Controllable Construction of Temperature-Sensitive Supramolecular Hydrogel Based on Cellulose and Cyclodextrin
Abstract
1. Introduction
2. Experimental
2.1. Materials
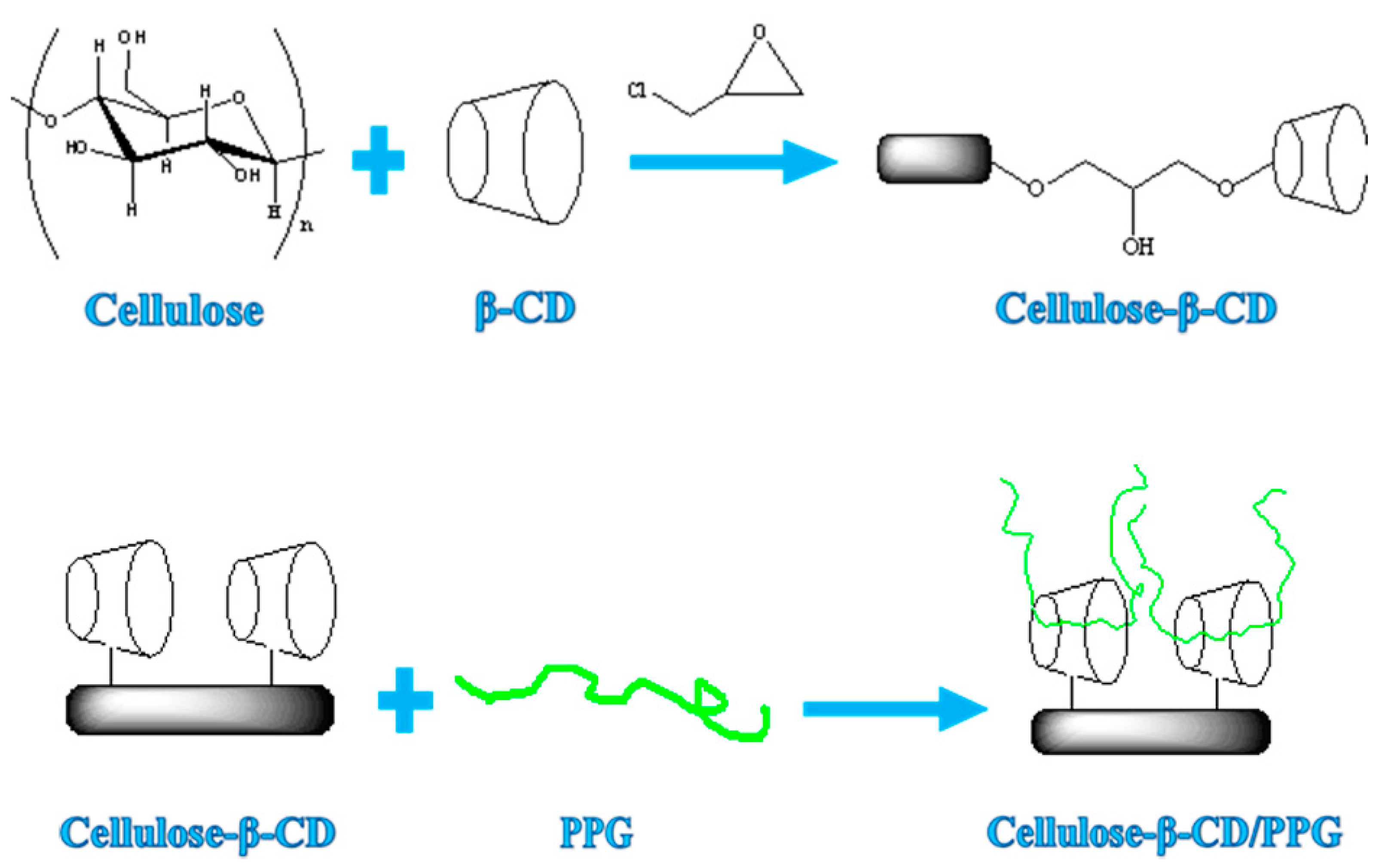
2.2. Preparation of a Cellulose-Based Supramolecular Hydrogel
2.3. Characterization
2.4. Rheology
2.5. Temperature Sensitivity
3. Result and Discussion
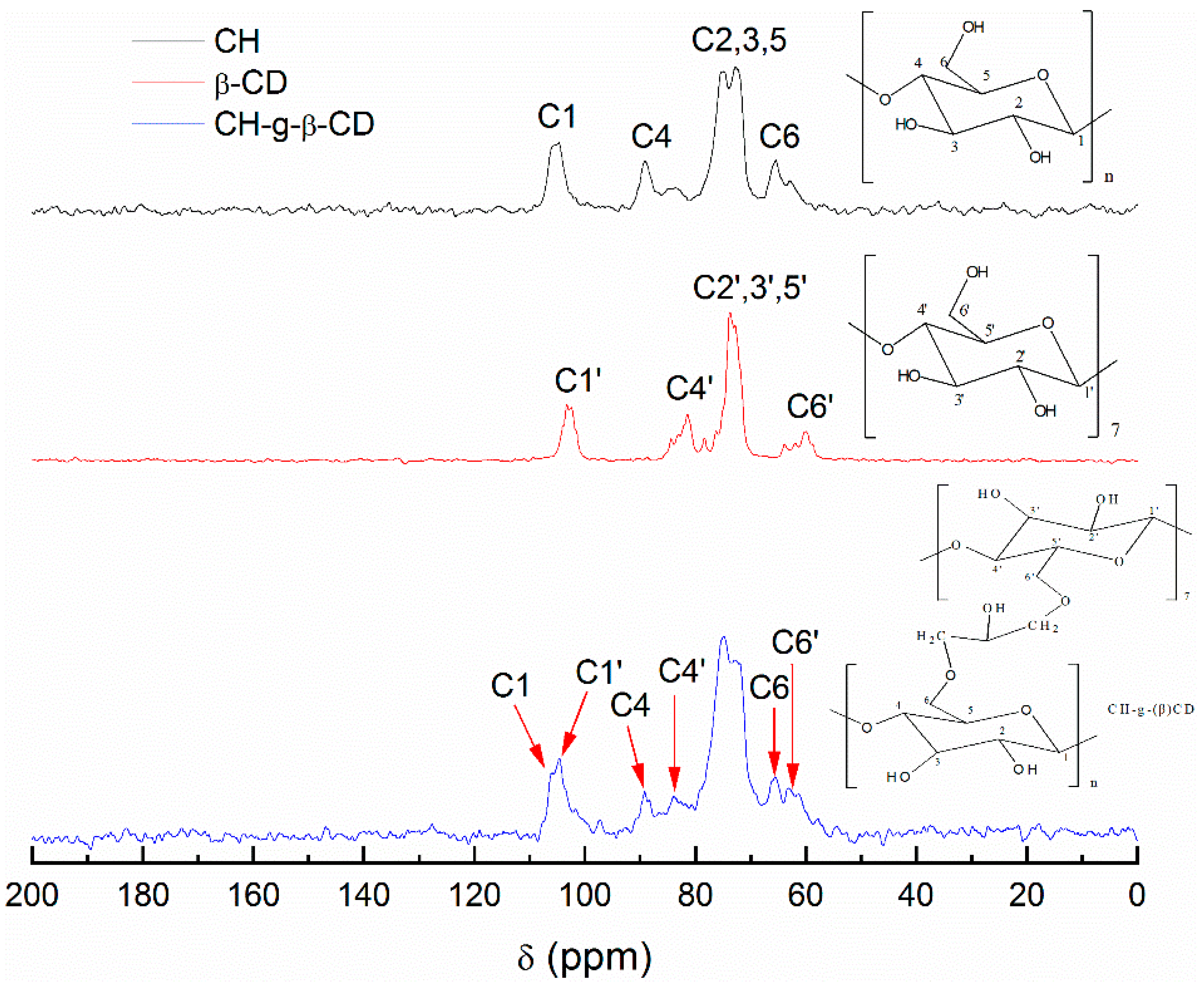
3.1. 13C NMR Analysis
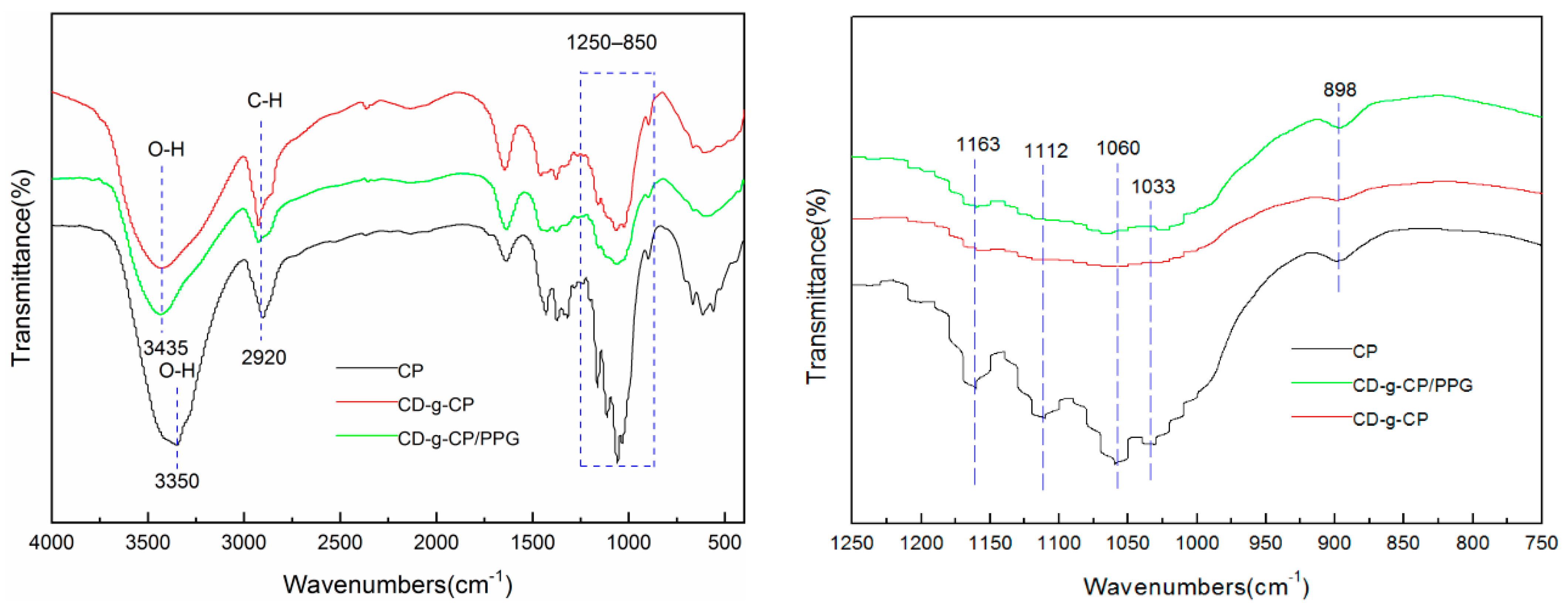
3.2. FTIR
3.3. Morphology
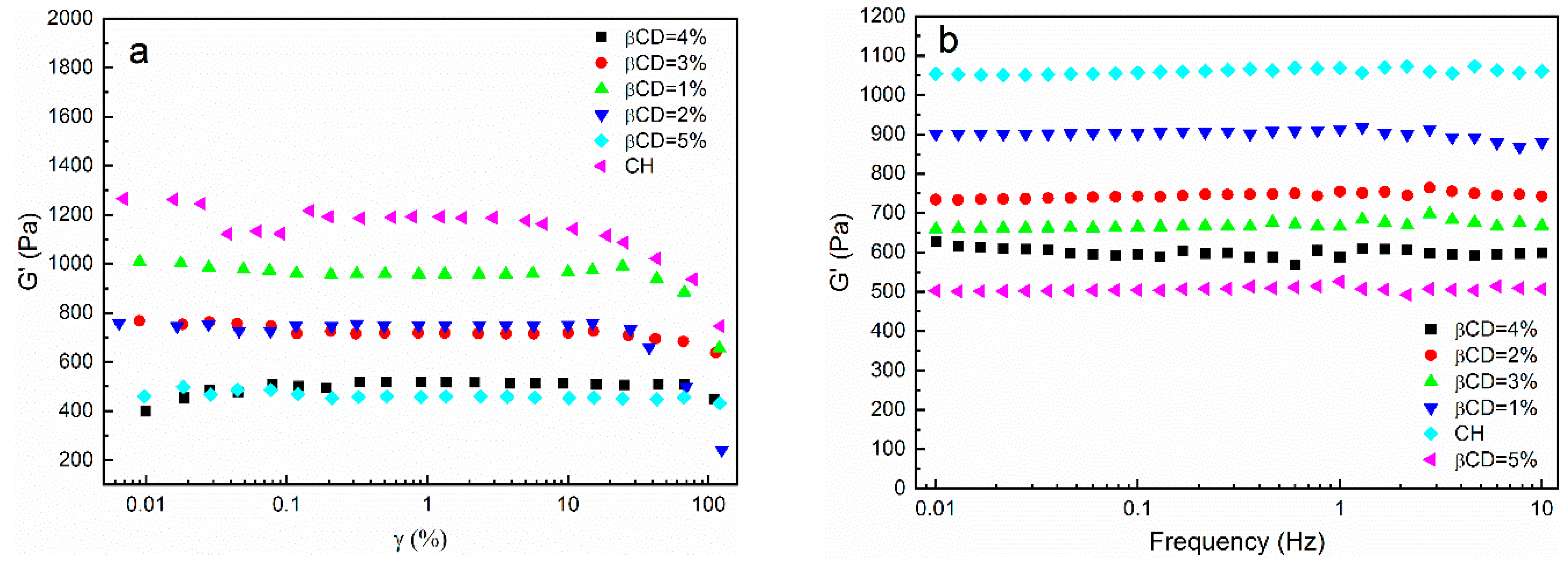
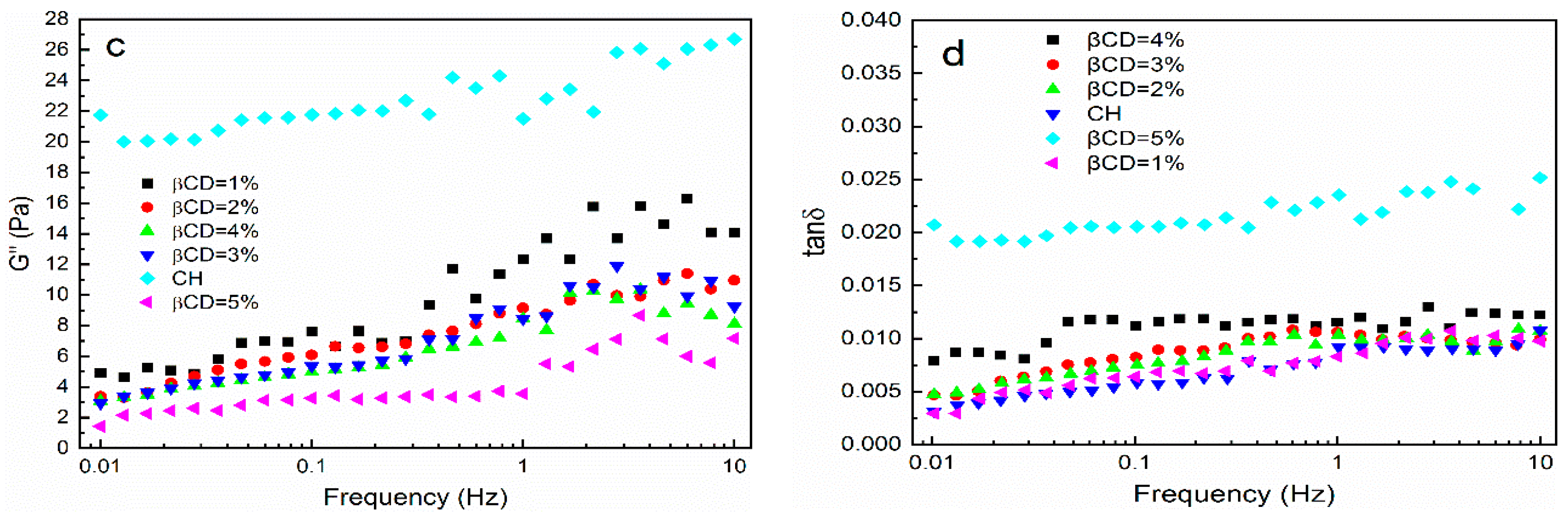
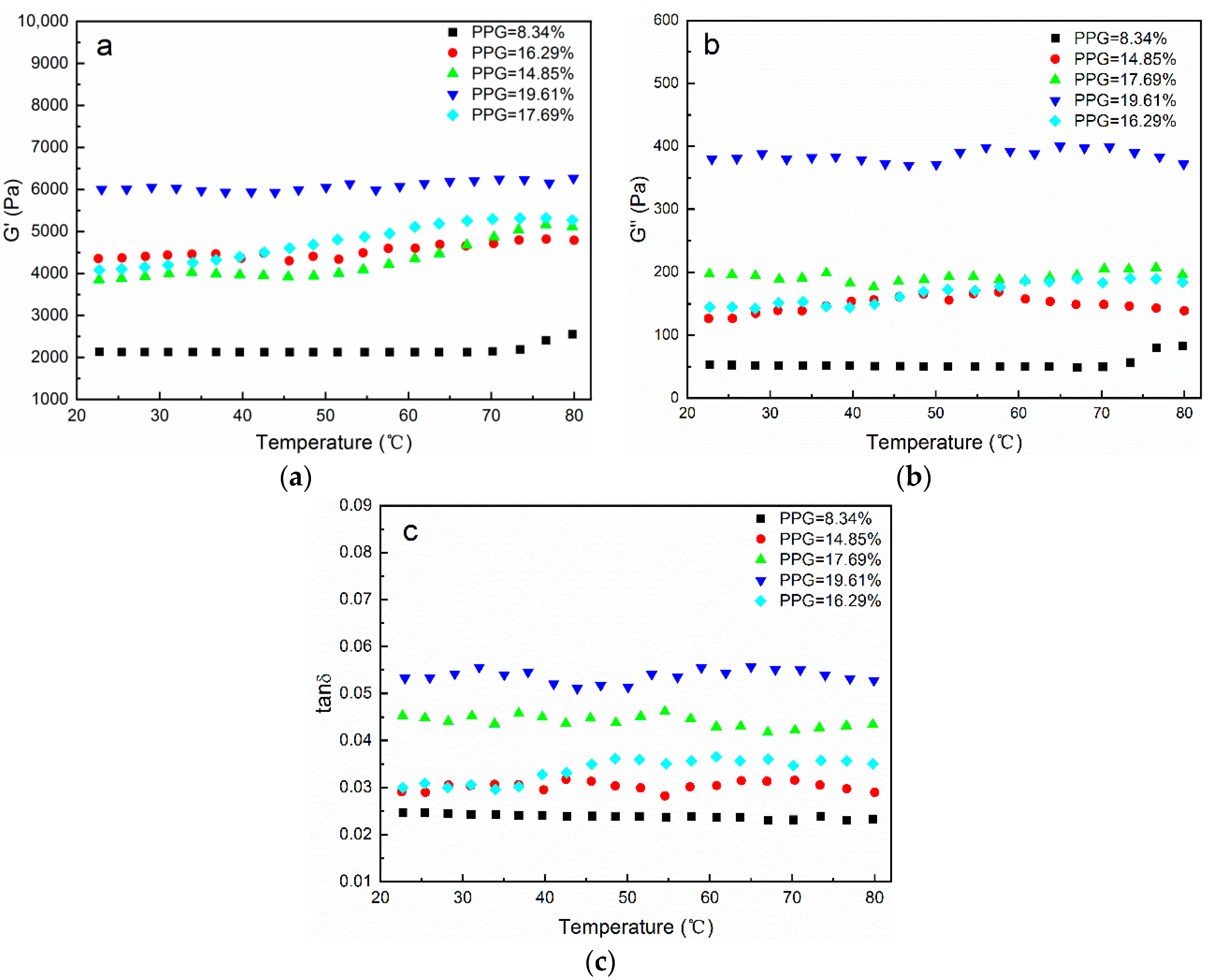
3.4. Rheological Analysis
3.5. TGA
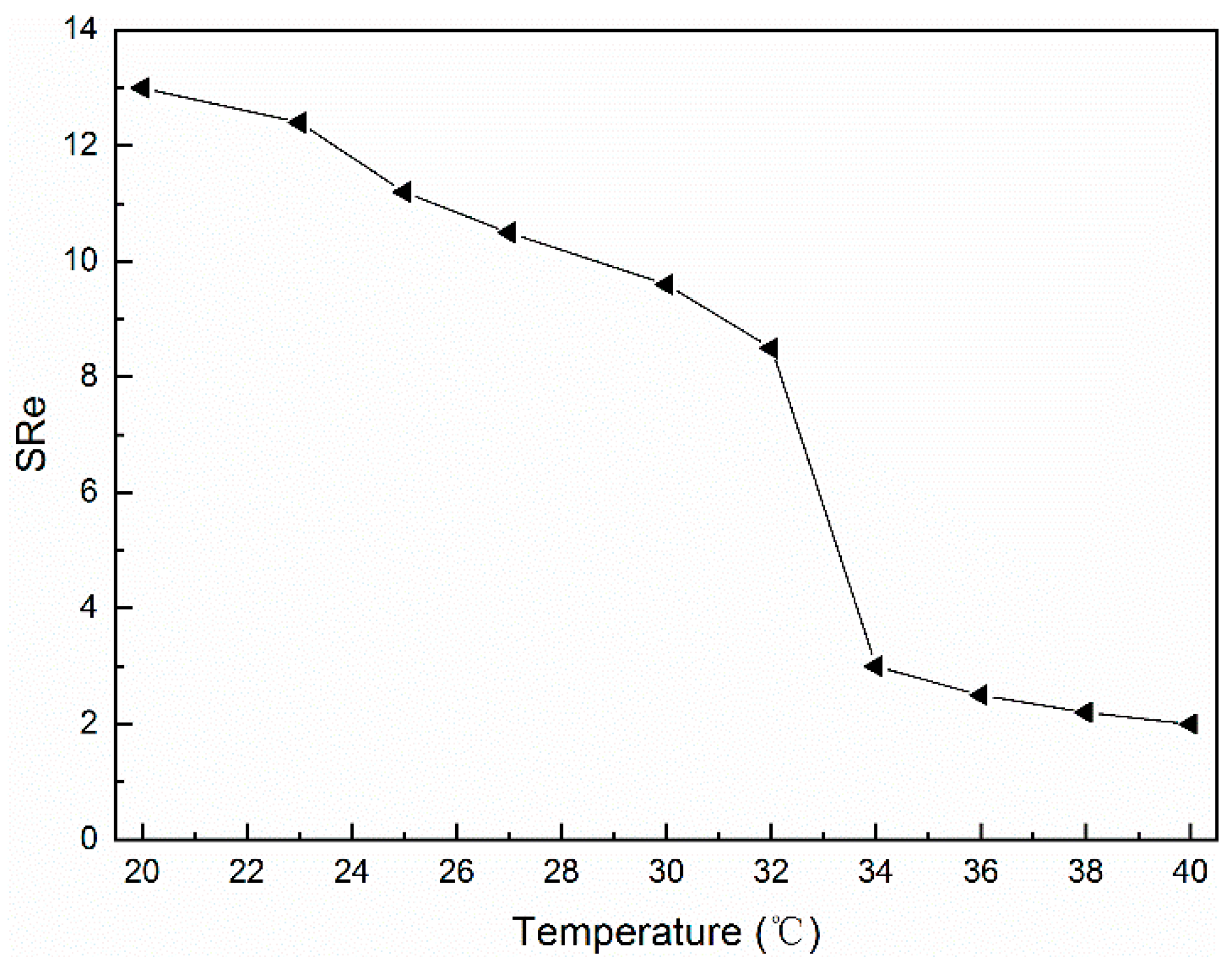
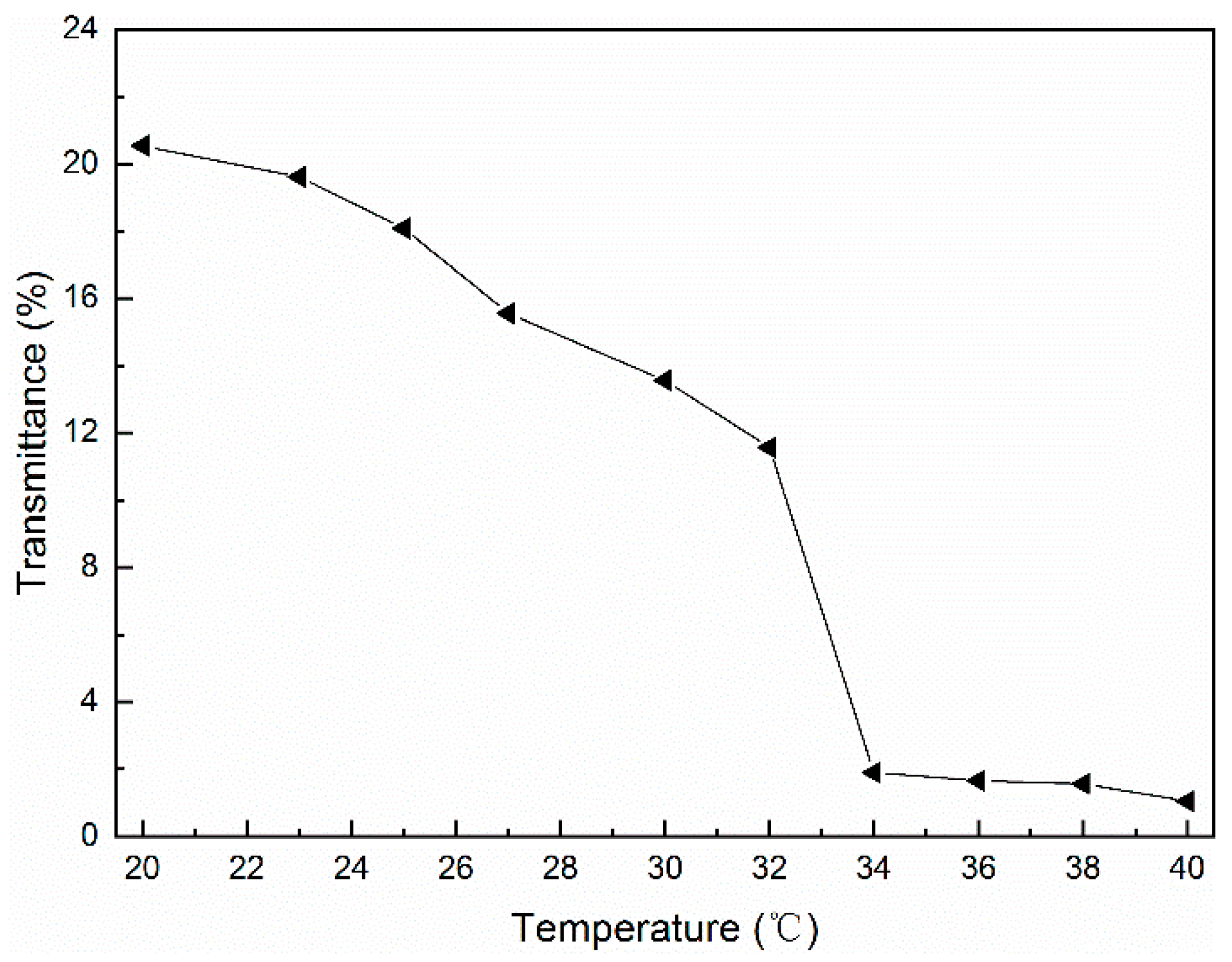
3.6. Thermosensitivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, W.; Li, M.; Zhou, J. Modeling programmable deformation of self-folding all-polymer structures with temperature-sensitive hydrogels. Smart Mater. Struct. 2013, 22, 115028. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Wang, W.; Ji, Q.; Xia, Y. Temperature-sensitive poly(N-isopropylacrylamide)/graphene oxide nanocomposite hydrogels by in situ polymerization with improved swelling capability and mechanical behavior. Eur. Polym. J. 2013, 49, 389–396. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, R.S.; Stiles, W.R.; Jo, M.; Zeng, L.; Rho, S.; Baek, Y.; Kim, J.; Kim, M.S.; Kang, H.; et al. Injectable thermosensitive hydrogels for a sustained release of iron nanochelators. Adv. Sci. 2022, 9, 2200872. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, Q.; Wang, S.; Mao, J.; Guo, Z.; Hu, Y. Synthesis of dual cross-linked ion conductive temperature-sensitive hydrogel and its application in tunable smart window. J. Mater. Sci. 2022, 57, 12672–12684. [Google Scholar] [CrossRef]
- Chen, F.; Lu, G.; Yuan, H.; Li, R.; Nie, J.; Zhao, Y.; Shu, X.; Zhu, X. Mechanism and regulation of LCST behavior in poly (hydroxypropyl acrylate)-based temperature-sensitive hydrogels. J. Mater. Chem. A 2022. advance article. [Google Scholar] [CrossRef]
- Bulut, E.; Turhan, Y. Synthesis and characterization of temperature-sensitive microspheres based on acrylamide grafted hydroxypropyl cellulose and chitosan for the controlled release of amoxicillin trihydrate. Int. J. Biol. Macromol. 2021, 191, 1191–1203. [Google Scholar] [CrossRef]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Ge, S.; Li, J.; Geng, J.; Liu, S.; Xu, H.; Gu, Z. Adjustable dual temperature-sensitive hydrogel based on a self-assembly cross-linking strategy with highly stretchable and healable properties. Mater. Horiz. 2021, 8, 1189–1198. [Google Scholar] [CrossRef]
- Käfer, F.; Liu, F.; Stahlschmidt, U.; Jérôme, V.; Freitag, R.; Karg, M.; Agarwal, S. LCST and UCST in one: Double thermoresponsive behavior of block copolymers of poly (ethylene glycol) and poly (acrylamide-co-acrylonitrile). Langmuir 2015, 31, 8940–8946. [Google Scholar] [CrossRef]
- Shi, X.; Wu, J.; Wang, Z.; Song, F.; Gao, W.; Liu, S. Synthesis and properties of a temperature-sensitive hydrogel based on physical crosslinking via stereocomplexation of PLLA-PDLA. RSC Adv. 2020, 10, 19759–19769. [Google Scholar] [CrossRef]
- Feki, A.; Hamdi, M.; Jaballi, I.; Zghal, S.; Nasri, M.; Amara, I.B. Conception and characterization of a multi-sensitive composite chitosan-red marine alga-polysaccharide hydrogels for insulin controlled-release. Carbohydr. Polym. 2020, 236, 116046. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Z.; Wu, M.; Xu, X.; Huang, F.; Zhang, L.; Liu, Y.; Shuai, Q. NIR as a “trigger switch” for rapid phase change, on-demand release, and photothermal synergistic antibacterial treatment with chitosan-based temperature-sensitive hydrogel. Int. J. Biol. Macromol. 2021, 191, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liang, X.; You, L.; Yang, Y.; Fen, G.; Gao, Y.; Cui, X. Temperature-sensitive poly (N-isopropylacrylamide)-chitosan hydrogel for fluorescence sensors in living cells and its antibacterial application. Int. J. Biol. Macromol. 2021, 189, 316–323. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.P.; Bruni, G.P.; Fabra, M.J.; da Rosa Zavareze, E.; López-Rubio, A.; Martínez-Sanz, M. Development of food packaging bioactive aerogels through the valorization of Gelidium sesquipedale seaweed. Food Hydrocoll. 2019, 89, 337–350. [Google Scholar] [CrossRef]
- Batista, R.A.; Espitia, P.J.P.; Quintans, J.D.S.S.; Freitas, M.M.; Cerqueira, M.Â.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S. Polymeric ionic liquid materials derived from natural source for adsorption purpose. Sep. Purif. Technol. 2018, 196, 208–216. [Google Scholar] [CrossRef]
- Gul, K.; Gan, R.Y.; Sun, C.X.; Jiao, G.; Wu, D.T.; Li, H.B.; Kenaan, A.; Corke, H.; Fang, Y.P. Recent advances in the structure, synthesis, and applications of natural polymeric hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 3817–3832. [Google Scholar] [CrossRef]
- Cao, J.; Wu, X.; Wang, L.; Shao, G.; Qin, B.; Wang, Z.; Wang, T.; Fu, Y. A cellulose-based temperature sensitivity molecular imprinted hydrogel for specific recognition and enrichment of paclitaxel. Int. J. Biol. Macromol. 2021, 181, 1231–1242. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, W.; Zhang, X.; Meng, X.; Tong, G.; Deng, Y. Temperature-sensitive poly-NIPAm modified cellulose nanofibril cryogel microspheres for controlled drug release. Cellulose 2016, 23, 415–425. [Google Scholar] [CrossRef]
- Crini, G. A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, V.B.; Patel, J.K. Cyclodextrin inclusion complex to enhance solubility of poorly water soluble drugs: A review. Int. J. Pharm. Sci. Res. 2013, 4, 68–76. [Google Scholar]
- Pandey, A. Cyclodextrin-based nanoparticles for pharmaceutical applications: A review. Environ. Chem. Lett. 2021, 19, 4297–4310. [Google Scholar] [CrossRef]
- Kayaci, F.; Sen, H.S.; Durgun, E.; Uyar, T. Functional electrospun polymeric nanofibers incorporating geraniol–cyclodextrin inclusion complexes: High thermal stability and enhanced durability of geraniol. Food Res. Int. 2014, 62, 424–431. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular polymeric materials via cyclodextrin–guest interactions. Acc. Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef]
- Hu, Q.D.; Tang, G.P.; Chu, P.K. Cyclodextrin-based host–guest supramolecular nanoparticles for delivery: From design to applications. Acc. Chem. Res. 2014, 47, 2017–2025. [Google Scholar] [CrossRef]
- Bernardinelli, O.D.; Lima, M.A.; Rezende, C.A.; Polikarpov, I.; deAzevedo, E.R. Quantitative 13C MultiCP solid-state NMR as a tool for evaluation of cellulose crystallinity index measured directly inside sugarcane biomass. Biotechnol. Biofuels 2015, 8, 1–11. [Google Scholar]
- Wang, T.; Hong, M. Solid-state NMR investigations of cellulose structure and interactions with matrix polysaccharides in plant primary cell walls. J. Exp. Bot. 2016, 20, 333–338. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Supramolecular hydrogels from in situ host–guest inclusion between chemically modified cellulose nanocrystals and cyclodextrin. Biomacromolecules 2013, 14, 871–880. [Google Scholar] [CrossRef]
- Mariano, M.; Bernardinelli, O.D.; Pires-Oliveira, R.; Ferreira, G.A.; Loh, W. Inclusion complexation between α-cyclodextrin and oligo (ethylene glycol) methyl ether methacrylate. ACS Omega 2020, 5, 9517–9528. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Rambabu, N.; PAnthapulakkal, S.; Sain, M.; Dalai, A.K. Production of nanocellulose fibers from pinecone biomass: Evaluation and optimization of chemical and mechanical treatment conditions on mechanical properties of nanocellulose films. Ind. Crops Prod. 2016, 83, 746–754. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, S.; Cheng, X.; Yam, R.C.; Kong, D.; Li, R.K. Surface modification of cellulose fiber via supramolecular assembly of biodegradable polyesters by the aid of host-guest inclusion complexation. Biomacromolecules 2010, 11, 1364–1369. [Google Scholar] [CrossRef]
- Yu, S.; Yuan, J.; Shi, J.; Ruan, X.; Wang, Y.; Gao, S.; Du, Y. One-pot synthesis of water-soluble, β-cyclodextrin-based polyrotaxanes in a homogeneous water system and its use in bio-applications. J. Mater. Chem. B 2015, 3, 5277–5283. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Ni, X.; Wang, X.; Li, H.; Leong, K.W. Self-assembled supramolecular hydrogels formed by biodegradable PEO–PHB–PEO triblock copolymers and α-cyclodextrin for controlled drug delivery. Biomaterials 2006, 27, 4132–4140. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H.; Xiao, N.; Ma, X.; Liu, C.; Zhong, L.; Xiao, G. Preparation and properties of epichlorohydrin-cross-linked chitosan/hydroxyethyl cellulose based CuO nanocomposite films. Cellulose 2022, 29, 4413–4426. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; Zhang, L. Structure and properties of β-cyclodextrin/cellulose hydrogels prepared in NaOH/urea aqueous solution. Carbohydr. Polym. 2013, 94, 386–393. [Google Scholar] [CrossRef]
- Duan, J.; Jiang, J.; Han, C.; Yang, J.; Liu, L.; Li, J. The study of intermolecular inclusion in cellulose physical gels. BioResources 2014, 9, 4006–4013. [Google Scholar] [CrossRef][Green Version]
- Lin, N.; Huang, J.; Dufresne, A. Polysaccharide nanocrystals-based materials for advanced applications. Polysacch.-Based Nanocryst. 2015, 6, 219–254. [Google Scholar]
- Yang, K.; Wan, S.; Chen, B.; Gao, W.; Chen, J.; Liu, M.; He, B.; Wu, H. Dual pH and temperature responsive hydrogels based on β-cyclodextrin derivatives for atorvastatin delivery. Carbohydr. Polym. 2016, 136, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Lee, D.S. In situ gelling pH-and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J. Control. Release 2014, 193, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Hu, J.; Xu, X.; Chen, X.; Shen, D. Thermo-and pH-sensitive hydrogels containing the β-cyclodextrin moiety for controlled protein release. Monatsh. Chem. 2014, 145, 39–46. [Google Scholar] [CrossRef]




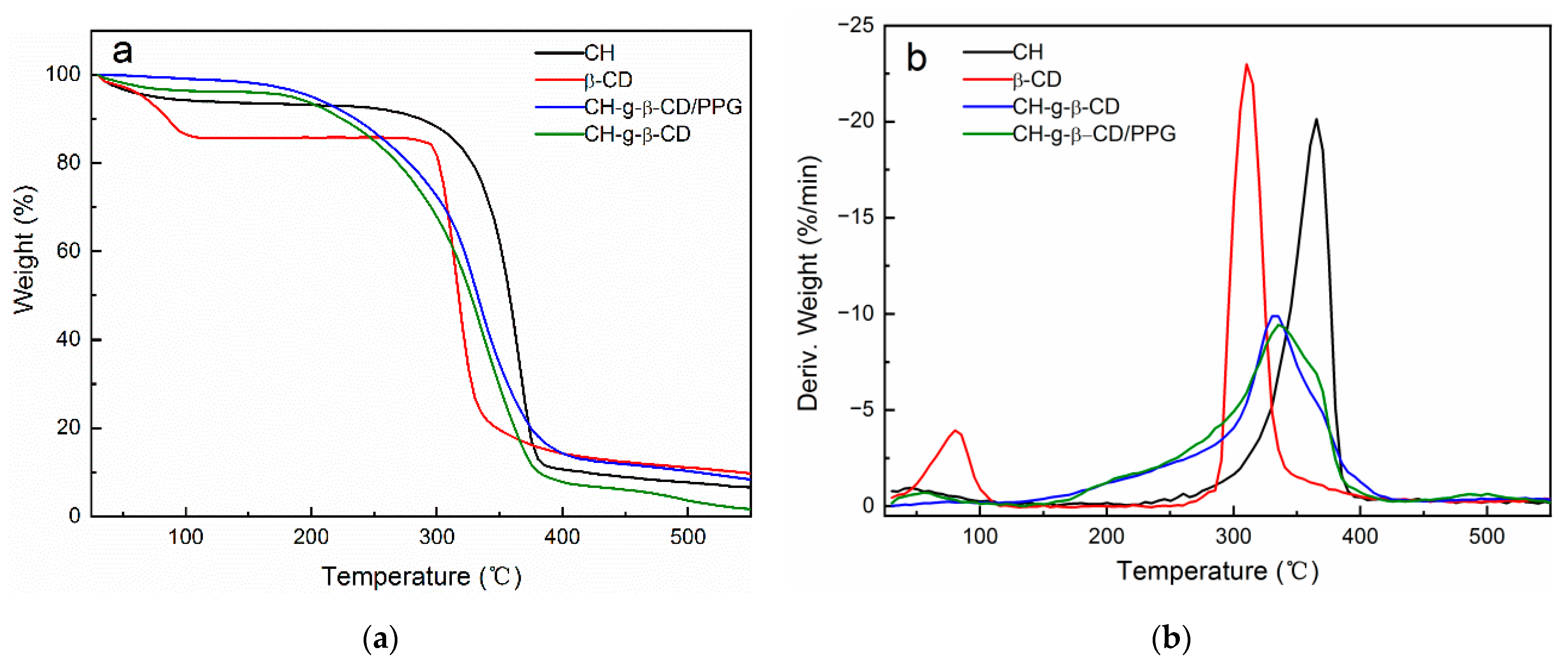
| Sample | Onset Temperature (°C) | Tmax (°C) | Redidual Mass (%) |
|---|---|---|---|
| cellulose | 326 | 354.7 | 6.39 |
| β-CD | 301.6 | 316.5 | 7.75 |
| CH-g-β-CD | 284 | 328.4 | 1.67 |
| CH-g-β-CD/PPG | 294.2 | 332.8 | 4.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Lu, Q.; Wang, H.; Lu, B.; Huang, B. Controllable Construction of Temperature-Sensitive Supramolecular Hydrogel Based on Cellulose and Cyclodextrin. Polymers 2022, 14, 3801. https://doi.org/10.3390/polym14183801
Wu J, Lu Q, Wang H, Lu B, Huang B. Controllable Construction of Temperature-Sensitive Supramolecular Hydrogel Based on Cellulose and Cyclodextrin. Polymers. 2022; 14(18):3801. https://doi.org/10.3390/polym14183801
Chicago/Turabian StyleWu, Jiayin, Qilin Lu, Hanchen Wang, Beili Lu, and Biao Huang. 2022. "Controllable Construction of Temperature-Sensitive Supramolecular Hydrogel Based on Cellulose and Cyclodextrin" Polymers 14, no. 18: 3801. https://doi.org/10.3390/polym14183801
APA StyleWu, J., Lu, Q., Wang, H., Lu, B., & Huang, B. (2022). Controllable Construction of Temperature-Sensitive Supramolecular Hydrogel Based on Cellulose and Cyclodextrin. Polymers, 14(18), 3801. https://doi.org/10.3390/polym14183801







