Application of Nano-Inspired Scaffolds-Based Biopolymer Hydrogel for Bone and Periodontal Tissue Regeneration
Abstract
:1. Introduction
2. Background of Use of Polysaccharide Hydrogel for Bone Defect Treatment
3. Significance of Scaffold-Based Polysaccharide Hydrogel
4. Architecture of the Natural Bone
5. Bone Tissue Engineering Manufacture
6. Nano-Inspired Scaffold-Based Polysaccharide Hydrogel for Bone Tissue Engineering
| Polysaccharides | Proteins |
|---|---|
| Alginate | Collagen |
| Starch | Gelatin |
| Cellulose | Silk |
| Chitosan | Soybean (Glycine max) |
| Hyaluronic acid | Fibrin |
| Xyloglucan | Albumin |
| Chondroitin sulfate | Casein |
| Cyclodextrin | Zein |
| Dextran | Gliadin |
| Heparin | Legumin |
| Kappa-carrageenan | Elastin |
| Gum polysaccharides | |
| Pectin | |
| Pullulan |
| Plants | Mucilage, Pectin, Hemicellulose, Gums Cellulose, Glucomannan, Starch |
|---|---|
| Algae | Carrageenans, alginates, galactans, agar |
| Animals | Cellulose, glycosaminoglycans, hyaluronic acid, chitosan, chitin |
| Bacteria | Cellulose, xanthan, polygalactosamine, gellan, levan, dextran |
| Fungal | Yeast glucans, chitosan, chitin, pollulan, elsinan |

| Application | Advantages of Nanoparticle–Hydrogel Superstructures |
|---|---|
| Drug delivery | Enhanced protection and stability of the drug Prolonged drug retention and drug release sustained Responsive drug release by internal and external stimuli responsive like pH |
| Detoxification | Detoxification agent confinement to the site of diseases Retention of prolonged and release of sustained released |
| Immune modulation | Off-target effects reduction Controlled therapeutic and drug dosages Responsive release of cargo by internal and external stimuli |
| Tissue engineering | Tunable mechanical properties Localized and controlled delivery of drugs Enhanced bioavailability |
| Natural Polysaccharide | Delivery System | Experiment Design | Outcome | Ref |
|---|---|---|---|---|
| Carrageenan | Nano-HA/gum arabic/k-carrageenan composite scaffold | Analysis of the mineralization process and the expression of osteogenic gene markers by osteoblast-like cells using Western blots | Osteoblast-like cells show significant osteogenic markers without cytotoxicity | [91] 2020 |
| Carrageenan | Ag/carrageenan/gelatin nanocomposite | In vitro examination of antibacterial against human pathogens, i.e., S. pyogenes 1210, S. agalactiae 1661, and E. coli | The antibacterial, drug delivery, and anticancer properties of the novel Ag/carrageenan/gelatin hydrogel | [92] 2021 |
| N-carboxyethyl chitosan/hyaluronic acid-aldehyde | N-carboxyethyl chitosan/hyaluronic acid-aldehyde loaded with nanohydroxyapatite | In vitro analysis for osteogenic differentiation. In vivo analysis for alveolar bone regeneration following dental extractions in rats | Maintaining dimensional alveolar ridge and promoting soft-tissue healing | [93] 2020 |
| Regenerated cellulose (rCL) nanofibers/chitosan (CS) | Regenerated cellulose (rCL) nanofibers/chitosan (CS) hydrogel | Alkaline phosphatase (ALP) and alizarin red (ARS) staining were used to assess osteogenic activity in vivo. | The rCL/CS scaffold promoted biomineralization and improved the viability, adhesion, and proliferation of preosteoblast cells (MC3T3-E1) | [94] 2021 |
| Chitosan/hyaluronic acid | Chitosan/hyaluronic acid nanopearl composite | In vivo Cell Counting Kit-8 and ALP activity assessment for preosteoblastic cells | Upregulation of RUNX2, OCN, and OPN genes. Best results were obtained with 10 wt% and 25 wt% nanopearl | [95] 2020 |
| Chitosan | Chitosan nanohydrogel/poly-ε-caprolactone (PCL) loaded with nanotriclosan and flurbiprofen | In vivo study of the NG on experimental periodontitis (EP) rats | Dual antibacterial and anti-inflammatory effects, which revealed an excellent therapeutic outcome | [96] 2019 |
| Gelatin/alginate | Gelatin–alginate–graphene oxide nanocomposite scaffold | In vivo mechanical evaluation and cell differentiation of MG-63 cells in vitro/evaluation of in vivo cone beam | Enhancement in the expression of osteoblast transcription factors and ALP | [97] 2019 |
| Carrageenan | Carrageenan/whitlockite nanocomposite hydrogel | In vivo evaluation of osteogenic activity in adipose-derived stem cells; immunocytochemical staining | Enhancement of osteogenic differentiation and ALP activity | [98] 2019 |
| Carrageenan | Carrageenan/nanohydroxyapatitecomposite scaffold | In vivo evaluation of osteoblast viability and adhesion by MTS viability testing | Promotion of osteoblast activity without any pharmaceutical medicaments | [99] 2018 |
| Chitosan | Chitosan gold nanoparticles combined with peroxisome proliferator-activated receptor g | In vivo testing of gene transfer on the improvement of osseointegration in dental implants in diabetic rats | Improving the prognosis of dental implants in diabetes patients (bone development and mineralization) | [100] 2017 |
| Alginate/chitosan | Alginate/chitosan loaded with nanohydroxylapatite | QuantiChrom ALP kit and alizarin red staining were used to assess MCT3 cell growth and mineralization in vivo. | Stimulation of MC3T3 cell differentiation and mineralization, particularly at increasing hydroxyapatite concentrations | [101] 2015 |
7. Bioarchitecture–Microribbon Hydrogel
8. Gelatin-Based Microribbons
9. Poly(ethylene glycol)-Based Microribbons
10. Bioarchitecture Microporous Polysaccharide Hydrogel
11. Microporous Annealed Particle (MAP) Hydrogels
11.1. Gelatin-Based MAP Hydrogels
11.2. PEG-Based MAP Hydrogels
12. Polysaccharide and Proteins in BTE
12.1. Collagen
- (1)
- Nonimmunogenic and noncytotoxic to prevent an inflammatory reaction;
- (2)
- Improved bone regeneration by being osteoinductive, osteoconductive, osteogenic, and osteocompatible;
- (3)
- To the greatest extent possible, replicating the natural ECM to aid cell adherence, propagation, and eventually osteogenic differentiation at the implant site;
- (4)
- Endogenous enzymes or hydrolysis degradable, synchronizing with new bone ingrowth to provide sufficient space for new bone formation;
- (5)
- For repairing load-bearing defects and avoiding denaturation during sterilization, structural stability and mechanical strength are required;
- (6)
- Suitable pore size and interconnected porosity, which can be improved by varying the concentration and variety of polymers and cross-linkers, to improve cell interaction, control the release of encapsulated bioactive factors, and allow the exchange of nutrients, oxygen, and metabolic waste within the hydrogels.
- (7)
- Patient compliance and injectable capacity to minimize discomfort and simplify the operation [165].
12.2. Alginate
12.3. Cellulosic Plant
12.4. Starch
12.5. Xyloglucan
12.6. Cyclodextrin
12.7. Dextran
12.8. Hyaluronic Acid
12.9. Chitosan
12.10. Carrageenan
12.11. Gum
12.12. Heparin
12.13. Chondroitin Sulfate
13. The Challenge and Future Direction of a New Generation of BTE Scaffold-Based Polysaccharide Hydrogel
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gopinath, V.; Kamath, S.M.; Priyadarshini, S.; Chik, Z.; Alarfaj, A.A.; Hirad, A.H. Multifunctional applications of natural polysaccharide starch and cellulose: An update on recent advances. Biomed. Pharmacother. 2022, 146, 112492. [Google Scholar] [CrossRef]
- Rajan, M. Functional Nanomaterials for Regenerative Tissue Medicines; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Kumar, N.; Chamoli, P.; Misra, M.; Manoj, M.K.; Sharma, A. Advanced metal and carbon nanostructures for medical, drug delivery and bio-imaging applications. Nanoscale 2022, 14, 3987–4017. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Fuh, J.Y.H.; Dheen, S.T.; Kumar, A.S. Functions and applications of metallic and metallic oxide nanoparticles in orthopedic implants and scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Pers, J.-O.; Saraux, A.; Pierre, R.; Youinou, P. Anti–TNF-α Immunotherapy Is Associated With Increased Gingival Inflammation Without Clinical Attachment Loss in Subjects With Rheumatoid Arthritis. J. Periodontol. 2008, 79, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Kirschneck, C.; Fanghänel, J.; Wahlmann, U.; Wolf, M.; Roldán, J.C.; Proff, P. Interactive effects of periodontitis and orthodontic tooth movement on dental root resorption, tooth movement velocity and alveolar bone loss in a rat model. Ann. Anat. Anat. Anz. 2017, 210, 32–43. [Google Scholar] [CrossRef]
- Pragati, S.; Ashok, S.; Kuldeep, S. Recent advances in periodontal drug delivery systems. Int. J. Drug Deliv. 2011, 1, 1–14. [Google Scholar]
- Pers, J.O.; Saraux, A.; Pierre, R.; Youinou, P. A multifunctional polymeric periodontal membrane with osteogenic and antibacterial characteristics. Adv. Funct. Mater. 2018, 28, 1703437. [Google Scholar]
- Trombino, S.; Curcio, F.; Cassano, R.; Curcio, M.; Cirillo, G.; Iemma, F. Polymeric Biomaterials for the Treatment of Cardiac Post-Infarction Injuries. Pharmaceutics 2021, 13, 1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef]
- Li, J.; Cui, X.; Lindberg, G.; Alcala-Orozco, C.R.; Hooper, G.J.; Lim, K.; Woodfield, T.B.F. Hybrid fabrication of photo-clickable vascular hydrogels with additive manufactured titanium implants for enhanced osseointegration and vascularized bone formation. Biofabrication 2022, 14, 34. [Google Scholar] [CrossRef]
- Wei, W.; Ma, Y.; Yao, X.; Zhou, W.; Wang, X.; Li, C.; Lin, J.; He, Q.; Leptihn, S.; Ouyang, H. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact. Mater. 2021, 6, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Chhaya, M.P.; Poh, P.S.P.; Balmayor, E.R.; van Griensven, M.; Schantz, J.-T.; Hutmacher, D.W. Additive manufacturing in biomedical sciences and the need for definitions and norms. Expert Rev. Med Devices 2015, 12, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Kasir, R.; Vernekar, V.N.; Laurencin, C.T. Regenerative Engineering of Cartilage Using Adipose-Derived Stem Cells. Regen. Eng. Transl. Med. 2015, 1, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Simon, V.; Cavalu, S.; Simon, S.; Mocuta, H.; Vanea, E.; Prinz, M.; Neumann, M. Surface functionalisation of sol–gel derived aluminosilicates in simulated body fluids. Solid State Ion. 2009, 180, 764–769. [Google Scholar] [CrossRef]
- Ruffini, A.; Sandri, M.; Dapporto, M.; Campodoni, E.; Tampieri, A.; Sprio, S. Nature-Inspired Unconventional Approaches to Develop 3D Bioceramic Scaffolds with Enhanced Regenerative Ability. Biomedicines 2021, 9, 916. [Google Scholar] [CrossRef]
- Ciolacu, D.E.; Nicu, R.; Ciolacu, F. Natural Polymers in Heart Valve Tissue Engineering: Strategies, Advances and Challenges. Biomedicines 2022, 10, 1095. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide hydrogels: Functionalization, construction and served as scaffold for tissue engineering. Carbohydr. Polym. 2022, 278, 118952. [Google Scholar] [CrossRef]
- Houhua, P.; Liping, H.; Xie, Y.; Pan, H.; Zhao, J.; Huang, L.; Zheng, X. Different response of osteoblastic cells to Mg2+, Zn2+ and Sr2+ doped calcium silicate coatings. J. Mater. Sci. Mater. Med. 2016, 27, 56. [Google Scholar] [CrossRef]
- Arkin, V.H.; Narendrakumar, U.; Madhyastha, H.; Manjubala, I. Characterization and In Vitro Evaluations of Injectable Calcium Phosphate Cement Doped with Magnesium and Strontium. ACS Omega 2021, 6, 2477–2486. [Google Scholar] [CrossRef]
- Li, J.; Lai, Y.; Li, M.; Chen, X.; Zhou, M.; Wang, W.; Li, J.; Cui, W.; Zhang, G.; Wang, K.; et al. Repair of infected bone defect with Clindamycin-Tetrahedral DNA nanostructure Complex-loaded 3D bioprinted hybrid scaffold. Chem. Eng. J. 2022, 435, 134855. [Google Scholar] [CrossRef]
- Dumitriu, D.; Menten, R.; Clapuyt, P. Ultrasonography of the bone surface in children: Normal and pathological findings in the bone cortex and periosteum. Pediatr. Radiol. 2022, 52, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Simon, V. Microstructure and bioactivity of acrylic bone cements for prosthetic surgery. J. Optoelectron. Adv. Mater. 2006, 8, 1520–1523. [Google Scholar]
- Blevins, K.M.; Danilkowicz, R.M.; Fletcher, A.N.; Allen, N.B.; Johnson, L.G.; Adams, S.B. In situ 3D bioprinting of musculoskeletal tissues in orthopedic surgery. J. 3D Print. Med. 2022, 6, 25–36. [Google Scholar] [CrossRef]
- Alam Ansari, M.A.; Golebiowska, A.A.; Dash, M.; Kumar, P.; Jain, P.K.; Nukavarapu, S.P.; Ramakrishna, S.; Nanda, H.S. Engineering biomaterials to 3D-print scaffolds for bone regeneration: Practical and theoretical consideration. Biomater. Sci. 2022, 10, 2789–2816. [Google Scholar] [CrossRef] [PubMed]
- Lemos, R.; Maia, F.R.; Reis, R.L.; Oliveira, J.M. Engineering of Extracellular Matrix-like Biomaterials at Nano- and Macroscale toward Fabrication of Hierarchical Scaffolds for Bone Tissue Engineering. Adv. NanoBiomed Res. 2022, 2, 2100116. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, H.; Zhang, Y.; Zhang, S.; Yang, X.; Wei, Y.; Huang, D.; Lian, X. Fabrication and characterization of double-layer asymmetric dressing through electrostatic spinning and 3D printing for skin wound repair. Mater. Des. 2022, 218, 110711. [Google Scholar] [CrossRef]
- Tiwari, S.; Patil, R.; Bahadur, P. Polysaccharide Based Scaffolds for Soft Tissue Engineering Applications. Polymers 2018, 11, 1. [Google Scholar] [CrossRef]
- Witzler, M.; Büchner, D.; Shoushrah, S.; Babczyk, P.; Baranova, J.; Witzleben, S.; Tobiasch, E.; Schulze, M. Polysaccharide-Based Systems for Targeted Stem Cell Differentiation and Bone Regeneration. Biomolecules 2019, 9, 840. [Google Scholar] [CrossRef]
- Park, J.S.; Suryaprakash, S.; Lao, Y.-H.; Leong, K.W. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods 2015, 84, 3–16. [Google Scholar] [CrossRef]
- Murphy, M.B.; Moncivais, K.; Caplan, A. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.; Zhang, Y.; Chen, Y. The application of natural polymer–based hydrogels in tissue engineering. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2019; pp. 273–307. [Google Scholar] [CrossRef]
- Basanth, A.; Mayilswamy, N.; Kandasubramanian, B. Bone regeneration by biodegradable polymers. Polym. Plast. Technol. Mater. 2022, 61, 816–845. [Google Scholar]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nip, J.; Subramanian, A.; James, R.; Harmon, M.D.; Yu, X.; Kumbar, S.G. Osteoinductive Small Molecules: Growth Factor Alternatives for Bone Tissue Engineering. Curr. Pharm. Des. 2013, 19, 3420–3428. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic acid-based hydrogels: From a natural polysaccharide to complex networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Fonticoli, L.; Trubiani, O.; Rajan, T.; Marconi, G.; Bramanti, P.; Mazzon, E.; Pizzicannella, J.; Diomede, F. Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials. Int. J. Mol. Sci. 2021, 22, 5236. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, P.; Rodríguez-Nogales, C.; Jordan, O.; Allémann, E. Combination of mesenchymal stem cells and bioactive molecules in hydrogels for osteoarthritis treatment. Eur. J. Pharm. Biopharm. 2022, 172, 41–52. [Google Scholar] [CrossRef]
- Parmentier, L.; van Vlierberghe, S. Natural hydrogels for bone tissue engineering. In Tissue Engineering Using Ceramics and Polymers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 743–770. [Google Scholar]
- Radulescu, D.-E.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. Novel Trends into the Development of Natural Hydroxyapatite-Based Polymeric Composites for Bone Tissue Engineering. Polymers 2022, 14, 899. [Google Scholar] [CrossRef]
- Minardi, S.; Corradetti, B.; Taraballi, F.; Sandri, M.; Van Eps, J.; Cabrera, F.; Weiner, B.K.; Tampieri, A.; Tasciotti, E. Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials 2015, 62, 128–137. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.; Epari, D.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef]
- Chen, X.; Fan, H.; Deng, X.; Wu, L.; Yi, T.; Gu, L.; Zhou, C.; Fan, Y.; Zhang, X. Scaffold Structural Microenvironmental Cues to Guide Tissue Regeneration in Bone Tissue Applications. Nanomaterials 2018, 8, 960. [Google Scholar] [CrossRef]
- De Witte, T.-M.; E Fratila-Apachitei, L.; Zadpoor, A.A.; A Peppas, N. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Arvidson, K.; Abdallah, B.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruder, S.P.; Fink, D.J.; Caplan, A. Mesenchymal stem cells in bone development, bone repair, and skeletal regenaration therapy. J. Cell. Biochem. 1994, 56, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Khan, F.; Ahmad, S.R. Polysaccharides and Their Derivatives for Versatile Tissue Engineering Application. Macromol. Biosci. 2013, 13, 395–421. [Google Scholar] [CrossRef]
- Ngiam, M.; Liao, S.; Patil, A.J.; Cheng, Z.; Chan, C.K.; Ramakrishna, S. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone 2009, 45, 4–16. [Google Scholar] [CrossRef]
- Türk, S.; Altınsoy, I.; Efe, G.Ç.; İpek, M.; Özacar, M.; Bindal, C. 3D porous collagen/functionalized multiwalled carbon nanotube/chitosan/hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2018, 92, 757–768. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Yadav, H.K.S.; Alsalloum, G.A.; al Halabi, N.A. Nanobionics and nanoengineered prosthetics. In Nanostructures for the Engineering of Cells, Tissues and Organs; Elsevier: Amsterdam, The Netherlands, 2018; pp. 513–587. [Google Scholar]
- Mosaad, K.E.; Shoueir, K.R.; Saied, A.H.; Dewidar, M.M. New Prospects in Nano Phased Co-substituted Hydroxyapatite Enrolled in Polymeric Nanofiber Mats for Bone Tissue Engineering Applications. Ann. Biomed. Eng. 2021, 49, 2006–2029. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Pal, V.K.; Jain, R.; Sen, S.; Kailasam, K.; Roy, S. Designing nanofibrillar cellulose peptide conjugated polymeric hydrogel scaffold for controlling cellular behaviour. Cellulose 2021, 28, 10335–10357. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. caffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Soundarya, S.P.; Menon, A.H.; Chandran, S.V.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef]
- Draghi, L.; Resta, S.; Pirozzolo, M.G.; Tanzi, M.C. Microspheres leaching for scaffold porosity control. J. Mater. Sci. Mater. Electron. 2005, 16, 1093–1097. [Google Scholar] [CrossRef]
- Lima, A.C.; Sher, P.; Mano, J.F. Production methodologies of polymeric and hydrogel particles for drug delivery applications. Expert Opin. Drug Deliv. 2012, 9, 231–248. [Google Scholar] [CrossRef]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, Y.H.; Chong, M.S.; Lee, Y.M. Preparation and characteristics of hybrid scaffolds composed of β-chitin and collagen. Biomaterials 2004, 25, 2309–2317. [Google Scholar] [CrossRef]
- Theron, S.; Yarin, A.; Zussman, E.; Kroll, E. Multiple jets in electrospinning: Experiment and modeling. Polymer 2005, 46, 2889–2899. [Google Scholar] [CrossRef]
- Vadillo, D.C.; Mathues, W.; Clasen, C. Microsecond relaxation processes in shear and extensional flows of weakly elastic polymer solutions. Rheol. Acta 2012, 51, 755–769. [Google Scholar] [CrossRef]
- Mittal, H.; Ray, S.S.; Kaith, B.S.; Bhatia, J.K.; Sukriti; Sharma, J.; Alhassan, S.M. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur. Polym. J. 2018, 109, 402–434. [Google Scholar] [CrossRef]
- Elbert, D.L. Liquid–liquid two-phase systems for the production of porous hydrogels and hydrogel microspheres for biomedical applications: A tutorial review. Acta Biomater. 2011, 7, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, D.; Zhao, X.; Pakvasa, M.; Tucker, A.B.; Luo, H.; Qin, K.H.; Hu, D.A.; Wang, E.J.; Li, A.J.; et al. Stem Cell-Friendly Scaffold Biomaterials: Applications for Bone Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 598607. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumari, K.; Kundu, P.P. Nanocellulose Biocomposites for Bone Tissue Engineering. In Handbook of Nanocelluloses: Classification, Properties, Fabrication, and Emerging Applications; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–51. [Google Scholar] [CrossRef]
- Bouet, G.; Marchat, D.; Cruel, M.; Malaval, L.; Vico, L. In Vitro Three-Dimensional Bone Tissue Models: From Cells to Controlled and Dynamic Environment. Tissue Eng. Part B Rev. 2015, 21, 133–156. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.L.; Gates, E.M.; Gilchrist, C.L.; Hoffman, B.D. Bio-Instructive Scaffolds for Musculoskeletal Tissue Engineering and Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2016; Volume 1. [Google Scholar]
- Zhang, F.; King, M.W. Biodegradable Polymers as the Pivotal Player in the Design of Tissue Engineering Scaffolds. Adv. Health Mater. 2020, 9, e1901358. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhu, C.; Fan, D.; Mi, Y.; Li, X.; Fu, R.Z.; Duan, Z.; Wang, Y.; Feng, R.R. A Novel Human-like Collagen Hydrogel Scaffold with Porous Structure and Sponge-like Properties. Polymers 2017, 9, 638, Corrigendum in 2018, 10, 304. [Google Scholar] [CrossRef]
- Chen, J.; Yu, M.; Guo, B.; Ma, P.X.; Yin, Z. Conductive nanofibrous composite scaffolds based on in-situ formed polyaniline nanoparticle and polylactide for bone regeneration. J. Colloid Interface Sci. 2018, 514, 517–527. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, J.; Fan, D. Fabrication of High-Strength and Porous Hybrid Scaffolds Based on Nano-Hydroxyapatite and Human-Like Collagen for Bone Tissue Regeneration. Polymers 2020, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Ni, P.; Wang, B.; Chu, B.; Zheng, L.; Luo, F.; Luo, J.; Qian, Z. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials 2012, 33, 4801–4809. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; El-Sattar, N.E.A.A. Radiation synthesis of rapidly self-healing hydrogel derived from poly (acrylic acid) with good mechanical strength. Macromol. Chem. Phys. 2020, 221, 2000218. [Google Scholar] [CrossRef]
- Nguyen, B.B.; Moriarty, R.A.; Kamalitdinov, T.; Etheridge, J.M.; Fisher, J.P. Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering. J. Biomed. Mater. Res. Part A 2017, 105, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.-J.; Chiu, K.-H.; Chen, C.-Y.; Huang, C.-H.; Yao, C.-H. Alginate-gelatin based core-shell capsule enhances the osteogenic potential of human osteoblast-like MG-63 cells. Mater. Des. 2021, 210, 110109. [Google Scholar] [CrossRef]
- Abbah, S.A.; Lu, W.W.; Chan, D.; Cheung, K.M.C.; Liu, W.G.; Zhao, F.; Li, Z.Y.; Leong, J.C.Y.; Luk, K.D.K. Osteogenic behavior of alginate encapsulated bone marrow stromal cells: An in vitro study. J. Mater. Sci. Mater. Electron. 2006, 19, 2113–2119. [Google Scholar] [CrossRef]
- Garske, M.D.S.; Schmidt-Bleek, K.; Ellinghaus, A.; Dienelt, A.; Gu, L.; Mooney, D.J.; Duda, G.N.; Cipitria, A. Alginate Hydrogels for In Vivo Bone Regeneration: The Immune Competence of the Animal Model Matters. Tissue Eng. Part A 2020, 26, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Mu, M.; Fan, R.; Zou, B.; Guo, G. Functionalized chitosan as a promising platform for cancer immunotherapy: A review. Carbohydr. Polym. 2022, 290, 119452. [Google Scholar] [CrossRef]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous bone grafts in oral implantology—Is it still a “gold standard”. A consecutive review of. Int. J. Implant. Dent. 2017, 3, 279. [Google Scholar] [CrossRef]
- Bernardo, M.P.; da Silva, B.C.R.; Hamouda, A.E.I.; de Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite scaffolds exhibit in vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
- Nambiar, J.; Jana, S.; Nandi, S.K. Strategies for Enhancing Vascularization of Biomaterial-Based Scaffold in Bone Regeneration. Chem. Rec. 2022, 22, e202200008. [Google Scholar] [CrossRef]
- Magagula, S.; Mohapi, M.; Jafta, N.; Mochane, M.; Lebelo, K.; Lenetha, G. Biopolymer-based biodegradable biomaterials for in vivo and in vitro biomedical applications. In Polymeric Biomaterials for Healthcare Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 165–210. [Google Scholar]
- Adeyemi, S.A.; Choonara, Y.E. Current advances in cell therapeutics: A biomacromolecules application perspective. Expert Opin. Drug Deliv. 2022, 19, 521–538. [Google Scholar] [CrossRef]
- Bai, X.; Gao, M.; Syed, S.; Zhuang, J.; Xu, X.; Zhang, X.-Q. Bioactive hydrogels for bone regeneration. Bioact. Mater. 2018, 3, 401–417. [Google Scholar] [CrossRef]
- Lima, I.B.; Moreno, L.C.; Silva-Filho, E.C.; Irache, J.M.; Veiga, F.J.; Rolim, H.M.; Nunes, L.C. Development of nanostructured systems using natural polymers to optimize the treatment of inflammatory bowel diseases: A prospective study. J. Drug Deliv. Sci. Technol. 2021, 64, 102590. [Google Scholar] [CrossRef]
- Verma, D.; Gulati, N.; Kaul, S.; Mukherjee, S.; Nagaich, U. Protein Based Nanostructures for Drug Delivery. J. Pharm. 2018, 2018, 9285854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle–hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.; Jolly, R.; Zia, I.; Umar, M.S.; Owais, M.; Shakir, M. Bioactive Gum Arabic/κ-Carrageenan-Incorporated Nano-Hydroxyapatite Nanocomposites and Their Relative Biological Functionalities in Bone Tissue Engineering. ACS Omega 2020, 5, 11279–11290. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Pavithra, U.; Sivaranjani, V.; Balasubramanian, N.; Sakthivel, K.M.; Pruncu, C.I. A novel Ag/carrageenan–gelatin hybrid hydrogel nanocomposite and its biological applications: Preparation and characterization. J. Mech. Behav. Biomed. Mater. 2021, 115, 104257. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Y.; Kuang, R.; Liu, H.; Sun, D.; Mao, T.; Jiang, K.; Yang, X.; Watanabe, N.; Mayo, K.H.; et al. Injectable hydrogel-loaded nano-hydroxyapatite that improves bone regeneration and alveolar ridge promotion. Mater. Sci. Eng. C 2020, 116, 111158. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Aminu, N.; Chan, S.-Y.; Yam, M.-F.; Toh, S.-M. A dual-action chitosan-based nanogel system of triclosan and flurbiprofen for localised treatment of periodontitis. Int. J. Pharm. 2019, 570, 118659. [Google Scholar] [CrossRef]
- Purohit, S.D.; Bhaskar, R.; Singh, H.; Yadav, I.; Gupta, M.K.; Mishra, N.C. Development of a nanocomposite scaffold of gelatin–alginate–graphene oxide for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 592–602. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Mohandas, A.; Hwang, N.S.; Jayakumar, R. Injectable angiogenic and osteogenic carrageenan nanocomposite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2019, 122, 320–328. [Google Scholar] [CrossRef] [PubMed]
- González Ocampo, J.I.; Bassous, N.; Ossa Orozco, C.P.; Webster, T.J. Evaluation of cytotoxicity and antimicrobial activity of an injectable bone substitute of carrageenan and nano hydroxyapatite. J. Biomed. Mater. Res. Part A 2018, 106, 2984–2993. [Google Scholar] [CrossRef] [PubMed]
- Moraes, G.; Zambom, C.; Siqueira, W. Nanoparticles in Dentistry: A Comprehensive Review. Pharmaceuticals 2021, 14, 752. [Google Scholar] [CrossRef]
- Kim, H.-L.; Jung, G.-Y.; Yoon, J.-H.; Han, J.-S.; Park, Y.-J.; Kim, D.-G.; Zhang, M.; Kim, D.-J. Preparation and characterization of nano-sized hydroxyapatite/alginate/chitosan composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 54, 20–25. [Google Scholar] [CrossRef] [PubMed]
- E Stanton, A.; Tong, X.; Jing, M.S.; Behn, A.W.; Storaci, H.; Yang, F. Aligned microribbon scaffolds with hydroxyapatite gradient for engineering bone-tendon interface. Tissue Eng. Part A 2022, 28, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Ghobashy, M.M.; Reheem, A.A.; Mazied, N.A. Ion Etching Induced Surface Patterns of Blend Polymer (Poly Ethylene Glycol–Poly Methyl Methacrylate) Irradiated with Gamma Rays. Int. Polym. Process. 2017, 32, 174–182. [Google Scholar] [CrossRef]
- Rogan, H.; Ilagan, F.; Tong, X.; Chu, C.R.; Yang, F. Microribbon-hydrogel composite scaffold accelerates cartilage regeneration in vivo with enhanced mechanical properties using mixed stem cells and chondrocytes. Biomaterials 2020, 228, 119579. [Google Scholar] [CrossRef]
- Boularaoui, S.; Al Hussein, G.; Khan, K.A.; Christoforou, N.; Stefanini, C. An overview of extrusion-based bioprinting with a focus on induced shear stress and its effect on cell viability. Bioprinting 2020, 20, e00093. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef]
- Conrad, B.; Han, L.-H.; Yang, F. Gelatin-Based Microribbon Hydrogels Accelerate Cartilage Formation by Mesenchymal Stem Cells in Three Dimensions. Tissue Eng. Part A 2018, 24, 1631–1640. [Google Scholar] [CrossRef]
- Tang, Y.; Tong, X.; Conrad, B.; Yang, F. Injectable and in situ crosslinkable gelatin microribbon hydrogels for stem cell delivery and bone regeneration in vivo. Theranostics 2020, 10, 6035–6047. [Google Scholar] [CrossRef] [PubMed]
- Han, L.-H.; Tong, X.; Yang, F. Photo-crosslinkable PEG-Based Microribbons for Forming 3D Macroporous Scaffolds with Decoupled Niche Properties. Adv. Mater. 2014, 26, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Marra, K.G.; Kobayashi, K.; Defail, A.J.; Chu, C.R. Controlled in vivo degradation of genipin crosslinked polyethylene glycol hydrogels within osteochondral defects. Tissue Eng. 2006, 12, 2657–2663. [Google Scholar] [CrossRef]
- Wang, C.; Sinha, S.; Jiang, X.; Murphy, L.; Fitch, S.; Wilson, C.; Grant, G.; Yang, F. Matrix Stiffness Modulates Patient-Derived Glioblastoma Cell Fates in Three-Dimensional Hydrogels. Tissue Eng. Part A 2021, 27, 390–401. [Google Scholar] [CrossRef]
- Elisseeff, J.; Anseth, K.; Sims, D.; McIntosh, W.; Randolph, M.; Yaremchuk, M.; Langer, R. Transdermal photopolymerization of poly (ethylene oxide)-based injectable hydrogels for tissue-engineered cartilage. Plast. Reconstr. Surg. 1999, 104, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, B.J.; Dormer, N.H.; Ingavle, G.C.; Roatch, C.H.; Lomakin, J.; Detamore, M.S.; Gehrke, S.H. Hierarchically Designed Agarose and Poly(Ethylene Glycol) Interpenetrating Network Hydrogels for Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2010, 16, 1533–1542. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Deshmukh, A.S. Polysaccharide-based hydrogels as biomaterials. In Polymeric Hydrogels as Smart Biomaterials; Springer: Berlin/Heidelberg, Germany, 2016; pp. 45–71. [Google Scholar]
- Diekjürgen, D.; Grainger, D.W. Polysaccharide matrices used in 3D in vitro cell culture systems. Biomaterials 2017, 141, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Tan, Z.; Zeng, W.; Wang, X.; Shi, C.; Liu, Y.; He, H.; Chen, R.; Ye, X. Recent Advances of Chitosan-Based Injectable Hydrogels for Bone and Dental Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 587658. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Vega, S.L.; Kwon, M.; Soulas, E.M.; Burdick, J.A. Dimensionality and spreading influence MSC YAP/TAZ signaling in hydrogel environments. Biomaterials 2016, 103, 314–323. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef]
- Chen, Y.; Udduttula, A.; Xie, X.; Zhou, M.; Sheng, W.; Yu, F.; Weng, J.; Wang, D.; Teng, B.; Manivasagam, G.; et al. A novel photocrosslinked phosphate functionalized Chitosan-Sr5(PO4)2SiO4 composite hydrogels and in vitro biomineralization, osteogenesis, angiogenesis for bone regeneration application. Compos. Part B Eng. 2021, 222, 109057. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.-D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Falacho, R.; Palma, P.; Marques, J.; Figueiredo, M.; Caramelo, F.; Dias, I.; Viegas, C.; Guerra, F. Collagenated Porcine Heterologous Bone Grafts: Histomorphometric Evaluation of Bone Formation Using Different Physical Forms in a Rabbit Cancellous Bone Model. Molecules 2021, 26, 1339. [Google Scholar] [CrossRef] [PubMed]
- Zoratto, N.; Di Lisa, D.; de Rutte, J.; Sakib, M.N.; Alves E Silva, A.R.; Tamayol, A.; Di Carlo, D.; Khademhosseini, A.; Sheikhi, A. In situ forming microporous gelatin methacryloyl hydrogel scaffolds from thermostable microgels for tissue engineering. Bioeng. Transl. Med. 2020, 5, e10180. [Google Scholar] [CrossRef]
- Arkenberg, M.R.; Nguyen, H.D.; Lin, C.-C. Recent advances in bio-orthogonal and dynamic crosslinking of biomimetic hydrogels. J. Mater. Chem. B 2020, 8, 7835–7855. [Google Scholar] [CrossRef]
- Isaac, A.; Jivan, F.; Xin, S.; Hardin, J.; Luan, X.; Pandya, M.; Diekwisch, T.G.H.; Alge, D.L. Microporous Bio-orthogonally Annealed Particle Hydrogels for Tissue Engineering and Regenerative Medicine. ACS Biomater. Sci. Eng. 2019, 5, 6395–6404. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Younis, S.A.; Elhady, M.A.; Serp, P. Radiation induced in-situ cationic polymerization of polystyrene organogel for selective absorption of cholorophenols from petrochemical wastewater. J. Environ. Manag. 2018, 210, 307–315. [Google Scholar] [CrossRef]
- Shabaka, S.H.; Marey, R.S.; Ghobashy, M.; Abushady, A.M.; Ismail, G.A.; Khairy, H.M. Thermal analysis and enhanced visual technique for assessment of microplastics in fish from an Urban Harbor, Mediterranean Coast of Egypt. Mar. Pollut. Bull. 2020, 159, 111465. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.S.; Campbell, G.T.; Shekiro, K.M.T.; Anseth, K.S. Clickable microgel scaffolds as platforms for 3D cell encapsulation. Adv. Healthc. Mater. 2017, 6, 1700254. [Google Scholar] [CrossRef]
- Li, F.; Truong, V.X.; Fisch, P.; Levinson, C.; Glattauer, V.; Zenobi-Wong, M.; Thissen, H.; Forsythe, J.S.; Frith, J.E. Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells. Acta Biomater. 2018, 77, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Manzoli, V.; Villa, C.; Bayer, A.; Morales, L.C.; Molano, R.D.; Torrente, Y.; Ricordi, C.; Hubbell, J.A.; Tomei, A.A. Immunoisolation of murine islet allografts in vascularized sites through conformal coating with polyethylene glycol. Am. J. Transplant. 2018, 18, 590–603. [Google Scholar] [CrossRef]
- Shikanov, A.; Smith, R.M.; Xu, M.; Woodruff, T.; Shea, L.D. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials 2011, 32, 2524–2531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khansari, M.M.; Sorokina, L.V.; Mukherjee, P.; Mukhtar, F.; Shirdar, M.R.; Shahidi, M.; Shokuhfar, T. Classification of Hydrogels Based on Their Source: A Review and Application in Stem Cell Regulation. JOM 2017, 69, 1340–1347. [Google Scholar] [CrossRef]
- David, A.; Day, J.; Shikanov, A. Immunoisolation to prevent tissue graft rejection: Current knowledge and future use. Exp. Biol. Med. 2016, 241, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Wyman, O. Development of a High Throughput Microsphere System for Annealed Hydrogels. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2016. [Google Scholar]
- Xin, S.; Gregory, C.A.; Alge, D.L. Interplay between degradability and integrin signaling on mesenchymal stem cell function within poly(ethylene glycol) based microporous annealed particle hydrogels. Acta Biomater. 2020, 101, 227–236. [Google Scholar] [CrossRef]
- Pruett, L.; Koehn, H.; Martz, T.; Churnin, I.; Bs, S.F.; Salopek, L.; Cottler, P.; Griffin, D.R.; Ms, J.J.D. Development of a microporous annealed particle hydrogel for long-term vocal fold augmentation. Laryngoscope 2020, 130, 2432–2441. [Google Scholar] [CrossRef]
- Fang, J.; Koh, J.; Fang, Q.; Qiu, H.; Archang, M.M.; Hasani-Sadrabadi, M.M.; Miwa, H.; Zhong, X.; Sievers, R.; Gao, D.; et al. Injectable Drug-Releasing Microporous Annealed Particle Scaffolds for Treating Myocardial Infarction. Adv. Funct. Mater. 2020, 30, 2004307. [Google Scholar] [CrossRef]
- Pinnaratip, R.; Meng, H.; Rajachar, R.M.; Lee, B.P. Effect of incorporating clustered silica nanoparticles on the performance and biocompatibility of catechol-containing PEG-based bioadhesive. Biomed. Mater. 2018, 13, 025003. [Google Scholar] [CrossRef]
- Atala, A.; Danilevskiy, M.; Lyundup, A.; Glybochko, P.; Butnaru, D.; Vinarov, A.; Yoo, J.J. The potential role of tissue-engineered urethral substitution: Clinical and preclinical studies. J. Tissue Eng. Regen. Med. 2016, 11, 3–19. [Google Scholar] [CrossRef]
- Zhang, L.; Falla, T.J. Antimicrobial peptides: Therapeutic potential. Expert Opin. Pharmacother. 2006, 7, 653–663. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, S.; Ikoma, T.; Monkawa, A.; Marukawa, E.; Sotome, S.; Shinomiya, K.; Tanaka, J. Three-dimensional porous hydroxyapatite/collagen composite with rubber-like elasticity. J. Biomater. Sci. Polym. Ed. 2007, 18, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Itoh, S.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials 2001, 22, 1705–1711. [Google Scholar] [CrossRef]
- Bürck, J.; Aras, O.; Bertinetti, L.; Ilhan, C.A.; Ermeydan, M.A.; Schneider, R.; Ulrich, A.S.; Kazanci, M. Observation of triple helix motif on electrospun collagen nanofibers and its effect on the physical and structural properties. J. Mol. Struct. 2018, 1151, 73–80. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef]
- Ekaputra, A.K.; Prestwich, G.D.; Cool, S.M.; Hutmacher, D.W. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (ε-caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials 2011, 32, 8108–8117. [Google Scholar] [CrossRef]
- Dippold, D.; Cai, A.; Hardt, M.; Boccaccini, A.R.; Horch, R.; Beier, J.P.; Schubert, D.W. Novel approach towards aligned PCL-Collagen nanofibrous constructs from a benign solvent system. Mater. Sci. Eng. C 2017, 72, 278–283. [Google Scholar] [CrossRef]
- Roeder, B.A.; Kokini, K.; Sturgis, J.E.; Robinson, J.P.; Voytik-Harbin, S.L. Tensile Mechanical Properties of Three-Dimensional Type I Collagen Extracellular Matrices With Varied Microstructure. J. Biomech. Eng. 2002, 124, 214–222. [Google Scholar] [CrossRef]
- Ferreira, S.A.; Young, G.; Jones, J.R.; Rankin, S. Bioglass/carbonate apatite/collagen composite scaffold dissolution products promote human osteoblast differentiation. Mater. Sci. Eng. C 2021, 118, 111393. [Google Scholar] [CrossRef]
- Harley, B.A.; Leung, J.H.; Silva, E.C.; Gibson, L.J. Mechanical characterization of collagen–glycosaminoglycan scaffolds. Acta Biomater. 2007, 3, 463–474. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering. Membranes 2018, 8, 62. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of Bioactive Glasses on Angiogenesis: A Review of In Vitro and In Vivo Evidences. Tissue Eng. Part B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass® 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Arcos, D.; Sakamoto, Y.; Terasaki, O.; López-Noriega, A.; Vallet-Regí, M. High-Performance Mesoporous Bioceramics Mimicking Bone Mineralization. Chem. Mater. 2008, 20, 3191–3198. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Xia, L.; Zhao, C.; Chen, L.; Yi, D.; Chang, J.; Huang, L.; Zheng, X.; Zhu, H.; et al. Fabrication of nano-structured calcium silicate coatings with enhanced stability, bioactivity and osteogenic and angiogenic activity. Colloids Surf. B Biointerfaces 2015, 126, 358–366. [Google Scholar] [CrossRef]
- Li, X.; Chang, J. Preparation and characterization of bioactive collagen/wollastonite composite scaffolds. J. Mater. Sci. Mater. Electron. 2005, 16, 361–365. [Google Scholar] [CrossRef]
- Oh, S.-A.; Lee, H.-Y.; Lee, J.H.; Kim, T.-H.; Jang, J.-H.; Kim, H.-W.; Wall, I. Collagen Three-Dimensional Hydrogel Matrix Carrying Basic Fibroblast Growth Factor for the Cultivation of Mesenchymal Stem Cells and Osteogenic Differentiation. Tissue Eng. Part A 2012, 18, 1087–1100. [Google Scholar] [CrossRef]
- Lu, H.; Kawazoe, N.; Kitajima, T.; Myoken, Y.; Tomita, M.; Umezawa, A.; Chen, G.; Ito, Y. Spatial immobilization of bone morphogenetic protein-4 in a collagen-PLGA hybrid scaffold for enhanced osteoinductivity. Biomaterials 2012, 33, 6140–6146. [Google Scholar] [CrossRef]
- Niu, L.; Jiao, K.; Qi, Y.; Nikonov, S.; Yiu, C.K.Y.; Arola, D.D.; Gong, S.; El-Marakby, A.; Carrilho, M.R.O.; Hamrick, M.W.; et al. Intrafibrillar silicification of collagen scaffolds for sustained release of stem cell homing chemokine in hard tissue regeneration. FASEB J. 2012, 26, 4517–4529. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Sculean, A. Does periodontal tissue regeneration really work? Periodontology 2000 2009, 51, 208–219. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Cavalu, S.; Antoniac, I.V.; Mohan, A.; Bodog, F.; Doicin, C.; Mates, I.; Ulmeanu, M.; Murzac, R.; Semenescu, A. Nanoparticles and Nanostructured Surface Fabrication for Innovative Cranial and Maxillofacial Surgery. Materials 2020, 13, 5391. [Google Scholar] [CrossRef]
- Wang, H.; Leeuwenburgh, S.C.; Li, Y.; Jansen, J.A. The Use of Micro- and Nanospheres as Functional Components for Bone Tissue Regeneration. Tissue Eng. Part B Rev. 2012, 18, 24–39. [Google Scholar] [CrossRef]
- Cavalu, S.; Ratiu, C.; Ponta, O.; Simon, V.; Rugina, D.; Miclaus, V.; Akin, I.; Goller, G. Improving osseointegration of alumina/zirconia ceramic implants by fluoride surface treatment. Dig. J. Nanomater. Biostruct. 2014, 9, 797–808. [Google Scholar]
- Lee, S.-H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Bai, S.; Li, B.; Liu, H.; Wu, G.; Liu, S. Fabrication of gelatin methacrylate/nanohydroxyapatite microgel arrays for periodontal tissue regeneration. Int. J. Nanomed. 2016, 11, 4707–4718. [Google Scholar] [CrossRef]
- Sun, W.; Liu, Y.; Miao, L.; Wang, Y.; Ren, S.; Yang, X.; Hu, Y.; Chen, X. Controlled release of recombinant human cementum protein 1 from electrospun multiphasic scaffold for cementum regeneration. Int. J. Nanomed. 2016, 11, 3145–3158. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; El-Wahab, H.A.; Ismail, M.A.; Naser, A.; Abdelhai, F.; El-Damhougy, B.K.; Nady, N.; Meganid, A.S.; Alkhursani, S.A. Characterization of Starch-based three components of gamma-ray cross-linked hydrogels to be used as a soil conditioner. Mater. Sci. Eng. B 2020, 260, 114645. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; El-Damhougy, B.K.; El-Wahab, H.A.; Madani, M.; Amin, M.A.; Naser, A.E.M.; Alshangiti, D.M. Controlling radiation degradation of a CMC solution to optimize the swelling of acrylic acid hydrogel as water and fertilizer carriers. Polym. Adv. Technol. 2021, 32, 514–524. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Massoumi, B.; Mozaffari, Z.; Jaymand, M. A starch-based stimuli-responsive magnetite nanohydrogel as de novo drug delivery system. Int. J. Biol. Macromol. 2018, 117, 418–426. [Google Scholar] [CrossRef]
- Younis, S.A.; Ghobashy, M.M.; Samy, M. Development of aminated poly(glycidyl methacrylate) nanosorbent by green gamma radiation for phenol and malathion contaminated wastewater treatment. J. Environ. Chem. Eng. 2017, 5, 2325–2336. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, L. New solvents and functional materials prepared from cellulose solutions in alkali/urea aqueous system. Food Res. Int. 2013, 52, 387–400. [Google Scholar] [CrossRef]
- Rozi, P.; Abuduwaili, A.; Mutailifu, P.; Gao, Y.; Rakhmanberdieva, R.; Aisa, H.A.; Yili, A. Sequential extraction, characterization and antioxidant activity of polysaccharides from Fritillaria pallidiflora Schrenk. Int. J. Biol. Macromol. 2019, 131, 97–106. [Google Scholar] [CrossRef]
- Titorencu, I.; Albu, M.; Nemecz, M.; Jinga, V. Natural Polymer-Cell Bioconstructs for Bone Tissue Engineering. Curr. Stem Cell Res. Ther. 2017, 12, 165–174. [Google Scholar] [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.-H.; Cho, C.-S.; Park, I.-K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef]
- Mealy, J.E.; Chung, J.J.; Jeong, H.; Issadore, D.; Lee, D.; Atluri, P.; Burdick, J.A. Injectable Granular Hydrogels with Multifunctional Properties for Biomedical Applications. Adv. Mater. 2018, 30, 1705912. [Google Scholar] [CrossRef]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Jayakrishnan, A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Su, W.; Ran, Y.; Ma, X.; Yi, Z.; Chen, G.; Chen, X.; Deng, Z.; Tong, Q.; Wang, X.; et al. Synthesis and characterization of injectable self-healing hydrogels based on oxidized alginate-hybrid-hydroxyapatite nanoparticles and carboxymethyl chitosan. Int. J. Biol. Macromol. 2020, 165, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yao, Y.; Wang, D.-A.; Chen, X. Effect of microcavitary alginate hydrogel with different pore sizes on chondrocyte culture for cartilage tissue engineering. Mater. Sci. Eng. C 2014, 34, 168–175. [Google Scholar] [CrossRef]
- Fang, X.; Lei, L.; Jiang, T.; Chen, Y.; Kang, Y. Injectable thermosensitive alginate/β-tricalcium phosphate/aspirin hydrogels for bone augmentation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 106, 1739–1751. [Google Scholar] [CrossRef]
- Min, Q.; Yu, X.; Liu, J.; Zhang, Y.; Wan, Y.; Wu, J. Controlled Delivery of Insulin-like Growth Factor-1 from Bioactive Glass-Incorporated Alginate-Poloxamer/Silk Fibroin Hydrogels. Pharmaceutics 2020, 12, 574. [Google Scholar] [CrossRef]
- Li, Y.; Fang, X.; Jiang, T. Minimally traumatic alveolar ridge augmentation with a tunnel injectable thermo-sensitive alginate scaffold. J. Appl. Oral Sci. 2015, 23, 215–223. [Google Scholar] [CrossRef]
- Li, Y.; Fang, X.; Jiang, T. 3D printing of porous alginate/gelatin hydrogel scaffolds and their mechanical property characterization. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 299–306. [Google Scholar] [CrossRef]
- Duruel, T.; Çakmak, A.S.; Akman, A.; Nohutcu, R.M.; Gümüşderelioğlu, M. Sequential IGF-1 and BMP-6 releasing chitosan/alginate/PLGA hybrid scaffolds for periodontal regeneration. Int. J. Biol. Macromol. 2017, 104, 232–241. [Google Scholar] [CrossRef]
- Diniz, I.M.A.; Chen, C.; Ansari, S.; Zadeh, H.H.; Moshaverinia, M.; Chee, D.; Marques, M.M.; Shi, S.; Moshaverinia, A.; Dds, M.I.M.A.D.; et al. Gingival Mesenchymal Stem Cell (GMSC) Delivery System Based on RGD-Coupled Alginate Hydrogel with Antimicrobial Properties: A Novel Treatment Modality for Peri-Implantitis. J. Prosthodont. 2016, 25, 105–115. [Google Scholar] [CrossRef]
- Kassem, A.A.; Issa, D.A.E.; Kotry, G.S.; Farid, R.M. Thiolated alginate-based multiple layer mucoadhesive films of metformin forintra-pocket local delivery: In vitro characterization and clinical assessment. Drug Dev. Ind. Pharm. 2017, 43, 120–131. [Google Scholar] [CrossRef]
- Madhumathi, K.; Rekha, L.J.; Kumar, T.S. Tailoring antibiotic release for the treatment of periodontal infrabony defects using bioactive gelatin-alginate/apatite nanocomposite films. J. Drug Deliv. Sci. Technol. 2018, 43, 57–64. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, L.; Zhou, Z.; Sun, Q.; Liu, D.; Chen, Y.; Hu, H.; Cai, Y.; Lin, S.; Yu, Z.; et al. Simultaneous incorporation of PTH(1–34) and nano-hydroxyapatite into Chitosan/Alginate Hydrogels for efficient bone regeneration. Bioact. Mater. 2020, 6, 1839–1851. [Google Scholar] [CrossRef]
- Chalitangkoon, J.; Wongkittisin, M.; Monvisade, P. Silver loaded hydroxyethylacryl chitosan/sodium alginate hydrogel films for controlled drug release wound dressings. Int. J. Biol. Macromol. 2020, 159, 194–203. [Google Scholar] [CrossRef]
- Prakash, J.; Kumar, T.S.; Venkataprasanna, K.; Niranjan, R.; Kaushik, M.; Samal, D.B.; Venkatasubbu, G.D. PVA/alginate/hydroxyapatite films for controlled release of amoxicillin for the treatment of periodontal defects. Appl. Surf. Sci. 2019, 495, 143543. [Google Scholar] [CrossRef]
- Sevari, S.P.; Shahnazi, F.; Chen, C.; Mitchell, J.C.; Ansari, S.; Moshaverinia, A. Bioactive glass-containing hydrogel delivery system for osteogenic differentiation of human dental pulp stem cells. J. Biomed. Mater. Res. Part A 2019, 108, 557–564. [Google Scholar] [CrossRef]
- Tohamy, K.M.; Mabrouk, M.; Soliman, I.E.; Beherei, H.H.; Aboelnasr, M.A. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In vitro cell viability and proliferation of human mesenchymal stem cells. Int. J. Biol. Macromol. 2018, 112, 448–460. [Google Scholar] [CrossRef]
- Tohamy, K.M.; Soliman, I.E.; Mabrouk, M.; ElShebiney, S.; Beherei, H.H.; Aboelnasr, M.A.; Das, D.B. Novel polysaccharide hybrid scaffold loaded with hydroxyapatite: Fabrication, bioactivity, and in vivo study. Mater. Sci. Eng. C 2018, 93, 1–11. [Google Scholar] [CrossRef]
- Dang, L.H.; Doan, P.; Nhi, T.T.Y.; Nguyen, D.T.; Nguyen, B.T.; Nguyen, T.P.; Tran, N.Q. Multifunctional injectable pluronic-cystamine-alginate-based hydrogel as a novel cellular delivery system towards tissue regeneration. Int. J. Biol. Macromol. 2021, 185, 592–603. [Google Scholar] [CrossRef]
- Qiu, L.; Li, Z.; Qiao, M.; Long, M.; Wang, M.; Zhang, X.; Tian, C.; Chen, D. Self-assembled pH-responsive hyaluronic acid–g-poly (l-histidine) copolymer micelles for targeted intracellular delivery of doxorubicin. Acta Biomater. 2014, 10, 2024–2035. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; You, T.; Wang, K.; Xu, F. Effects of polymorphs on dissolution of cellulose in NaOH/urea aqueous solution. Carbohydr. Polym. 2015, 125, 85–91. [Google Scholar] [CrossRef]
- Choe, D.; Kim, Y.M.; Nam, J.E.; Nam, K.; Shin, C.S.; Roh, Y.H. Synthesis of high-strength microcrystalline cellulose hydrogel by viscosity adjustment. Carbohydr. Polym. 2018, 180, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Soenen, S.J.; van Gulck, E.; Vanham, G.; Rejman, J.; Van Calenbergh, S.; Vervaet, C.; Coenye, T.; Verstraelen, H.; Temmerman, M.; et al. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials 2012, 33, 962–969. [Google Scholar] [CrossRef]
- Miao, J.; Pangule, R.C.; Paskaleva, E.E.; Hwang, E.E.; Kane, R.S.; Linhardt, R.J.; Dordick, J.S. Lysostaphin-functionalized cellulose fibers with antistaphylococcal activity for wound healing applications. Biomaterials 2011, 32, 9557–9567. [Google Scholar] [CrossRef]
- Ion, R.; Necula, M.G.; Mazare, A.; Mitran, V.; Neacsu, P.; Schmuki, P.; Cimpean, A. Drug Delivery Systems Based on Titania Nanotubes and Active Agents for Enhanced Osseointegration of Bone Implants. Curr. Med. Chem. 2020, 27, 854–902. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef]
- Bozoğlan, B.K.; Duman, O.; Tunç, S. Preparation and characterization of thermosensitive chitosan/carboxymethylcellulose/scleroglucan nanocomposite hydrogels. Int. J. Biol. Macromol. 2020, 162, 781–797. [Google Scholar] [CrossRef]
- Deng, L.; Liu, Y.; Yang, L.; Yi, J.-Z.; Deng, F.; Zhang, L.-M. Injectable and bioactive methylcellulose hydrogel carrying bone mesenchymal stem cells as a filler for critical-size defects with enhanced bone regeneration. Colloids Surf. B Biointerfaces 2020, 194, 111159. [Google Scholar] [CrossRef]
- Nguyen, T.H.M.; Abueva, C.; Van Ho, H.; Lee, S.-Y.; Lee, B.-T. In vitro and in vivo acute response towards injectable thermosensitive chitosan/TEMPO-oxidized cellulose nanofiber hydrogel. Carbohydr. Polym. 2018, 180, 246–255. [Google Scholar] [CrossRef]
- Ding, L.; Huang, Q.; Li, H.; Wang, Z.; Fu, X.; Zhang, B. Controlled gelatinization of potato parenchyma cells under excess water condition: Structural and in vitro digestion properties of starch. Food Funct. 2019, 10, 5312–5322. [Google Scholar] [CrossRef] [PubMed]
- Katoch, A.; Choudhury, A.R. Understanding the rheology of novel guar-gellan gum composite hydrogels. Mater. Lett. 2020, 263, 127234. [Google Scholar] [CrossRef]
- Morrison, W.R.; Tester, R.F.; Snape, C.E.; Law, R.; Gidley, M.J. Swelling and gelatinization of cereal starches. IV: Some effects of lipid-complexed amylose and free amylose in waxy and normal barley starches. Cereal Chem. 1993, 70, 385–391. [Google Scholar]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Structure-function relationships of starch components. Starch-Stärke 2015, 67, 55–68. [Google Scholar] [CrossRef]
- Nigam, P.; Singh, D. Enzyme and microbial systems involved in starch processing. Enzym. Microb. Technol. 1995, 17, 770–778. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Alshangiti, D.M.; Alkhursani, S.A.; Al-Gahtany, S.A.; Shokr, F.S.; Madani, M. Improvement of In Vitro Dissolution of the Poor Water-Soluble Amlodipine Drug by Solid Dispersion with Irradiated Polyvinylpyrrolidone. ACS Omega 2020, 5, 21476–21487. [Google Scholar] [CrossRef]
- Ali, A.; Rehman, A.; Shehzad, Q.; Khan, S.; Karim, A.; Afzal, N.; Hussain, A.; Yang, F.; Xia, W. Development and Characterization of Nanoemulsions Incorporating Tuna Fish Oil. Int. J. Res. Agric. Sci. 2020, 7, 2348–3997. [Google Scholar]
- Dang, J.M.; Sun, D.D.; Shin-Ya, Y.; Sieber, A.N.; Kostuik, J.P.; Leong, K.W. Temperature-responsive hydroxybutyl chitosan for the culture of mesenchymal stem cells and intervertebral disk cells. Biomaterials 2006, 27, 406–418. [Google Scholar] [CrossRef]
- Bi, S.; Feng, C.; Wang, M.; Kong, M.; Liu, Y.; Cheng, X.; Wang, X.; Chen, X. Temperature responsive self-assembled hydroxybutyl chitosan nanohydrogel based on homogeneous reaction for smart window. Carbohydr. Polym. 2020, 229, 115557. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tehrani, Z.M.; Hosseini, S.H. Dendritic magnetite decorated by pH-responsive PEGylated starch: A smart multifunctional nanocarrier for the triggered release of anti-cancer drugs. Int. J. Res. Agric. Sci. 2015, 5, 48586–48595. [Google Scholar] [CrossRef]
- Dehghanbaniani, D.; Bagheri, R.; Solouk, A. Preparation and characterization of a composite biomaterial including starch micro/nano particles loaded chitosan gel. Carbohydr. Polym. 2017, 174, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.E.; Diez, A.M.D.R.; González, J.A.; Pérez, C.J.; Orrego, M.; Piehl, L.; Teves, S.; Copello, G.J. Antimicrobial Activity of Starch Hydrogel Incorporated with Copper Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 16280–16288. [Google Scholar] [CrossRef]
- Namazi, H.; Hasani, M.; Yadollahi, M. Antibacterial oxidized starch/ZnO nanocomposite hydrogel: Synthesis and evaluation of its swelling behaviours in various pHs and salt solutions. Int. J. Biol. Macromol. 2019, 126, 578–584. [Google Scholar] [CrossRef]
- Gholamali, I.; Hosseini, S.N.; Alipour, E.; Yadollahi, M. Preparation and characterization of oxidized starch/CuO nanocomposite hydrogels applicable in a drug delivery system. Starch-Stärke 2019, 71, 1800118. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Nemati, S.; Kalateh, A. Nanocomposite hydrogel based on carrageenan-coated starch/cellulose nanofibers as a hemorrhage control material. Carbohydr. Polym. 2021, 251, 117013. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Nemati, S.; Kalateh, A. Hybrid nanocomposites for optical applications. Solid State Sci. 2009, 11, 1810–1814. [Google Scholar]
- Park, Y.B.; Cosgrove, D.J. Xyloglucan and its Interactions with Other Components of the Growing Cell Wall. Plant Cell Physiol. 2015, 56, 180–194. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Joshi, A.A.; Patil, C.L.; Amale, P.D.; Patel, H.M.; Surana, S.J.; Belgamwar, V.S.; Chaudhari, K.S.; Pardeshi, C.V. Xyloglucan: A functional biomacromolecule for drug delivery applications. Int. J. Biol. Macromol. 2017, 104, 799–812. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Kulkarni, A.D.; Belgamwar, V.S.; Surana, S.J. Xyloglucan for drug delivery applications. In Fundamental Biomaterials: Polymers; Elsevier: Amsterdam, The Netherlands, 2018; pp. 143–169. [Google Scholar]
- Mahajan, H.S.; Tyagi, V.K.; Patil, R.R.; Dusunge, S.B. Thiolated xyloglucan: Synthesis, characterization and evaluation as mucoadhesive in situ gelling agent. Carbohydr. Polym. 2013, 91, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Guo, Q.; Ji, F.; Tian, X.; Cui, J.; Song, Y.; Sun, H.; Li, J.; Yao, F. Thermoresponsive polysaccharide-based composite hydrogel with antibacterial and healing-promoting activities for preventing recurrent adhesion after adhesiolysis. Acta Biomater. 2018, 74, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Ajovalasit, A.; Sabatino, M.A.; Todaro, S.; Alessi, S.; Giacomazza, D.; Picone, P.; Di Carlo, M.; Dispenza, C. Xyloglucan-based hydrogel films for wound dressing: Structure-property relationships. Carbohydr. Polym. 2018, 179, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.A.; Richard, C.; Ducouret, G.; Bessodes, M.; Scherman, D.; Merten, O.-W. Xyloglucan-Derivatized Films for the Culture of Adherent Cells and Their Thermocontrolled Detachment: A Promising Alternative to Cells Sensitive to Protease Treatment. Biomacromolecules 2013, 14, 512–519. [Google Scholar] [CrossRef]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.; Patel, G.; Abu-Thabit, N.Y. Responsive cyclodextrins as polymeric carriers for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 555–580. [Google Scholar]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.S.; Quintans-Júnior, L.J.; Veiga Júnior, V.F.D.; Neves de Lima, A. Cyclodextrin–drug inclusion complexes: In vivo and in vitro approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Blach, P.; Fourmentin, S.; Landy, D.; Cazier, F.; Surpateanu, G. Cyclodextrins: A new efficient absorbent to treat waste gas streams. Chemosphere 2008, 70, 374–380. [Google Scholar] [CrossRef]
- Wijetunge, S.S.; Wen, J.; Yeh, C.-K.; Sun, Y. Wheat germ agglutinin liposomes with surface grafted cyclodextrins as bioadhesive dual-drug delivery nanocarriers to treat oral cells. Colloids Surf. B Biointerfaces 2020, 185, 110572. [Google Scholar] [CrossRef]
- Ramírez Barragán, C.A.; Macías Balleza, E.R.; García-Uriostegui, L.; Andrade Ortega, J.A.; Toríz, G.; Delgado, E. Rheological characterization of new thermosensitive hydrogels formed by chitosan, glycerophosphate, and phosphorylated β-cyclodextrin. Carbohydr. Polym. 2018, 201, 471–481. [Google Scholar] [CrossRef]
- S Soe, H.M.S.H.; Luckanagul, J.A.; Pavasant, P.; Jansook, P. Development of in situ gel containing asiaticoside/cyclodextrin complexes. Evaluation in culture human periodontal ligament cells (HPLDCs). Int. J. Pharm. 2020, 586, 119589. [Google Scholar] [CrossRef] [PubMed]
- de Paula, G.S.; Oliveira, M.C.; Sales, L.S.; Boriollo, M.; Rodrigues, L.K.A.; Nobre-Dos-Santos, M.; Steiner-Oliveira, C. Antimicrobial photodynamic therapy mediated by methylene blue coupled to β-cyclodextrin reduces early colonizing microorganisms from the oral biofilm. Photodiagn. Photodyn. Ther. 2021, 34, 102283. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T.; Liebert, T.; Heublein, B.; Hornig, S. Functional Polymers Based on Dextran; Springer: Berlin/Heidelberg, Germany, 2006; pp. 199–291. [Google Scholar]
- Sun, G.; Mao, J.J. Engineering dextran-based scaffolds for drug delivery and tissue repair. Nanomedicine 2012, 7, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; He, L.; Tang, Y.; Yang, L.; Wu, B.; Ni, J. Facile design and development of photoluminescent graphene quantum dots grafted dextran/glycol-polymeric hydrogel for thermoresponsive triggered delivery of buprenorphine on pain management in tissue implantation. J. Photochem. Photobiol. B Biol. 2019, 197, 111530. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- Raia, N.R.; Jia, D.; Ghezzi, C.E.; Muthukumar, M.; Kaplan, D.L. Characterization of silk-hyaluronic acid composite hydrogels towards vitreous humor substitutes. Biomaterials 2020, 233, 119729. [Google Scholar] [CrossRef]
- Laurent, T.C.; Fraser, J.R.E. The Properties and Turnover of Hyaluronan. In Ciba Foundation Symposium 124-Functions of the Proteoglycans: Functions of the Proteoglycans: Ciba Foundation Symposium 124; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 9–29. [Google Scholar] [CrossRef]
- Yazdani, M.; Shahdadfar, A.; Jackson, C.J.; Utheim, T.P. Hyaluronan-Based Hydrogel Scaffolds for Limbal Stem Cell Transplantation: A Review. Cells 2019, 8, 245. [Google Scholar] [CrossRef]
- Allison, D.D.; Grande-Allen, K.J. Hyaluronan: A powerful tissue engineering tool. Tissue Eng. 2006, 12, 2131–2140. [Google Scholar] [CrossRef]
- Prestwich, G.D. Engineering a clinically-useful matrix for cell therapy. Organogenesis 2008, 4, 42–47. [Google Scholar] [CrossRef]
- Prestwich, G.D.; Kuo, J.W. Chemically-modified HA for therapy and regenerative medicine. Curr. Pharm. Biotechnol. 2008, 9, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D. Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: A pragmatic approach. J. Cell. Biochem. 2007, 101, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. J. Control. Release 2011, 155, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Bedi, A.; Manjoo, A.; Niazi, F.; Shaw, P.; Mease, P. Anti-Inflammatory Effects of Intra-Articular Hyaluronic Acid: A Systematic Review. Cartilage 2019, 10, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Park, S.S.; Saleh, T.; Ahn, K.M.; Lee, B.T. In vitro and in vivo evaluation of Ca/P-hyaluronic acid/gelatin based novel dental plugs for one-step socket preservation. Mater. Des. 2020, 194, 108891. [Google Scholar] [CrossRef]
- Cui, N.; Qian, J.; Liu, T.; Zhao, N.; Wang, H. Hyaluronic acid hydrogel scaffolds with a triple degradation behavior for bone tissue engineering. Carbohydr. Polym. 2015, 126, 192–198. [Google Scholar] [CrossRef]
- Motokawa, K.; Hahn, S.K.; Nakamura, T.; Miyamoto, H.; Shimoboji, T. Selectively crosslinked hyaluronic acid hydrogels for sustained release formulation of erythropoietin. J. Biomed. Mater. Res. Part A 2006, 78A, 459–465. [Google Scholar] [CrossRef]
- Ozçelik, H.; Batool, F.; Corre, M.; Garlaschelli, A.; Conzatti, G.; Stutz, C.; Petit, C.; Delpy, E.; Zal, F.; Leize-Zal, E.; et al. Characterization of a hyaluronic acid-based hydrogel containing an extracellular oxygen carrier (M101) for periodontitis treatment: An in vitro study. Int. J. Pharm. 2021, 605, 120810. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Acar, O.K.; Kayitmazer, A.B.; Kose, G.T. Hyaluronic Acid/Chitosan Coacervate-Based Scaffolds. Biomacromolecules 2018, 19, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Huang, J.; Zhang, Z. Hydrogels based on chitosan in tissue regeneration: How do they work? A mini review. J. Appl. Polym. Sci. 2019, 136, 47235. [Google Scholar] [CrossRef]
- Samadian, H.; Maleki, H.; Fathollahi, A.; Salehi, M.; Gholizadeh, S.; Derakhshankhah, H.; Allahyari, Z.; Jaymand, M. Naturally occurring biological macromolecules-based hydrogels: Potential biomaterials for peripheral nerve regeneration. Int. J. Biol. Macromol. 2020, 154, 795–817. [Google Scholar] [CrossRef] [PubMed]
- Ghobashy, M.M.; Elkodous, M.A.; Shabaka, S.H.; Younis, S.A.; Alshangiti, D.M.; Madani, M.; Al-Gahtany, S.A.; Elkhatib, W.F.; Noreddin, A.M.; Nady, N.; et al. An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment. Nanotechnol. Rev. 2021, 10, 954–977. [Google Scholar] [CrossRef]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Weiner, M.L. Toxicological properties of carrageenan. Agents Actions 1991, 32, 46–51. [Google Scholar] [CrossRef]
- Catanzaro, P.J.; Schwartz, H.J.; Graham, R.C. Spectrum and possible mechanism of carrageenan cytotoxicity. Am. J. Pathol. 1971, 64, 387–404. [Google Scholar]
- Grenha, A.; Gomes, M.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. Part A 2010, 92, 1265–1272. [Google Scholar] [CrossRef]
- Mihaila, S.M.; Popa, E.G.; Reis, R.L.; Marques, A.; Gomes, M.E. Fabrication of Endothelial Cell-Laden Carrageenan Microfibers for Microvascularized Bone Tissue Engineering Applications. Biomacromolecules 2014, 15, 2849–2860. [Google Scholar] [CrossRef] [PubMed]
- González Ocampo, J.I.; Machado de Paula, M.M.; Bassous, N.J.; Lobo, A.O.; Ossa Orozco, C.P.; Webster, T.J. Osteoblast responses to injectable bone substitutes of kappa-carrageenan and nano hydroxyapatite. Molecules 2019, 83, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Feng, S.; Tang, K.; He, X.; Jing, A.; Liang, G. A novel composite of collagen-hydroxyapatite/kappa-carrageenan. J. Alloys Compd. 2017, 693, 482–489. [Google Scholar] [CrossRef]
- Li, D.; Hu, X.; Zhang, S. Biodegradation of graphene-based nanomaterials in blood plasma affects their biocompatibility, drug delivery, targeted organs and antitumor ability. Biomaterials 2019, 202, 12–25. [Google Scholar] [CrossRef]
- Yasui, T.; Matsuki, J.; Sasaki, T.; Yamamori, M. Amylose and lipid contents, amylopectin structure, and gelatinisation properties of waxy wheat (Triticum aestivum) starch. J. Cereal Sci. 1996, 24, 131–137. [Google Scholar]
- Weaver, C.L.; Cui, X.T. Directed Neural Stem Cell Differentiation with a Functionalized Graphene Oxide Nanocomposite. Adv. Health Mater. 2015, 4, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, H.; Liu, J.; Zhai, G. Advanced Nanocarriers Based on Heparin and Its Derivatives for Cancer Management. Biomacromolecules 2015, 16, 423–436. [Google Scholar] [CrossRef]
- Jeong, D.; Na, K. Chondroitin sulfate based nanocomplex for enhancing the stability and activity of anthocyanin. Carbohydr. Polym. 2012, 90, 507–515. [Google Scholar] [CrossRef]
- Jardim, K.V.; Siqueira, J.L.N.; Báo, S.N.; Sousa, M.H.; Parize, A.L. The role of the lecithin addition in the properties and cytotoxic activity of chitosan and chondroitin sulfate nanoparticles containing curcumin. Carbohydr. Polym. 2020, 227, 115351. [Google Scholar] [CrossRef]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Gruppuso, M.; Turco, G.; Marsich, E.; Porrelli, D. Polymeric wound dressings, an insight into polysaccharide-based electrospun membranes. Appl. Mater. Today 2021, 24, 101148. [Google Scholar] [CrossRef]
- Hu, C.; Ashok, D.; Nisbet, D.R.; Gautam, V. Bioinspired surface modification of orthopedic implants for bone tissue engineering. Biomaterials 2019, 219, 119366. [Google Scholar] [CrossRef]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef]
- Indurkar, A.; Pandit, A.; Jain, R.; Dandekar, P. Plant-based biomaterials in tissue engineering. Bioprinting 2021, 21, e00127. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Bassioni, G. pH stimuli-responsive poly (acrylamide-co-sodium alginate) hydrogels prepared by γ-radiation for improved compressive strength of concrete. Adv. Polym. Technol. 2018, 37, 2123–2133. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; El-Sawy, N.M.; Kodous, A.S. Nanocomposite of cosubstituted carbonated hydroxyapatite fabricated inside Poly(sodium hyaluronate-acrylamide) hydrogel template prepared by gamma radiation for osteoblast cell regeneration. Radiat. Phys. Chem. 2021, 183, 109408. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Elbarbary, A.M.; Hegazy, D.E. Gamma radiation synthesis of a novel amphiphilic terpolymer hydrogel pH-responsive based chitosan for colon cancer drug delivery. Carbohydr. Polym. 2021, 263, 117975. [Google Scholar] [CrossRef]
- Simionescu, B.C.; Ivanov, D. Natural and synthetic polymers for designing composite materials. In Handbook of Bioceramics and Biocomposites; Springer: Berlin/Heidelberg, Germany, 2016; pp. 233–286. [Google Scholar]
- Zhang, Z.; Ortiz, O.; Goyal, R.; Kohn, J. Biodegradable polymers. In Handbook of Polymer Applications in Medicine and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2014; pp. 303–335. [Google Scholar]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef]
- Park, S.-B.; Lih, E.; Park, K.-S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Lutzweiler, G.; Halili, A.N.; Vrana, N.E. The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceutics 2020, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bhaduri, S.; Webster, T.J. Biomaterials in Translational Medicine; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Sivakumar, P.M.; Yetisgin, A.A.; Sahin, S.B.; Demir, E.; Cetinel, S. Bone tissue engineering: Anionic polysaccharides as promising scaffolds. Carbohydr. Polym. 2022, 283, 119142. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Deng, Y.; Su, J. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2009432. [Google Scholar] [CrossRef]
- Dinoro, J.; Maher, M.; Talebian, S.; Jafarkhani, M.; Mehrali, M.; Orive, G.; Foroughi, J.; Lord, M.S.; Dolatshahi-Pirouz, A. Sulfated polysaccharide-based scaffolds for orthopaedic tissue engineering. Biomaterials 2019, 214, 119214. [Google Scholar] [CrossRef]
- Pereira, D.; Canadas, R.; Correia, J.S.; Morais, A.D.S.; Oliveira, M.; Dias, I.; Mano, J.; Marques, A.; Reis, R.; Oliveira, J.M. Injectable gellan-gum/hydroxyapatite-based bilayered hydrogel composites for osteochondral tissue regeneration. Appl. Mater. Today 2018, 12, 309–321. [Google Scholar] [CrossRef] [Green Version]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Mukherjee, I. Recent Development of Polysaccharide-Derived Hydrogel: Properties, Stimuli-Responsiveness and Bioapplications. ChemRxiv 2022. [Google Scholar] [CrossRef]
- Agrawal, G.; Kumar, A. Polysaccharides-Based Biomaterials for Surgical Applications. In Polysaccharides of Microbial Origin; Springer: Cham, Switzerland, 2022; pp. 943–974. [Google Scholar] [CrossRef]
- Xing, F.; Chi, Z.; Yang, R.; Xu, D.; Cui, J.; Huang, Y.; Zhou, C.; Liu, C. Chitin-hydroxyapatite-collagen composite scaffolds for bone regeneration. Int. J. Biol. Macromol. 2021, 184, 170–180. [Google Scholar] [CrossRef]
- Chen, J.; Nichols, B.L.B.; Norris, A.M.; Frazier, C.E.; Edgar, K.J. All-Polysaccharide, Self-Healing Injectable Hydrogels Based on Chitosan and Oxidized Hydroxypropyl Polysaccharides. Biomacromolecules 2020, 21, 4261–4272. [Google Scholar] [CrossRef] [PubMed]
- Pentlavalli, S.; McCarthy, H.O.; Dunne, N.J. Hydrogels for Bone Regeneration: An Overview. In Hydrogels; CRC Press: Boca Raton, FL, USA, 2017; pp. 102–127. [Google Scholar] [CrossRef]
- Sánchez-Téllez, D.A.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 2017, 9, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, V.; Kamath, S.M.; Priyadarshini, S.; Chik, Z.; Alarfaj, A.A.; Hirad, A.H. Hydrogel as a biomaterial for bone tissue engineering: A review. Nanomaterials 2020, 10, 1511. [Google Scholar]
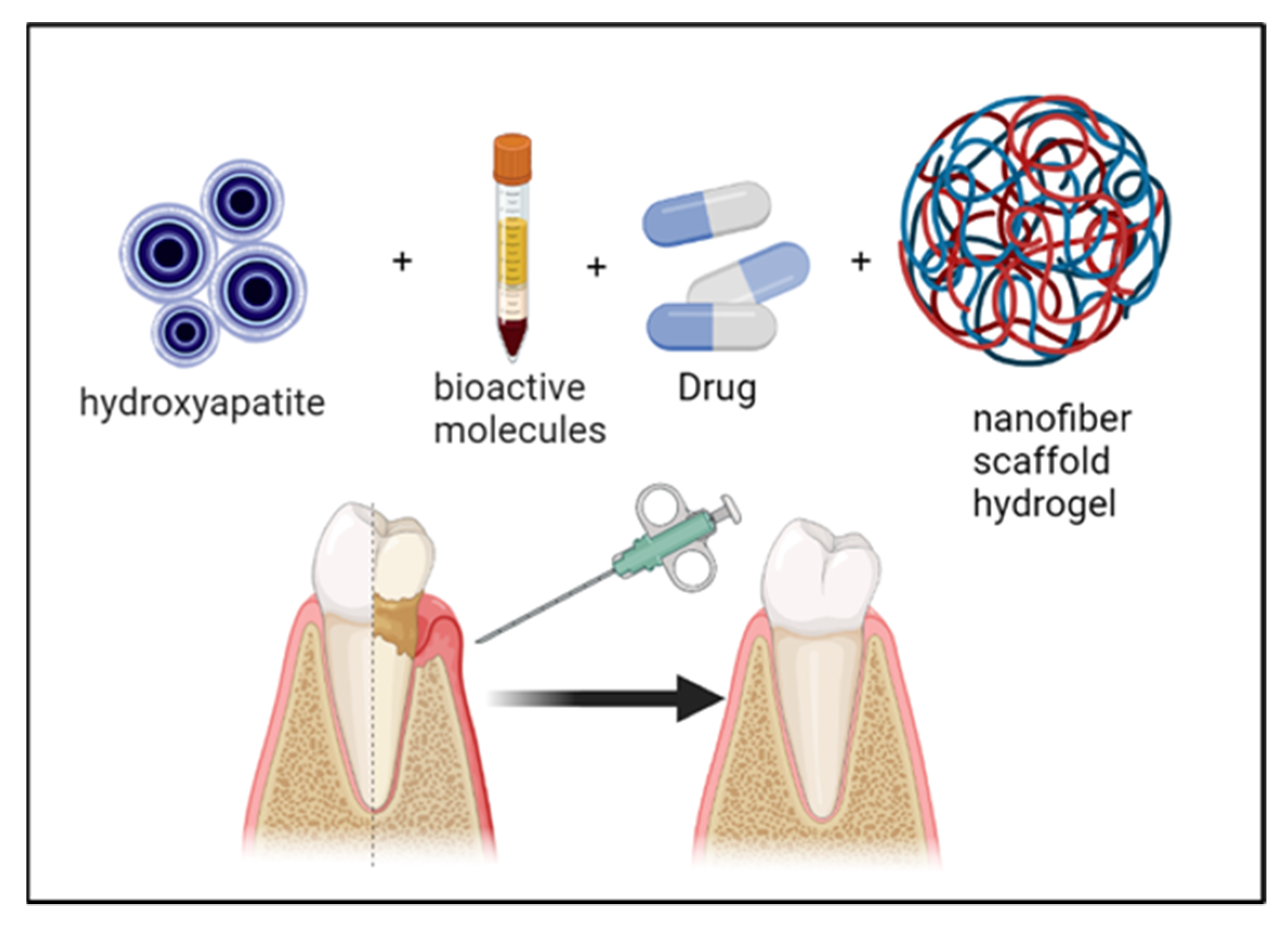

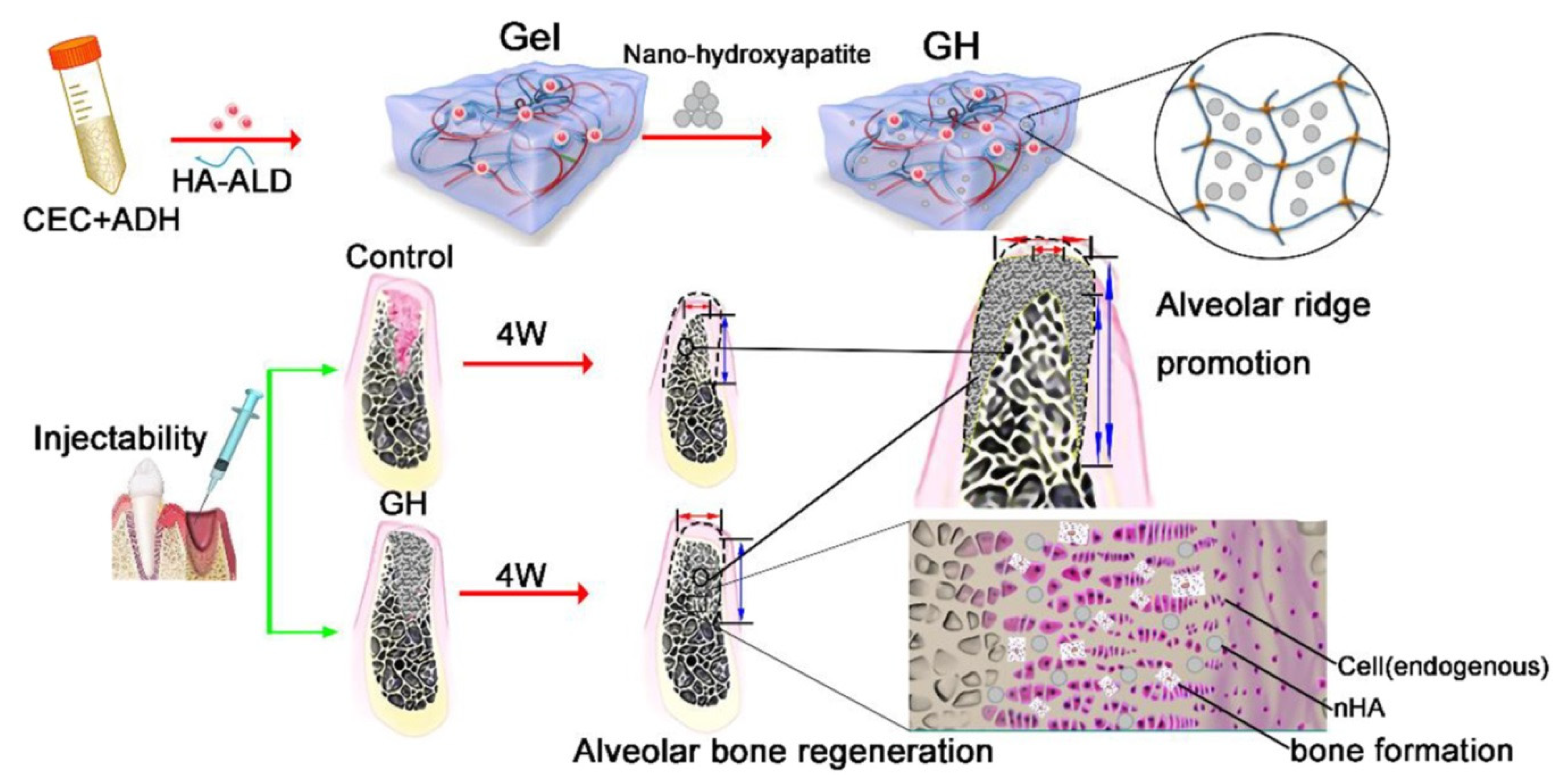

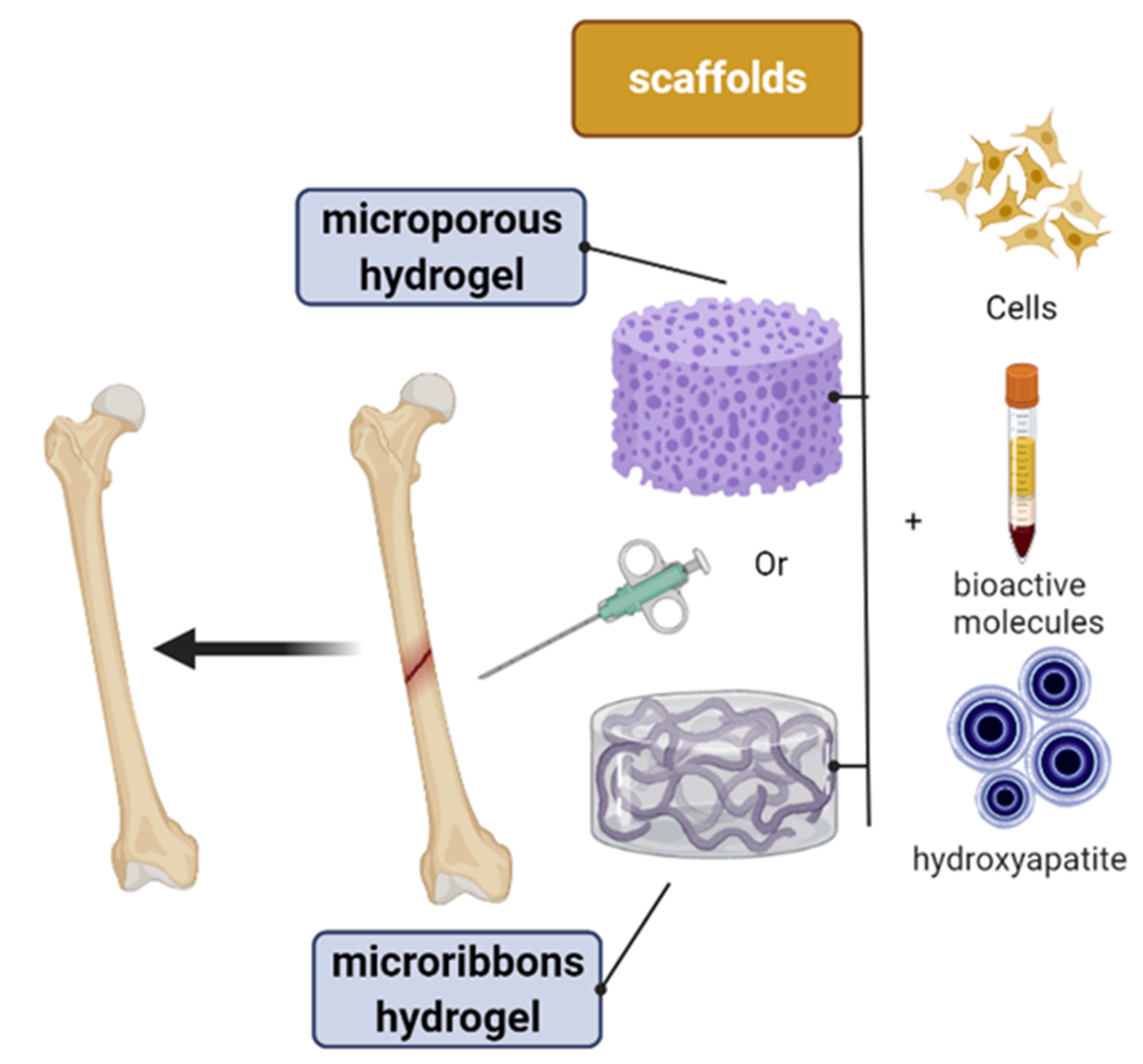
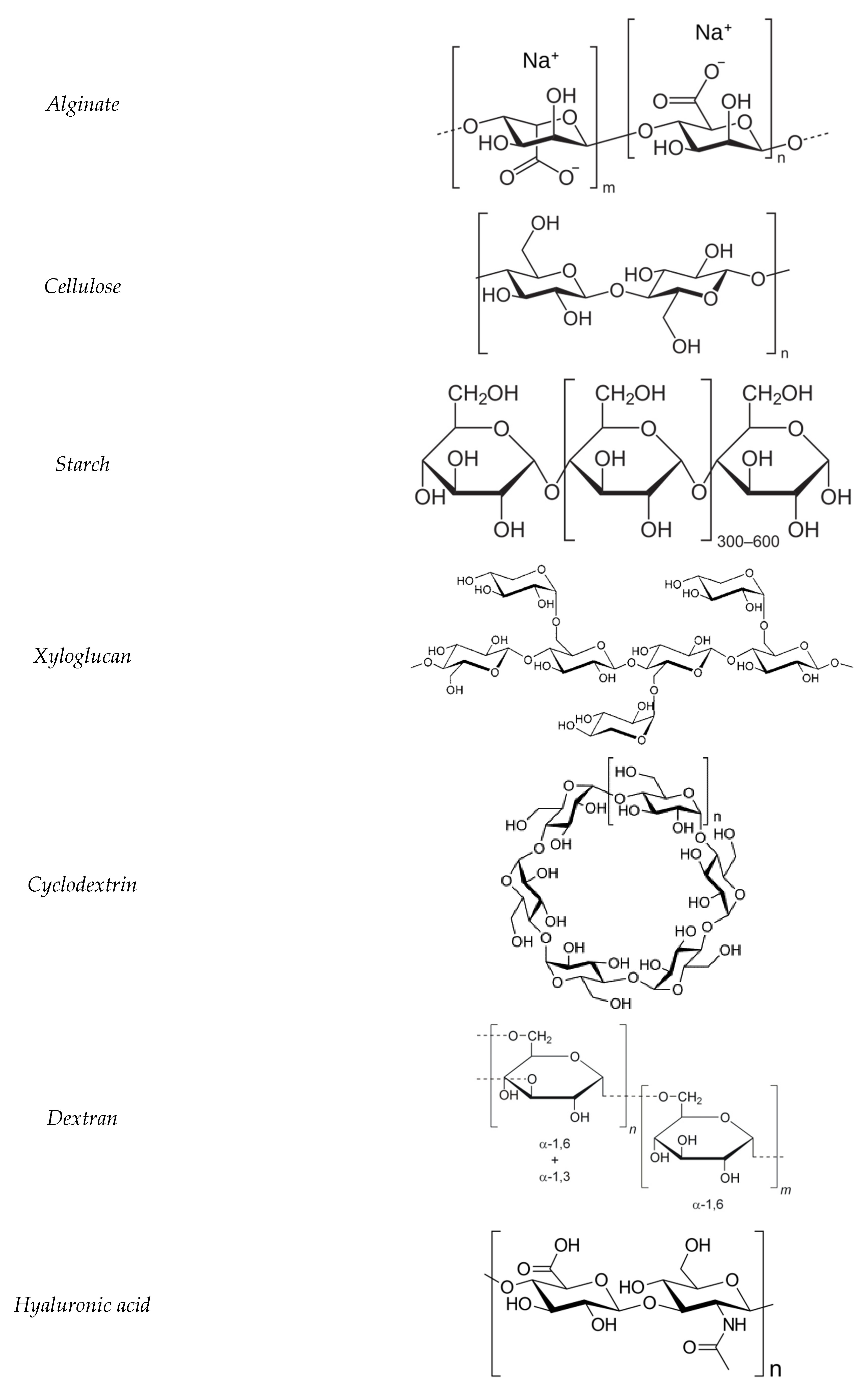

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhursani, S.A.; Ghobashy, M.M.; Al-Gahtany, S.A.; Meganid, A.S.; Abd El-Halim, S.M.; Ahmad, Z.; Khan, F.S.; Atia, G.A.N.; Cavalu, S. Application of Nano-Inspired Scaffolds-Based Biopolymer Hydrogel for Bone and Periodontal Tissue Regeneration. Polymers 2022, 14, 3791. https://doi.org/10.3390/polym14183791
Alkhursani SA, Ghobashy MM, Al-Gahtany SA, Meganid AS, Abd El-Halim SM, Ahmad Z, Khan FS, Atia GAN, Cavalu S. Application of Nano-Inspired Scaffolds-Based Biopolymer Hydrogel for Bone and Periodontal Tissue Regeneration. Polymers. 2022; 14(18):3791. https://doi.org/10.3390/polym14183791
Chicago/Turabian StyleAlkhursani, Sheikha A., Mohamed Mohamady Ghobashy, Samera Ali Al-Gahtany, Abeer S. Meganid, Shady M. Abd El-Halim, Zubair Ahmad, Farhat S. Khan, Gamal Abdel Nasser Atia, and Simona Cavalu. 2022. "Application of Nano-Inspired Scaffolds-Based Biopolymer Hydrogel for Bone and Periodontal Tissue Regeneration" Polymers 14, no. 18: 3791. https://doi.org/10.3390/polym14183791
APA StyleAlkhursani, S. A., Ghobashy, M. M., Al-Gahtany, S. A., Meganid, A. S., Abd El-Halim, S. M., Ahmad, Z., Khan, F. S., Atia, G. A. N., & Cavalu, S. (2022). Application of Nano-Inspired Scaffolds-Based Biopolymer Hydrogel for Bone and Periodontal Tissue Regeneration. Polymers, 14(18), 3791. https://doi.org/10.3390/polym14183791








