xu, G.; Yu, L.; Ding, J. Sustained release of lipophilic gemcitabine from an injectable polymeric hydrogel for synergistically enhancing tumor chemoradiotherapy. Chem. Eng. J. 2020, 396, 125320. [Google Scholar] [CrossRef]
- Wang, M.; Zhan, J.; Xu, L.; Wang, Y.; Lu, D.; Li, Z.; Li, J.; Luo, F.; Tan, H. Synthesis and characterization of PLGA-PEG-PLGA based thermosensitive polyurethane micelles for potential drug delivery. J. Biomater. Sci. Polym. Ed. 2021, 32, 613–634. [Google Scholar] [CrossRef]
- Wu, X.; Wang, X.; Chen, X.; Yang, X.; Ma, Q.; Xu, G.; Yu, L.; Ding, J. Injectable and thermosensitive hydrogels mediating a universal macromolecular contrast agent with radiopacity for noninvasive imaging of deep tissues. Bioact. Mater. 2021, 6, 4717–4728. [Google Scholar] [CrossRef]
- Khaledi, S.; Jafari, S.; Hamidi, S.; Molavi, O.; Davaran, S. Preparation and characterization of PLGA-PEG-PLGA polymeric nanoparticles for co-delivery of 5-Fluorouracil and Chrysin. J. Biomater. Sci. Polym. Ed. 2020, 31, 1107–1126. [Google Scholar] [CrossRef]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Chen, X.; Gu, S.; Cui, S.; Yang, X.; Yu, L.; Ding, J. Decisive Influence of Hydrophobic Side Chains of Polyesters on Thermoinduced Gelation of Triblock Copolymer Aqueous Solutions. Macromolecules 2021, 54, 7421–7433. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Sinh, L.H.; Huynh, D.P.; Nguyen, D.H.; Huynh, C. Self-Assemblable Polymer Smart-Blocks for Temperature-Induced Injectable Hydrogel in Biomedical Applications. Front. Chem. 2020, 8, 19. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Z.; Zhang, H.; Ding, J. Mixing a sol and a precipitate of block copolymers with different block ratios leads to an injectable hydrogel. Biomacromolecules 2009, 10, 1547–1553. [Google Scholar] [CrossRef]
- Qu, J.Z.; Kao, L.W.; Smith, J.E.; Kuo, A.; Xue, A.; Iyer, M.H.; Essandoh, M.K.; Dalia, A.A. Brain Protection in Aortic Arch Surgery: An Evolving Field. J. Cardiothorac Vasc. Anesth. 2021, 35, 1176–1188. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, J.; Cai, S.; Bao, X.; Li, Q.; Xu, G. Setting Characteristics and High Compressive Strength of an Anti-washout, Injectable Calcium Phosphate Cement Combined with Thermosensitive Hydrogel. Materials 2020, 13, 5779. [Google Scholar] [CrossRef]
- Wei, W.; Li, H.; Yin, C.; Tang, F. Research progress in the application of in situ hydrogel system in tumor treatment. Drug Deliv. 2020, 27, 460–468. [Google Scholar] [CrossRef]
- Liang, P.; Wang, G.; Liu, X.; Wang, Z.; Wang, J.; Gao, W. Spatiotemporal combination of thermosensitive polypeptide fused interferon and temozolomide for post-surgical glioblastoma immunochemotherapy. Biomaterials 2021, 264, 120447. [Google Scholar] [CrossRef]
- Mirabdaly, S.; Elieh, D.; Shakiba, Y.; Moini, A.; Kiani, A. Effects of temozolomide on U87MG glioblastoma cell expression of CXCR4, MMP2, MMP9, VEGF, anti-proliferatory cytotoxic and apoptotic properties. Mol. Biol. Rep. 2020, 47, 1187–1197. [Google Scholar] [CrossRef]
- Sidell, D.; Ward, J.A.; Pordal, A.; Quimby, C.; Nassar, M.; Choo, D.I. Combination therapies using an intratympanic polymer gel delivery system in the guinea pig animal model: A safety study. Int. J. Pediatr. Otorhinolaryngol. 2016, 84, 132–136. [Google Scholar] [CrossRef]
- Cuming, R.S.; Abarca, E.M.; Duran, S.; Wooldridge, A.A.; Stewart, A.J.; Ravis, W.; Babu, R.J.; Lin, Y.J.; Hathcock, T. Development of a Sustained-Release Voriconazole-Containing Thermogel for Subconjunctival Injection in Horses. Invest. Ophthalmol. Vis. Sci. 2017, 58, 2746–2754. [Google Scholar] [CrossRef] [PubMed]
- Mora-Pereira, M.; Abarca, E.M.; Duran, S.; Ravis, W.; Mcmullen, R.J., Jr.; Fischer, B.M.; Lee, Y.P. Wooldridge A A. Sustained-release voriconazole-thermogel for subconjunctival injection in horses: Ocular toxicity and in-vivo studies. BMC Vet. Res. 2020, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Ata, F.; Akkam, V.S.F.; Gaber, M.; Omar, N.E.; Madani, A.; Mah, A.H.; Aldardouri, M.M.; Amer, A.; Kohla, S.; Zar, G.A.R. Fatal temozolomide induced aplastic anemia in a female with Glioblastoma multiforme : A case report and literature review. Clin. Case Rep. 2021, 9, 1641–1646. [Google Scholar] [CrossRef]
- Patel, N.; Ji, N.; Wang, Y.; Li, X.; Langley, N.; Tan, C. Subcutaneous Delivery of Albumin: Impact of Thermosensitive Hydrogels. AAPS Pharm. Sci. Tech. 2021, 22, 120. [Google Scholar] [CrossRef]
- Afzalipour, R.; Khoei, S.; Khoee, S.; Shirvalilou, S.; Raoufi, N.J.; Motevalian, M.; Karimi, M.Y. Thermosensitive magnetic nanoparticles exposed to alternating magnetic field and heat-mediated chemotherapy for an effective dual therapy in rat glioma model. Nanomedicine 2021, 31, 102319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, J.; Lu, Y. Doxorubicin and CDCUR inclusion complex coloaded in thermosensitive hydrogel PLGAPEGPLGA localized administration for osteosarcoma. Int. J. Oncol. 2020, 57, 433–444. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Shen, W.; Li, K.; Yu, L.; Chen, Q.; Ding, J. Controlled release of liraglutide using thermogelling polymers in treatment of diabetes. Sci. Rep. 2016, 6, 31593. [Google Scholar] [CrossRef]
- Shi, J.; Yu, L.; Ding, J. PEG-based thermosensitive and biodegradable hydrogels. Acta Biomater. 2021, 128, 42–59. [Google Scholar] [CrossRef]
- Chen, X.; Wang, M.; Yang, X.; Wang, Y.; Yu, L.; Sun, J.; Ding, J. Injectable hydrogels for the sustained delivery of a HER2-targeted antibody for preventing local relapse of HER2+ breast cancer after breast-conserving surgery. Theranostics 2019, 9, 6080–6098. [Google Scholar] [CrossRef]
- Xu, W.K.; Tang, J.-Y.; Yuan, Z.; Cai, C.-Y.; Chen, X.-B.; Cui, S.-Q.; Liu, P.; Yu, L.; Cai, K.-Y.; Ding, J.-D. Accelerated Cutaneous Wound Healing Using an Injectable Teicoplanin-loaded PLGA-PEG-PLGA Thermogel Dressing. Chin. J. Polym. Sci. 2019, 37, 548–559. [Google Scholar] [CrossRef]
- Giraudeau, P.; Baguet, E. Improvement of the inverse-gated-decoupling sequence for a faster quantitative analysis of various samples by 13C NMR spectroscopy. J. Magn. Reson. 2006, 180, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Giraudeau, P.; Wang, J.L.; Baguet, É. Improvement of the inverse-gated-decoupling sequence for a faster quantitative analysis by 13C NMR. Comptes Rendus Chim. 2006, 9, 525–529. [Google Scholar] [CrossRef]
- Yan, X.; Fang, W.W.; Xue, J.; Sun, T.C.; Dong, L.; Zha, Z.; Qian, H.; Song, Y.H.; Zhang, M.; Gong, X.; et al. Thermoresponsive in Situ Forming Hydrogel with Sol-Gel Irreversibility for Effective Methicillin-Resistant Staphylococcus aureus Infected Wound Healing. ACS Nano 2019, 13, 10074–10084. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Hu, K.; Guo, Z.; Zhang, Q.; Chen, J.; Zhang, T.; Gu, N. Thermo-Sensitive PLGA-PEG-PLGA Tri-Block Copolymer Hydrogel as Three-Dimensional Cell Culture Matrix for Ovarian Cancer Cells. J. Nanosci. Nanotechnol. 2018, 18, 5252–5255. [Google Scholar] [CrossRef] [PubMed]
- Vojtova, L.; Michlovska, L.; Valova, K.; Zboncak, M.; Trunec, M.; Castkova, K.; Krticka, M.; Pavlinakova, V.; Polacek, P.; Dzurov, M.; et al. The Effect of the Thermosensitive Biodegradable PLGA(-)PEG(-)PLGA Copolymer on the Rheological, Structural and Mechanical Properties of Thixotropic Self-Hardening Tricalcium Phosphate Cement. Int. J. Mol. Sci. 2019, 20, 391. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Q.; Zhu, Y.; Liu, Y.; Xie, X.; Li, S.; Lin, H.; Chen, W.; Zhu, F. Co-delivery of metformin and levofloxacin hydrochloride using biodegradable thermosensitive hydrogel for the treatment of corneal neovascularization. Drug Deliv. 2019, 26, 522–531. [Google Scholar] [CrossRef]
- Chan, P.S.; Xian, J.W.; Li, Q.; Chan, C.W.; Leung, S.S.Y.; To, K.K.W. Biodegradable Thermosensitive PLGA-PEG-PLGA Polymer for Non-irritating and Sustained Ophthalmic Drug Delivery. AAPS J. 2019, 21, 59. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Li, S.; Guo, Q.; Xie, J.; Yu, L.; Xu, X.; Ding, C.; Li, J.; Ding, J. Calcitonin-Loaded Thermosensitive Hydrogel for Long-Term Antiosteopenia Therapy. ACS Appl. Mater. Interfaces 2017, 9, 23428–23440. [Google Scholar] [CrossRef]
- Huang, C.; Fu, C.; Qi, Z.P.; Guo, W.L.; You, D.; Li, R.; Zhu, Z. Localised delivery of quercetin by thermo-sensitive PLGA-PEG-PLGA hydrogels for the treatment of brachial plexus avulsion. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1010–1021. [Google Scholar] [CrossRef]
- Wang, S.J.; Qin, J.Z.; Zhang, T.E.; Xia, C. Intra-articular Injection of Kartogenin-Incorporated Thermogel Enhancing Osteoarthritis Treatment. Front. Chem. 2019, 7, 677. [Google Scholar] [CrossRef]
- Yang, X.; Shah, S.J.; Wang, Z.; Agrahari, V.; Pal, D.; Mitra, A.K. Nanoparticle-based topical ophthalmic formulation for sustained release of stereoisomeric dipeptide prodrugs of ganciclovir. Drug Deliv. 2016, 23, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chang, G.T.; Zhang, H.; Ding, J.D. Injectable block copolymer hydrogels for sustained release of a PEGylated drug. Int. J. Pharm. 2008, 348, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Yap, L.S.; Yang, M.C. Thermo-reversible injectable hydrogel composing of pluronic F127 and carboxymethyl hexanoyl chitosan for cell-encapsulation. Colloids Surf. B Biointerfaces 2020, 185, 110606. [Google Scholar] [CrossRef]
- Mellati, A.; Valizadeh, K.M.; Dai, S.; Bi, J.; Jin, B.; Zhang, H. Influence of polymer molecular weight on the in vitro cytotoxicity of poly (N-isopropylacrylamide). Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, L.; Tan, L.; Wang, M.; Ren, X.; Pi, J.; Jiang, M.; Li, N. Preparation and Characterization of PLGA-PEG-PLGA Nanoparticles Containing Salidroside and Tamoxifen for Breast Cancer Therapy. AAPS Pharm. Sci. Tech. 2020, 21, 85. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.S.; Li, Q.; Zhang, B.; To, K.K.W.; Leung, S.S.Y. In vivo biocompatibility and efficacy of dexamethasone-loaded PLGA-PEG-PLGA thermogel in an alkali-burn induced corneal neovascularization disease model. Eur. J. Pharm. Biopharm. 2020, 155, 190–198. [Google Scholar] [CrossRef]
- Yogev, S.; Shabtay-Orbach, A.; Nyska, A.; Mizrahi, B. Local Toxicity of Topically Administrated Thermoresponsive Systems: In Vitro Studies with In Vivo Correlation. Toxicol. Pathol. 2019, 47, 426–432. [Google Scholar] [CrossRef]
- Lin, W.; Xu, T.; Wang, Z.; Chen, J. Sustained intrathecal delivery of amphotericin B using an injectable and biodegradable thermogel. Drug Deliv. 2021, 28, 499–509. [Google Scholar] [CrossRef]
- Liu, P.; Wang, B.; Qiao, W.; Li, J. Novel thermosensitive nano-micelles from triblock copolymer for drug delivery. J. Biomed. Nanotechnol. 2009, 5, 310–313. [Google Scholar] [CrossRef]
- Dutta, K.; Das, R.; Ling, J.; Monibas, R.M.; Carballo-Jane, E.; Kekec, A.; Feng, D.D.; Lin, S.; Mu, J.; Saklatvala, R.; et al. In Situ Forming Injectable Thermoresponsive Hydrogels for Controlled Delivery of Biomacromolecules. ACS Omega 2020, 5, 17531–17542. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Xu, W.; Xiao, G.; Ding, J.; Chen, X. Tumor microenvironment-labile polymer-doxorubicin conjugate thermogel combined with docetaxel for in situ synergistic chemotherapy of hepatoma. Acta Biomater. 2018, 77, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, T.N.S.; Larasati, D.; Nugroho, A.K.; Choiri, S. Assessment of the Effect of PLGA Co-polymers and PEG on the Formation and Characteristics of PLGA-PEG-PLGA Co-block Polymer Using Statistical Approach. Adv. Pharm. Bull. 2019, 9, 382–392. [Google Scholar] [CrossRef]
- Yan, Q.; Xiao, L.Q.; Tan, L.; Sun, W.; Wu, T.; Chen, L.W.; Mei, Y.; Shi, B. Controlled release of simvastatin-loaded thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue regeneration: In vitro and in vivo characteristics. J. Biomed. Mater. Res. A 2015, 103, 3580–3589. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cano, J.J.; Sigen, A.; Andres-Guerrero, V.; Tai, H.; Bravo-Osuna, I.; Molina-Martinez, I.T.; Wang, W.; Herrero-Vanrell, R. Thermo-Responsive PLGA-PEG-PLGA Hydrogels as Novel Injectable Platforms for Neuroprotective Combined Therapies in the Treatment of Retinal Degenerative Diseases. Pharmaceutics 2021, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Zhen, W.; Zhu, Y.; Wang, W.; Hou, Z. Synthesis and Properties of Amphipathic Poly(D,L-lactide-co-glycolide)-polyethylene glycol-poly(D,L-lactide-co-glycolide) Triblock Copolymers. Aust. J. Chem. 2015, 68, 1593–1598. [Google Scholar] [CrossRef]
- Steinman, N.Y.; Haim, Z.M.; Goldstein, I.A.; Goldberg, A.H.; Haber, T.; Berlin, J.M.; Domb, A.J. Effect of PLGA block molecular weight on gelling temperature of PLGA-PEG-PLGA thermoresponsive copolymers. J. Polym. Sci. Part. A Polym. Chem. 2018, 57, 35–39. [Google Scholar] [CrossRef]
- Wang, P.; Chu, W.; Zhuo, X.; Zhang, Y.; Gou, J.; Ren, T.; He, H.; Yin, T.; Tang, X. Modified PLGA-PEG-PLGA thermosensitive hydrogels with suitable thermosensitivity and properties for use in a drug delivery system. J. Mater. Chem. B 2017, 5, 1551–1565. [Google Scholar] [CrossRef]
- Chen, L.; Ci, T.; Yu, L.; Ding, J. Effects of Molecular Weight and Its Distribution of PEG Block on Micellization and Thermogellability of PLGA–PEG–PLGA Copolymer Aqueous Solutions. Macromolecules 2015, 48, 3662–3671. [Google Scholar] [CrossRef]
- Qiao, M.; Chen, D.; Hao, T.; Zhao, X.; Hu, H.; Ma, X. Effect of bee venom peptide-copolymer interactions on thermosensitive hydrogel delivery systems. Int. J. Pharm. 2007, 345, 116–124. [Google Scholar] [CrossRef]
- Newlands, E.S.; Stevens, M.F.; Wedge, S.R.; Wheelhouse, R.T.; Brock, C. Temozolomide: A review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat. Rev. 1997, 23, 35–61. [Google Scholar] [CrossRef]
- Ahad, A.; Shakeel, F.; Raish, M.; Ahmad, A.; Bin Jardan, Y.A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Thermodynamic Solubility Profile of Temozolomide in Different Commonly Used Pharmaceutical Solvents. Molecules 2022, 27, 1437. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lin, C.C.; Parker, D.; Veals, J.; Lim, J.; Likhari, P.; Statkevich, P.; Marco, A.; Nomeir, A.A. High-performance liquid chromatographic determination and stability of 5-(3-methyltriazen-1-yl)-imidazo-4-carboximide, the biologically active product of the antitumor agent temozolomide, in human plasma. J. Chromatogr. B Biomed. Sci. Appl. 1997, 703, 225–233. [Google Scholar] [CrossRef]
- Andrasi, M.; Bustos, R.; Gaspar, A.; Gomez, F.A.; Klekner, A. Analysis and stability study of temozolomide using capillary electrophoresis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Uebbing, L.; Klumpp, L.; Webster, G.K.; Lobenberg, R. Justification of disintegration testing beyond current FDA criteria using in vitro and in silico models. Drug Des. Devel. 2017, 11, 1163–1174. [Google Scholar] [CrossRef]
- Gao, Y.; Ji, H.; Peng, L.; Gao, X.; Jiang, S. Development of PLGA-PEG-PLGA Hydrogel Delivery System for Enhanced Immunoreaction and Efficacy of Newcastle Disease Virus DNA Vaccine. Molecules 2020, 25, 2505. [Google Scholar] [CrossRef]
- Graham-Gurysh, E.G.; Murthy, A.B.; Moore, K.M.; Hingtgen, S.D.; Bachelder, E.M.; Ainslie, K.M. Synergistic drug combinations for a precision medicine approach to interstitial glioblastoma therapy. J. Control. Release 2020, 323, 282–292. [Google Scholar] [CrossRef]
- Sun, J.; Walker, J.; Beck-Broichsitter, M.; Schwendeman, S.P. Characterization of commercial PLGAs by NMR spectroscopy. Drug Deliv. Transl. Res. 2021, 12, 720–729. [Google Scholar] [CrossRef]
- Cespi, M.; Bonacucina, G.; Tiboni, M.; Casettari, L.; Cambriani, A.; Fini, F.; Perinelli, D.R.; Palmieri, G.F. Insights in the rheological properties of PLGA-PEG-PLGA aqueous dispersions: Structural properties and temperature-dependent behaviour. Polymer 2021, 213, 123216. [Google Scholar] [CrossRef]
- Shapira-Furman, T.; Serra, R.; Gorelick, N.; Doglioli, M.; Tagliaferri, V.; Cecia, A.; Peters, M.; Kumar, A.; Rottenberg, Y.; Langer, R.; et al. Biodegradable wafers releasing Temozolomide and Carmustine for the treatment of brain cancer. J. Control. Release 2019, 295, 93–101. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, J.; Zhang, L.; Wang, L.; Xu, H.; Han, Y.; Jia, J.; Lu, Y.; Yu, R.; Liu, H. Injectable postoperative enzyme-responsive hydrogels for reversing temozolomide resistance and reducing local recurrence after glioma operation. Biomater. Sci. 2020, 8, 5306–5316. [Google Scholar] [CrossRef]
- Ngo, M.T.; Karvelis, E.; Harley, B.A.C. Multidimensional hydrogel models reveal endothelial network angiocrine signals increase glioblastoma cell number, invasion, and temozolomide resistance. Integr. Biol. 2020, 12, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Garrett, M.C.; O’shea, T.M.; Wollenberg, A.L.; Bernstein, A.M.; Hung, D.; Staarman, B.; Soto, H.; Deming, T.J.; Sofroniew, M.V.; Kornblum, H.I. Injectable diblock copolypeptide hydrogel provides platform to deliver effective concentrations of paclitaxel to an intracranial xenograft model of glioblastoma. PLoS ONE 2020, 15, e0219632. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Perazzoli, G.; Cabeza, L.; Jimenez-Luna, C.; Luque, R.; Prados, J.; Melguizo, C. Temozolomide: An Updated Overview of Resistance Mechanisms, Nanotechnology Advances and Clinical Applications. Curr. Neuropharmacol. 2021, 19, 513–537. [Google Scholar] [CrossRef] [PubMed]




| Label | MPEG | NLA | NGA | RLA/GA | MPLGA | RPLGA/PEG | Mn (NMR) | Mw (GPC) | Mn (GPC) | PDI | Tgel (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Copolymer–2 | 1616.78 | 21.84 | 6.22 | 3.51 | 1933.89 | 2.39 | 5484.56 | 8321 | 7004 | 1.19 | 35.61 |
| B–0.5 | 1389.96 | 18.56 | 5.84 | 3.18 | 1675.04 | 2.41 | 4740.04 | 7201 | 6224 | 1.16 | 32.65 |
| B–1 | 1539.34 | 20.60 | 6.46 | 3.18 | 1857.81 | 2.41 | 5254.96 | 6858 | 5823 | 1.18 | 31.35 |
| B–1.5 | 1445.62 | 18.97 | 6.20 | 3.06 | 1724.79 | 2.39 | 4895.2 | 7058 | 6454 | 1.09 | 30.01 |
| B–2 | 1483.46 | 19.44 | 6.41 | 3.04 | 1771.53 | 2.39 | 5026.52 | 7240 | 6162 | 1.17 | 29.63 |
| B–2.5 | 1409.54 | 18.48 | 6.12 | 3.02 | 1685.52 | 2.39 | 4780.58 | 6997 | 5917 | 1.18 | 28.90 |
| B–3 | 1553.42 | 20.38 | 6.66 | 3.06 | 1853.28 | 2.39 | 5259.98 | 7085 | 6014 | 1.18 | 28.70 |
| B–3.5 | 1565.74 | 20.57 | 6.78 | 3.03 | 1874.28 | 2.39 | 5314.3 | 6953 | 5769 | 1.20 | 28.60 |
| B–4 | 1367.3 | 18.04 | 6.04 | 2.99 | 1648.91 | 2.41 | 4665.12 | 6870 | 5573 | 1.23 | 28.51 |
| Copolymer–1 | 1424.5 | 18.28 | 6.26 | 2.92 | 1679.89 | 2.36 | 4784.28 | 6855 | 5810 | 1.17 | 27.25 |
| Copolymer–3 | 1585.10 | 19.40 | 5.68 | 3.42 | 1726.24 | 2.18 | 5037.58 | 7579 | 6143 | 1.23 | 37.97 |
| Copolymer–4 | 1563.54 | 21.98 | 6.26 | 3.51 | 1946 | 2.49 | 5455.54 | 9465 | 7869 | 1.20 | I * |
| Copolymer–5 | 1480.82 | 17.79 | 5.58 | 3.19 | 1603.87 | 2.17 | 4688.56 | 8273 | 6603 | 1.25 | 39.14 |
| Drug Loading (mg) | 3 | 50 | 100 | 150 | 250 | |
|---|---|---|---|---|---|---|
| Zero–Order Model | R2 | 0.6202 | 0.8832 | 0.9934 | 0.9889 | 0.9972 |
| K0 (mg/h) | 0.9445 | 0.7246 | 0.6122 | 0.5372 | 0.3910 | |
| F | 8.1644 | 60.4785 | 1360.3078 | 889.7366 | 4233.0078 | |
| p | 0.0355 | 0.0001 * | 0.0000 ** | 0.0000 ** | 0.0000 ** | |
| First–Order Model | R2 | 0.4715 | 0.7478 | 0.8096 | 0.8909 | 0.8383 |
| K1 (h−1) | 0.0168 | 0.0178 | 0.0184 | 0.0148 | 0.0116 | |
| F | 4.4616 | 23.7203 | 38.2707 | 81.6265 | 62.2299 | |
| p | 0.0884 | 0.0012 * | 0.0002 * | 0.0000 ** | 0.0000 ** | |
| Higuchi Model | R2 | 0.8073 | 0.9569 | 0.9665 | 0.9553 | 0.9696 |
| Kh (h−1/2) | 10.2892 | 9.8076 | 8.4369 | 7.8597 | 6.3814 | |
| F | 20.9452 | 177.5321 | 259.4737 | 213.4981 | 382.2087 | |
| p | 0.0060 * | 0.0000 ** | 0.0000 ** | 0.0000 ** | 0.0000 ** | |
| Korsmeyer–Peppas Model | R2 | 0.8836 | 0.9805 | 0.9922 | 0.9467 | 0.9843 |
| Kp (h−n) | 1.8571 | 3.4940 | 4.8246 | 4.0696 | 4.3371 | |
| n | 2.5723 | 1.6442 | 1.4379 | 1.7075 | 1.7062 | |
| F | 37.9687 | 401.5021 | 1151.3027 | 177.6524 | 754.6880 | |
| p | 0.0016 * | 0.0000 ** | 0.0000 ** | 0.0000 ** | 0.0000 ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, W.; Fan, R.; Quan, J.; Cheng, Y.; Wang, S.; Zhang, H.; Zheng, A.; Song, S. Intracranial In Situ Thermosensitive Hydrogel Delivery of Temozolomide Accomplished by PLGA–PEG–PLGA Triblock Copolymer Blending for GBM Treatment. Polymers 2022, 14, 3368. https://doi.org/10.3390/polym14163368
Gu W, Fan R, Quan J, Cheng Y, Wang S, Zhang H, Zheng A, Song S. Intracranial In Situ Thermosensitive Hydrogel Delivery of Temozolomide Accomplished by PLGA–PEG–PLGA Triblock Copolymer Blending for GBM Treatment. Polymers. 2022; 14(16):3368. https://doi.org/10.3390/polym14163368
Chicago/Turabian StyleGu, Weinan, Ranran Fan, Jingnan Quan, Yi Cheng, Shanshan Wang, Hui Zhang, Aiping Zheng, and Shenghan Song. 2022. "Intracranial In Situ Thermosensitive Hydrogel Delivery of Temozolomide Accomplished by PLGA–PEG–PLGA Triblock Copolymer Blending for GBM Treatment" Polymers 14, no. 16: 3368. https://doi.org/10.3390/polym14163368
APA StyleGu, W., Fan, R., Quan, J., Cheng, Y., Wang, S., Zhang, H., Zheng, A., & Song, S. (2022). Intracranial In Situ Thermosensitive Hydrogel Delivery of Temozolomide Accomplished by PLGA–PEG–PLGA Triblock Copolymer Blending for GBM Treatment. Polymers, 14(16), 3368. https://doi.org/10.3390/polym14163368