A Review on Research Progress in Plasma-Controlled Superwetting Surface Structure and Properties
Abstract
:1. Introduction
2. Low Temperature Plasma Control Technology
2.1. Dielectric Barrier Discharge
2.2. Atmospheric Pressure Plasma Jet
2.3. Glow Discharge
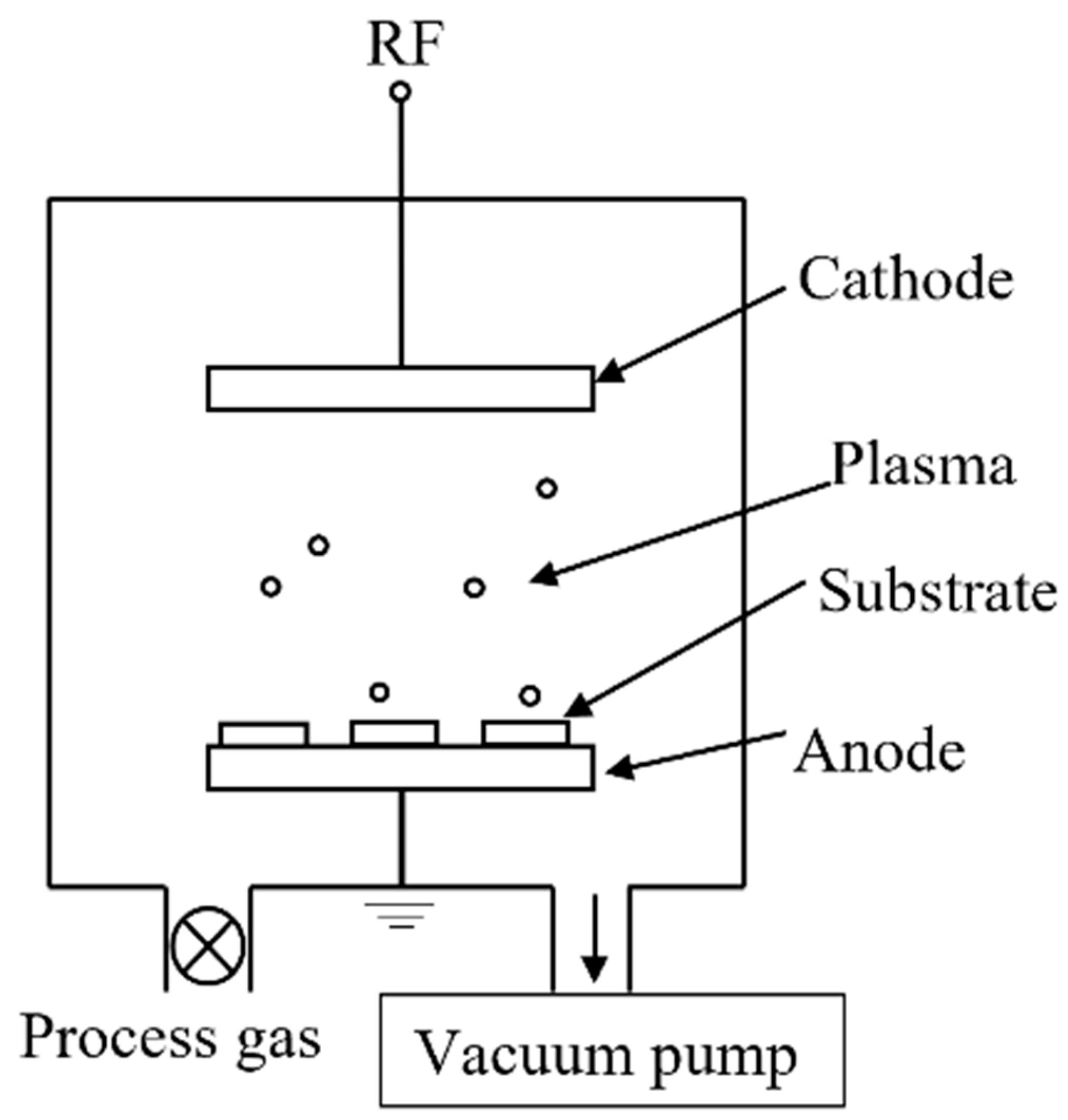
2.4. Radio Frequency Plasma Discharge
2.5. Inductively Coupled Plasma
3. Results and Discussion
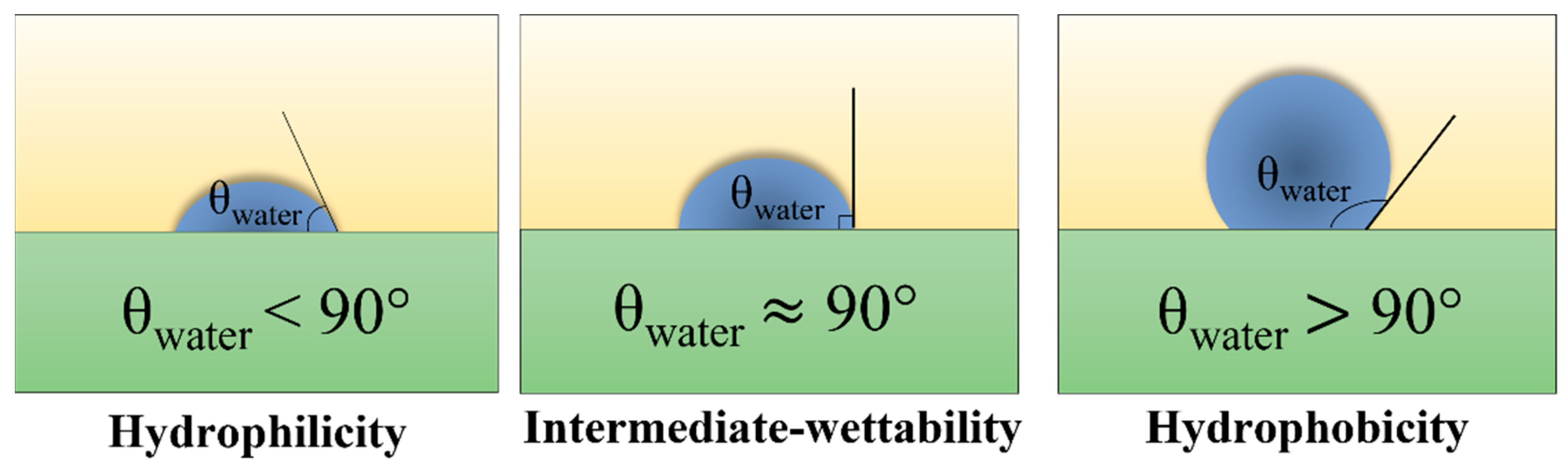
3.1. Modification Mechanism of Superwetting Surface
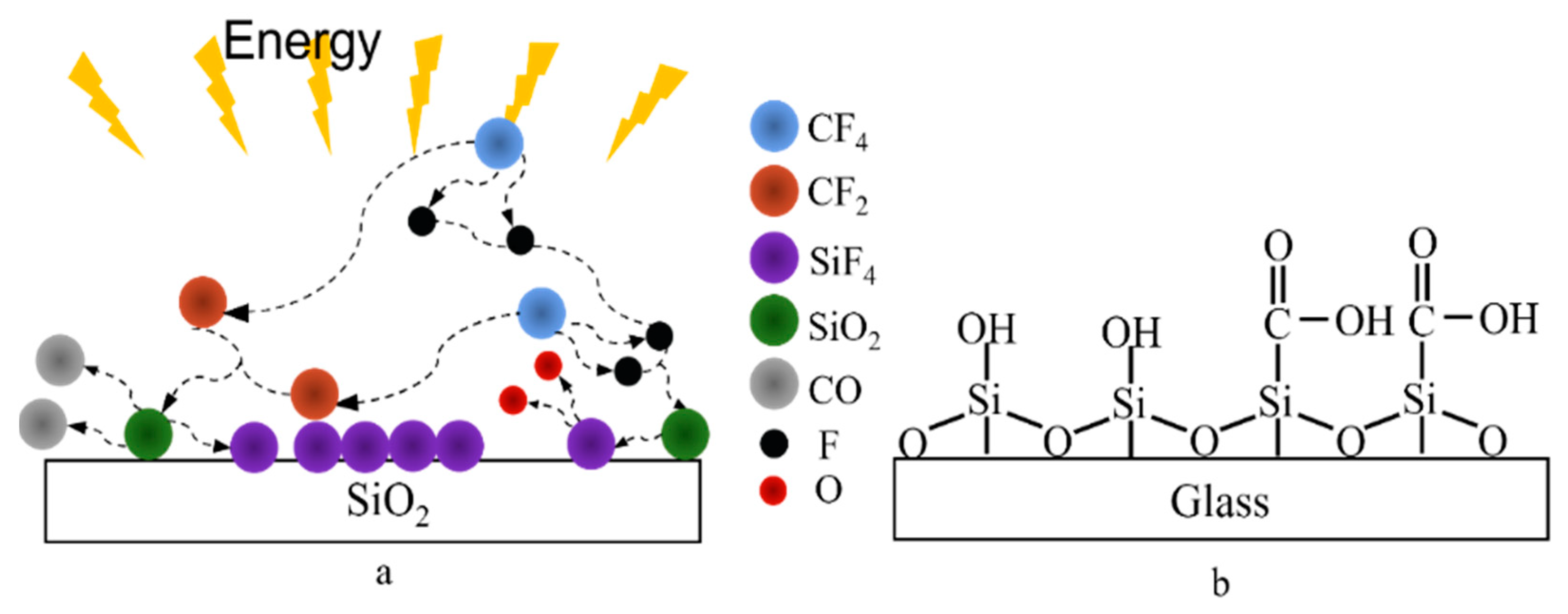
3.1.1. Changes in Surface Chemical Composition
3.1.2. Changes in Surface Roughness
3.2. Modification Method of Superwetting Structure on Surface of Different Materials by Plasma
3.2.1. Textile Fiber Surface
3.2.2. Porous Material Surface
3.2.3. Porous Material Surface
3.2.4. Wood Surface
3.2.5. Glass Surface
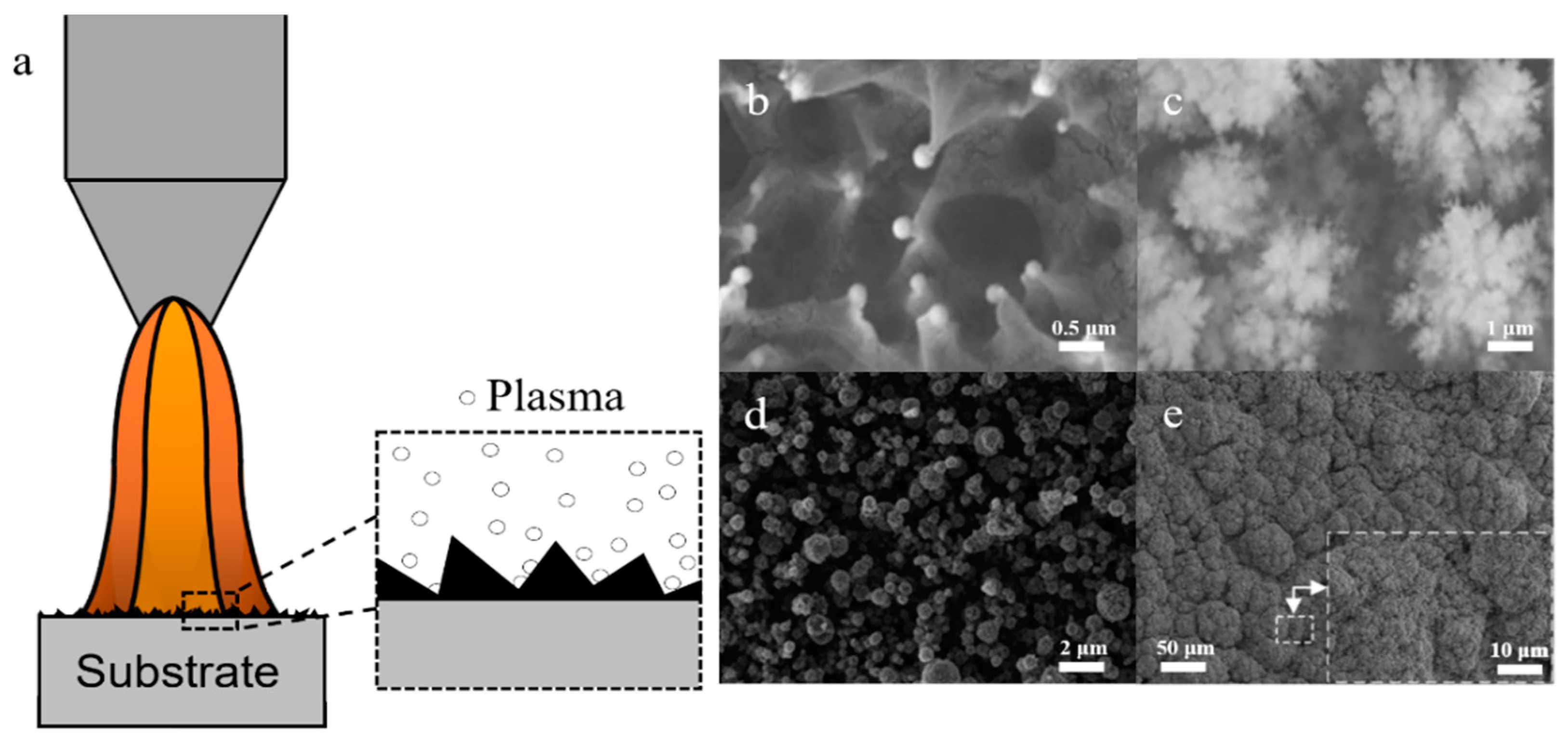
3.2.6. Particle/Powder Surface
4. Application of Superwetting Materials
4.1. Oil-Water Separation
4.2. Ice Prevention
4.3. Self-Cleaning Antifouling Performance
4.4. Biological Field
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anupriyanka, T.; Shanmugavelayutham, G.; Sarma, B.; Mariammal, M. A single step approach of fabricating superhydrophobic PET fabric by using low pressure plasma for oil-water separation. Colloids Surf. A 2020, 600, 124949. [Google Scholar] [CrossRef]
- Chen, T.C.; Cai, M.L.; Xu, H.; Zhou, L.P.; Zhang, J.L.; Hu, N.N. Fabrication of superwetting, anti-icing nickel-cobalt carbonate hydroxide coated-aluminosilicate fiber paper for oil-water separation. Colloids Surf. A 2021, 627, 127002. [Google Scholar]
- Yuan, X.; Du, Y.; Su, J. Approaches and potentials for pool boiling enhancement with superhigh heat flux on responsive smart surfaces: A critical review. Renew. Sustain. Energy Rev. 2021, 156, 111974. [Google Scholar] [CrossRef]
- Montes, L.; Román, J.M.; García-Casas, X.; Castillo-Seoane, J.; Sánchez-Valencia, J.R.; Barranco, Á.; López-Santos, C.; Borrás, A. Plasma-Assisted Deposition of TiO2 3D Nanomembranes: Selective Wetting, Superomniphobicity, and Self-Cleaning. Adv. Mater. Interf. 2021, 8, 2100767. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Q.; Luo, Y.; Murad, M.S.; Zhu, L.; Mu, G. Improved packing performance and structure-stability of casein edible films by dielectric barrier discharges (DBD) cold plasma. Food Packag. Shelf Life 2020, 24, 100471. [Google Scholar] [CrossRef]
- Vijayan, V.M.; Tucker, B.S.; Baker, P.A.; Vohra, Y.K.; Thomas, V. Non-equilibrium hybrid organic plasma processing for superhydrophobic PTFE surface towards potential bio-interface applications. Colloids Surf. B Biointerfaces 2019, 183, 110463. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, G.; Wang, M.; Zhang, Y.; Yin, W.; He, Q. A simple preparation method for superhydrophobic surface on silicon rubber and its properties. Prog. Org. Coat. 2020, 143, 105612. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.; Zhang, D.; Ou, J. Effect of surface nanostructure on enhanced atmospheric corrosion resistance of a superhydrophobic surface. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 647, 129058. [Google Scholar] [CrossRef]
- Al-Shatty, W.; Hill, D.A.; Kiani, S.; Stanulis, A.; Winston, S.; Powner, I.; Alexander, S.; Barron, A.R. Superhydrophilic surface modification of fabric via coating with cysteic acid mineral oxide. Appl. Surf. Sci. 2021, 580, 152306. [Google Scholar] [CrossRef]
- He, H.; Zhang, T.C.; Li, Z.; Liang, Y.; Yuan, S. Superhydrophilic fish-scale-like CuC2O4 nanosheets wrapped copper mesh with underwater super oil-repellent properties for effective separation of oil-in-water emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127133. [Google Scholar] [CrossRef]
- Lin, X.; Heo, J.; Choi, M.; Hong, J. Simply realizing durable dual Janus superwettable membranes integrating underwater low-oil-adhesive with super-water-repellent surfaces for controlled oil–water permeation. J. Membr. Sci. 2019, 580, 248–255. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Yang, J.; Yue, Y.; Zhang, H. Fabrication of superhydrophobic surface on stainless steel by two-step chemical etching. Chem. Phys. Lett. 2022, 797, 139567. [Google Scholar] [CrossRef]
- Charoenyuenyao, P.; Chaleawpong, R.; Borwornpornmetee, N.; Paosawatyanyong, B.; Sittimart, P.; Yoshitake, T.; Promros, N. Investigation of morphological surface features, wetting behavior and mechanical traits under various substrate temperatures for beta iron disilicide prepared via facing-targets sputtering. Mater. Sci. Semicond. Process. 2022, 146, 106604. [Google Scholar] [CrossRef]
- Lei, Z.; Xin, C.; Yang, J.; Zhang, L.; Hao, S.; Min, L.; Yusu, W.; Xin, W. The denitration mechanism of fly ash catalysts prepared by low-temperature plasma technology. Vacuum 2020, 181, 109695. [Google Scholar] [CrossRef]
- Kim, J.; Hwang, J.H.; Kwon, Y.W.; Bae, H.W.; An, M.; Lee, W.; Lee, D. Hydrogen-assisted low-temperature plasma-enhanced chemical vapor deposition of thin film encapsulation layers for top-emission organic light-emitting diodes. Org. Electron. 2021, 97, 106261. [Google Scholar] [CrossRef]
- Leszczynski, S.; Strobel, C.; Leszczynska, B.; Waurenschk, S.; Röhlecke, S.; Stahr, F.; Albert, M.; Bartha, J.W. Dynamic deposition system for fabrication of amorphous/crystalline silicon heterojunction solar cells combining linear hot-wire and plasma enhanced chemical vapor deposition methods. Thin Solid Films 2022, 754, 139296. [Google Scholar] [CrossRef]
- Sarapirom, S.; Yu, L. Low-pressure and atmospheric plasma treatments of sunflower seeds. Surf. Coatings Technol. 2020, 406, 126638. [Google Scholar] [CrossRef]
- Takenaka, K.; Machida, R.; Bono, T.; Jinda, A.; Toko, S.; Uchida, G.; Setsuhara, Y. Development of a non-thermal atmospheric pressure plasma-assisted technology for the direct joining of metals with dissimilar materials. J. Manuf. Process. 2022, 75, 664–669. [Google Scholar] [CrossRef]
- Haye, E.; Job, N.; Wang, Y.; Penninckx, S.; Stergiopoulos, V.; Tumanov, N.; Cardinal, M.; Busby, Y.; Colomer, J.-F.; Su, B.-L.; et al. ZnO/Carbon xerogel photocatalysts by low-pressure plasma treatment, the role of the carbon substrate and its plasma functionalization. J. Colloid Interface Sci. 2020, 570, 312–321. [Google Scholar] [CrossRef]
- Chang, F.; Nishijima, D.; Tynan, G. Rough-surface effect on sputtering of Cr bombarded by low-energy He plasma. Nucl. Mater. Energy 2021, 29, 101077. [Google Scholar] [CrossRef]
- Ma, X.; Mao, Z.; Xu, D.; Ding, Y.; Xu, C. High-rate synthesis of SiCN films using single-source silicon precursor with high-density helicon plasma. Vacuum 2020, 177, 109397. [Google Scholar] [CrossRef]
- Surucu, S.; Sasmazel, H.T. DBD atmospheric plasma-modified, electrospun, layer-by-layer polymeric scaffolds for L929 fibroblast cell cultivation. J. Biomater. Sci. Polym. Ed. 2015, 27, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Lerdprom, W.; Grasso, S.; Jayaseelan, D.D.; Reece, M.J.; Lee, W. Densification behaviour and physico-mechanical properties of porcelains prepared using spark plasma sintering. Adv. Appl. Ceram. 2017, 116, 307–315. [Google Scholar] [CrossRef]
- Niu, G.; Knodel, A.; Burhenn, S.; Brandt, S.; Franzke, J. Review: Miniature dielectric barrier discharge (DBD) in analytical atomic spectrometry. Anal. Chim. Acta 2020, 1147, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Trinh, Q.H.; Nguyen, D.B.; Sudhakaran, M.; Mok, Y.S. Formation of plasma-polymerized superhydrophobic coating using an atmospheric-pressure plasma jet. Thin Solid Films 2019, 675, 34–42. [Google Scholar] [CrossRef]
- Lin, L.; Rui, L.; Li, C.; Liu, Q.; Li, S.; Xia, Y.; Hu, H.; Yang, W.; Xu, H. Study on CO2-based plasmas for surface modification of polytetrafluoroethylene and the wettability effects. J. CO2 Util. 2021, 53, 101752. [Google Scholar] [CrossRef]
- Matsusaka, S. Control of particle charge by atmospheric pressure plasma jet (APPJ): A review. Adv. Powder Technol. 2019, 30, 2851–2858. [Google Scholar] [CrossRef]
- Chen, F.; Liu, S.; Liu, J.; Huang, S.; Xia, G.; Song, J.; Xu, W.; Sun, J.; Liu, X. Surface modification of tube inner wall by transferred atmospheric pressure plasma. Appl. Surf. Sci. 2016, 389, 967–976. [Google Scholar] [CrossRef]
- Chen, F.; Liu, J.; Cui, Y.; Huang, S.; Song, J.; Sun, J.; Xu, W.; Liu, X. Stability of plasma treated superhydrophobic surfaces under different ambient conditions. J. Colloid Interface Sci. 2016, 470, 221–228. [Google Scholar] [CrossRef]
- Winchester, M.R.; Payling, R. Radio-frequency glow discharge spectrometry:: A critical review. Spectrochim. Acta Part B At. Spectrosc. 2004, 59, 607–666. [Google Scholar] [CrossRef]
- Morelli, A.; Hawker, M.J. Utilizing Radio Frequency Plasma Treatment to Modify Polymeric Materials for Biomedical Applications. ACS Biomater. Sci. Eng. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dai, D.; Wu, X. Fabrication of superhydrophobic surfaces on aluminum. Appl. Surf. Sci. 2008, 254, 5599–5601. [Google Scholar] [CrossRef]
- Banura, S.; Thirumdas, R.; Kaur, A.; Deshmukh, R.; Annapure, U. Modification of starch using low pressure radio frequency air plasma. LWT 2018, 89, 719–724. [Google Scholar] [CrossRef]
- Lim, T.; Ju, S. Control of graphene surface wettability by using CF 4 plasma. Surf. Coatings Technol. 2017, 328, 89–93. [Google Scholar] [CrossRef]
- Gürsoy, M.; Karaman, M. Hydrophobic coating of expanded perlite particles by plasma polymerization. Chem. Eng. J. 2016, 284, 343–350. [Google Scholar] [CrossRef]
- Lei, M.K.; Liu, Y.; Li, Y.P. Controllable wettability of poly (ethylene terephthlate) film modified by oxygen combined inductively and capacitively coupled radio-frequency plasma. Appl. Surf.Sci. 2011, 257, 7350–7358. [Google Scholar] [CrossRef]
- Le, T.; Singto, S.; Sajomsang, W.; Mongkolnavin, R.; Nuisin, R.; Painmanakul, P.; Sairiam, S. Hydrophobic PVDF hollow fiber membrane modified with pulse inductively coupling plasma activation and chloroalkylsilanes for efficient dye wastewater treatment by ozonation membrane contactor. J. Membr. Sci. 2021, 635, 119443. [Google Scholar]
- Paras; Kumar, A. Smart bioinspired anti-wetted surfaces: Perspectives, fabrication, stability and applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100139. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, W.; Sarshar, M.A.; Choi, C. Generalized models for advancing and receding contact angles of fakir droplets on pillared and pored surfaces. J. Colloid. Interf. Sci. 2019, 552, 359–371. [Google Scholar] [CrossRef]
- Zhu, B.; Ou, R.; Liu, J.; Yang, Y.; Chen, S.; Wei, G.; Zhang, Z. Fabrication of superhydrophobic surfaces with hierarchical structure and their corrosion resistance and self-cleaning properties. Surf. Interf. 2021, 28, 101608. [Google Scholar] [CrossRef]
- Ding, F.; Gao, M. Pore wettability for enhanced oil recovery, contaminant adsorption and oil/water separation: A review. Adv. Colloid Interf. 2021, 289, 102377. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Y.; Yan, W.; Wang, K.; Zhou, Y.; Gao, C. Amphiphobic polytetrafluoroethylene membrane with a ring-on-string-like micro/nano structure for air purification. J. Membr. Sci. 2022, 652, 120476. [Google Scholar] [CrossRef]
- Yonemoto, Y.; Tomimitsu, I.; Shimizu, K.; Kunugi, T. Wettability model for water-ethanol binary mixture droplet on roughened low-surface-energy solids. Int. J. Multiph. Flow 2021, 137, 103569. [Google Scholar] [CrossRef]
- Knizikevičius, R.; Kopustinskas, V. Influence of temperature on the etching rate of SiO2 in CF4+O2 plasma. Microelectron. Eng. 2006, 83, 193–196. [Google Scholar] [CrossRef]
- Li, D.; Xiong, M.; Wang, S.; Chen, X.; Wang, S.; Zeng, Q. Effects of low-temperature plasma treatment on wettability of glass surface: Molecular dynamic simulation and experimental study. Appl. Surf. Sci. 2019, 503, 144257. [Google Scholar] [CrossRef]
- Kang, W.S.; Hur, M.; Lee, J.-O.; Song, Y.-H. Controlling hydrophilicity of polymer film by altering gas flow rate in atmospheric-pressure homogeneous plasma. Appl. Surf. Sci. 2014, 295, 198–202. [Google Scholar] [CrossRef]
- Peng, S.; Ma, Y. Fabrication of hydrophilic and oil-repellent surface via CF4 plasma treatment. Mater. Des. 2018, 139, 293–297. [Google Scholar] [CrossRef]
- Sarshar, M.A.; Jiang, Y.; Xu, W.; Choi, C. Depinning force of a receding droplet on pillared superhydrophobic surfaces: Analytical models. J. Colloid. Interf. Sci. 2019, 543, 122–129. [Google Scholar] [CrossRef]
- Bormashenko, E. Young, Boruvka–Neumann, Wenzel and Cassie–Baxter equations as the transversality conditions for the variational problem of wetting. Colloids Surf. A 2009, 345, 163–165. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, K.; Park, J.; Hwang, B.G.; Ko, Y.; Kim, H.; Han, J.; Seo, E.; Park, Y.; Lee, S.J. Nearly Perfect Durable Superhydrophobic Surfaces Fabricated by a Simple One-Step Plasma Treatment. Sci. Rep. 2017, 7, 1981. [Google Scholar] [CrossRef]
- Asadollahi, S.; Profili, J.; Farzaneh, M.; Stafford, L. Multi-pass deposition of organosilicon-based superhydrophobic coatings in atmospheric pressure plasma jets. Thin Solid Films 2020, 714, 138369. [Google Scholar] [CrossRef]
- Uricchio, A.; Nadal, E.; Plujat, B.; Plantard, G.; Massines, F.; Fanelli, F. Low-temperature atmospheric pressure plasma deposition of TiO2-based nanocomposite coatings on open-cell polymer foams for photocatalytic water treatment. Appl. Surf. Sci. 2021, 561, 150014. [Google Scholar] [CrossRef]
- Xu, P.Y.; Coyle, T.W.; Pershin, L.; Mostaghimi, J. Fabrication of micro-/nano-structured superhydrophobic ceramic coating with reversible wettability via a novel solution precursor vacuum plasma spray process. Mater. Design 2018, 160, 974–984. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Evaluation of atmospheric-pressure plasma parameters to achieve superhydrophobic and self-cleaning HTV silicone rubber surfaces via a single-step, eco-friendly approach. Surf. Coat. Technol. 2019, 375, 100–111. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Shao, T.; Xie, Q.; Xu, J.; Yang, W. Hydrophobic treatment on polymethylmethacrylate surface by nanosecond-pulse DBDs in CF4 at atmospheric pressure. Appl. Surf. Sci. 2014, 311, 468–477. [Google Scholar] [CrossRef]
- Ullah, M.; Akther, H.; Rahman, M.; Foisal, A.; Hasan, M.; Zumahi, S.A.-A.; Amri, A. Surface modification and improvements of wicking properties and dyeability of grey jute-cotton blended fabrics using low-pressure glow discharge air plasma. Heliyon 2021, 7, e07893. [Google Scholar] [CrossRef]
- Gao, M.; Wang, Y.; Zhang, Y.; Li, Y.; Tang, Y.; Huang, Y. Deposition of thin films on glass fiber fabrics by atmospheric pressure plasma jet. Surf. Coatings Technol. 2020, 404, 126498. [Google Scholar] [CrossRef]
- Bigan, M.; Mutel, B. Cold remote plasma modification of wood: Optimization process using experimental design. Appl. Surf. Sci. 2018, 453, 423–435. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, M.; Zong, Y.; Li, Z. Modification of fabric via co-grafted with fluorine-free carbene polymer and its hydrophobicity. Polymer 2022, 247, 124802. [Google Scholar] [CrossRef]
- Vaswani, S.; Koskinen, J.; Hess, D.W. Surface modification of paper and cellulose by plasma-assisted deposition of fluorocarbon films. Surf. Coatings Technol. 2005, 195, 121–129. [Google Scholar] [CrossRef]
- Katouah, H.; El-Metwaly, N.M. Plasma treatment toward electrically conductive and superhydrophobic cotton fibers by in situ preparation of polypyrrole and silver nanoparticles. React. Funct. Polym. 2021, 159, 104810. [Google Scholar] [CrossRef]
- Sohbatzadeh, F.; Shakerinasab, E.; Eshghabadi, M.; Ghasemi, M. Characterization and performance of coupled atmospheric pressure argon plasma jet with n-hexane electrospray for hydrophobic layer coatings on cotton textile. Diam. Relat. Mater. 2018, 91, 34–45. [Google Scholar] [CrossRef]
- Yang, J.; Pu, Y.; He, H.W.; Cao, R.G.; Miao, D.G.; Ning, X. Superhydrophobic cotton nonwoven fabrics through atmospheric plasma treatmentfor applications in self-cleaning and oil–water separation. Cellulose 2019, 26, 7507–7522. [Google Scholar] [CrossRef]
- Asadian, M.; Onyshchenko, I.; Thukkaram, M.; Tabaei, P.S.E.; Van Guyse, J.; Cools, P.; Declercq, H.; Hoogenboom, R.; Morent, R.; De Geyter, N. Effects of a dielectric barrier discharge (DBD) treatment on chitosan/polyethylene oxide nanofibers and their cellular interactions. Carbohydr. Polym. 2018, 201, 402–415. [Google Scholar] [CrossRef]
- Flynn, C.; Byrne, C.; Meenan, B. Surface modification of cellulose via atmospheric pressure plasma processing in air and ammonia–nitrogen gas. Surf. Coatings Technol. 2013, 233, 108–118. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, Z.; Shen, C.; Meng, Q.; Zhang, G.; Gao, C. Porous composite membrane based on organic substrate for molecular sieving: Current status, opportunities and challenges. Adv. Membr. 2022, 2, 100027. [Google Scholar] [CrossRef]
- Asadollahi, S.; Profili, J.; Farzaneh, M.; Stafford, L. Development of Organosilicon-Based Superhydrophobic Coatings through Atmospheric Pressure Plasma Polymerization of HMDSO in Nitrogen Plasma. Materials 2019, 12, 219. [Google Scholar] [CrossRef]
- Alaizoki, A.; Phillips, C.; Parker, D.; Hardwick, C.; McGettrick, J.; Deganello, D. Improvement in liquid absorption of open-cell polymeric foam by plasma treatment for food packaging applications. J. Appl. Polym. Sci. 2021, 139, 52015. [Google Scholar] [CrossRef]
- Volkov, V.V.; Ibragimov, R.G.; Abdullin, I.S.; Gallyamov, R.T.; Ovcharova, A.A.; Bildyukevich, A.V. Modification of polysulfone porous hollow fiber membranes by air plasma treatment. J. Phys. Conf. Ser. 2016, 751, 12028. [Google Scholar] [CrossRef]
- Jiang, L.; Li, S.; Wang, J.; Yang, L.; Sun, Q.; Li, Z. Surface Wettability of Oxygen Plasma Treated Porous Silicon. J. Nanomater. 2014, 2014, 526149. [Google Scholar] [CrossRef]
- Banković, P.; Demarquette, N.; Da Silva, M. Obtention of selective membranes for water and hydrophobic liquids by plasma enhanced chemical vapor deposition on porous substrates. Mater. Sci. Eng. B 2004, 112, 165–170. [Google Scholar] [CrossRef]
- Yang, M.; Li, Q.; Zhang, X.; Bilotti, E.; Zhang, C.; Xu, C.; Gan, S.; Dang, Z.-M. Surface engineering of 2D dielectric polymer films for scalable production of High-Energy-Density films. Prog. Mater. Sci. 2022, 128, 100968. [Google Scholar] [CrossRef]
- Syafutra, H.; Pandey, M.; Kumari, N.; Pandey, S.S.; Benten, H.; Nakamura, M. Assisted alignment of conjugated polymers in floating film transfer method using polymer blend. Thin Solid Films 2021, 734, 138814. [Google Scholar] [CrossRef]
- Ellinas, K.; Smyrnakis, A.; Malainou, A.; Tserepi, A.; Gogolides, E. “Mesh-assisted” colloidal lithography and plasma etching: A route to large-area, uniform, ordered nano-pillar and nanopost fabrication on versatile substrates. Microelectron. Eng. 2011, 88, 2547–2551. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Shi, W.; Lei, M. Hydrophobic over-recovery during aging of polyethylene modified by oxygen capacitively coupled radio frequency plasma: A new approach for stable superhydrophobic surface with high water adhesion. Surf. Coat. Technol. 2012, 206, 4952–4958. [Google Scholar] [CrossRef]
- Vandencasteele, N.; Broze, B.; Collette, S.; De Vos, C.; Viville, P.; Lazzaroni, R.; Reniers, F. Evidence of the Synergetic Role of Charged Species and Atomic Oxygen in the Molecular Etching of PTFE Surfaces for Hydrophobic Surface Synthesis. Langmuir 2010, 26, 16503–16509. [Google Scholar] [CrossRef]
- Liu, K.; Lei, J.; Zheng, Z.; Zhu, Z.; Liu, S. The hydrophilicity improvement of polytetrafluoroethylene by Ar plasma jet: The relationship of hydrophilicity, ambient humidity and plasma parameters. Appl. Surf. Sci. 2018, 458, 183–190. [Google Scholar] [CrossRef]
- Porto, C.L.; Mundo, R.D.; Veronico, V.; Trizio, I.; Barucca, G.; Palumbo, F. Easy plasma nano-texturing of PTFE surface: From pyramid to unusual spherules-on-pyramid features. Appl. Surf. Sci. 2019, 483, 60–68. [Google Scholar] [CrossRef]
- Mundo, R.D.; Bottiglione, F.; Palumbo, F.; Notarnicola, M.; Carbone, G. Filamentary superhydrophobic Teflon surfaces: Moderate apparent contact angle but superior air-retaining properties. J. Colloid Interf. Sci. 2016, 482, 175–182. [Google Scholar] [CrossRef]
- Ellinas, K.; Gogolides, E. Ultra-low friction, superhydrophobic, plasma micro-nanotextured fluorinated ethylene propylene (FEP) surfaces. Micro Nano Eng. 2022, 14, 100104. [Google Scholar] [CrossRef]
- Yoshida, A.; Shoho, S.; Kinoshita, S. Evaluation of reduction effect on thermal load inside and outside of concrete building with wooden decoration by numerical analysis. Energy Procedia 2017, 132, 435–440. [Google Scholar] [CrossRef]
- Niu, K.; Song, K. Hot waxing treatment improves the aging resistance of wood surface under UV radiation and water. Prog. Org. Coat. 2021, 161, 106468. [Google Scholar] [CrossRef]
- Sohbatzadeh, F.; Shabannejad, A.; Ghasemi, M.; Mahmoudsani, Z. Deposition of halogen-free flame retardant and water-repellent coatings on firwood surfaces using the new version of DBD. Prog. Org. Coat. 2020, 151, 106070. [Google Scholar] [CrossRef]
- Profili, J.; Levasseur, O.; Koronai, A.; Stafford, L.; Gherardi, N. Deposition of nanocomposite coatings on wood using cold discharges at atmospheric pressure. Surf. Coat. Technol. 2017, 309, 729–737. [Google Scholar] [CrossRef]
- Xie, L.; Tang, Z.; Jiang, L.; Breedveld, V.; Hess, D.W. Creation of superhydrophobic wood surfaces by plasma etching and thin-film deposition. Surf. Coat. Technol. 2015, 281, 125–132. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, X.; Zhang, X.; Bian, J.; Shi, S.; Nguyen, T.; Chen, M.; Wan, J. Fast enhancement on hydrophobicity of poplar wood surface using low-pressure dielectric barrier discharges (DBD) plasma. Appl. Surf. Sci. 2017, 407, 412–417. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Jin, S.; Jiang, Z.; Wang, B. Reliability and sensitivity analysis of cold-bent curtain wall glass. J. Build. Eng. 2022, 49, 104116. [Google Scholar] [CrossRef]
- Hossain, M.; Trinh, Q.H.; Sudhakaran, M.; Sultana, L.; Mok, Y.S. Improvement of mechanical strength of hydrophobic coating on glass surfaces by an atmospheric pressure plasma jet. Surf. Coat. Technol. 2018, 357, 12–22. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Shao, T.; Fang, Z.; Yan, P. Formation of hydrophobic coating on PMMA surface using unipolar nanosecond-pulse DBD in atmospheric air. J. Electrost. 2013, 71, 435–439. [Google Scholar] [CrossRef]
- Cui, X.; Yan, B.; Zhang, B.; Fang, Z. Improving surface hydrophobicity of glass using an atmospheric pressure plasma jet array in Ar/TMS. Vacuum 2018, 151, 15–24. [Google Scholar] [CrossRef]
- Wang, R.; Shen, Y.; Zhang, C.; Yan, P.; Shao, T. Comparison between helium and argon plasma jets on improving the hydrophilic property of PMMA surface. Appl. Surf. Sci. 2016, 367, 401–406. [Google Scholar] [CrossRef]
- Ng, W.M.; Lim, J.K. Complex interplay between colloidal stability, transport, chemical reactivity and magnetic separability of polyelectrolyte-functionalized nanoscale zero-valent iron particles (nZVI) toward their environmental engineering application. Colloids Interface Sci. Commun. 2022, 46, 100582. [Google Scholar] [CrossRef]
- Kim, S.; Bilgili, E.; Davé, R.N. Impact of altered hydrophobicity and reduced agglomeration on dissolution of micronized poorly water-soluble drug powders after dry coating. Int. J. Pharm. 2021, 606, 120853. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Choi, C.-H. Superhydrophobic Sands for the Preservation and Purification of Water. Coatings 2021, 11, 151. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Huang, J.; Mao, J.; Chen, Z.; Mao, Q.; Ge, M.; Lai, Y. Smart surfaces with reversibly switchable wettability: Concepts, synthesis and applications. Adv. Colloid Interface Sci. 2021, 300, 102584. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R. Plasma treatment allows water suspending of the natural hydrophobic powder (lycopodium). Colloids Surf. B Biointerfaces 2012, 97, 171–174. [Google Scholar] [CrossRef]
- Akhavan, B.; Jarvis, K.; Majewski, P. Tuning the hydrophobicity of plasma polymer coated silica particles. Powder Technol. 2013, 249, 403–411. [Google Scholar] [CrossRef]
- Sardella, E.; Trulli, M.G.; Palumbo, F.; Cosmai, S.; Gristina, R.; Armenise, V.; Favia, P. Plasma deposition of long-lasting hydrophilic coatings on alumina micro-particles. Thin Solid Films 2019, 686, 137410. [Google Scholar] [CrossRef]
- Matsubara, K.; Danno, M.; Inoue, M.; Nishizawa, H.; Honda, Y.; Abe, T. Hydrophobization of polymer particles by tetrafluoromethane (CF4) plasma irradiation using a barrel-plasma-treatment system. Appl. Surf. Sci. 2013, 284, 340–347. [Google Scholar] [CrossRef]
- Abourayana, H.M.; Dobbyn, P.J.; Whyte, P.; Dowling, D.P. Investigation of the performance of a pilot-scale barrel atmospheric plasma system for plasma activation of polymer particles. Nanotechnol. Precis. Eng. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Yu, H.; Gong, L.; Qu, Z.; Hao, P.; Liu, J.; Fu, L. Wettability enhancement of hydrophobic artificial sandstones by using the pulsed microwave plasma jet. Colloids Interface Sci. Commun. 2020, 36, 100266. [Google Scholar] [CrossRef]
- Yan, L.; Li, J.; Li, W.; Zha, F.; Feng, H.; Hu, D. A photo-induced ZnO coated mesh for on-demand oil/water separation based on switchable wettability. Mater. Lett. 2016, 163, 247–249. [Google Scholar] [CrossRef]
- Liao, Y.; Tian, M.; Wang, R. A high-performance and robust membrane with switchable super-wettability for oil/water separation under ultralow pressure. J. Membr. Sci. 2017, 543, 123–132. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Zhao, Z.; He, Z.; Lin, T.; Zhao, Y.; Li, G.; Qu, J.; Huang, L.; Peng, X.; et al. A facile, clean construction of biphilic surface on filter paper via atmospheric air plasma for highly efficient separation of water-in-oil emulsions. Sep. Purif. Technol. 2021, 255, 117672. [Google Scholar] [CrossRef]
- Kim, D.; Mauchauffé, R.; Kim, J.; Moon, S. Publisher Correction: Simultaneous, efficient and continuous oil–water separation via antagonistically functionalized membranes prepared by atmospheric pressure cold plasma. Sci. Rep. 2021, 11, 9782. [Google Scholar] [CrossRef]
- Wei, C.; Jin, B.; Zhang, Q.; Zhan, X.; Chen, F. Anti-icing performance of super-wetting surfaces from icing-resistance to ice-phobic aspects: Robust hydrophobic or slippery surfaces. J. Alloy. Compd. 2018, 765, 721–730. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.-S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-Infused Nanostructured Surfaces with Extreme Anti-Ice and Anti-Frost Performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef]
- Yang, J.; Li, W. Preparation of superhydrophobic surfaces on Al substrates and the anti-icing behavior. J. Alloy. Compd. 2013, 576, 215–219. [Google Scholar] [CrossRef]
- Wang, Z.; Kwon, D.; DeVries, K.L.; Park, J. Frost formation and anti-icing performance of a hydrophobic coating on aluminum. Exp. Therm. Fluid. Sci. 2015, 60, 132–137. [Google Scholar] [CrossRef]
- Piispanen, M.; Hupa, L. Comparison of self-cleaning properties of three titania coatings on float glass. Appl. Surf. Sci. 2011, 258, 1126–1131. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Zhou, P.; Zhu, L.; Tang, H. Superhydrophobic copper coating: Switchable wettability, on-demand oil-water separation, and antifouling. Chem. Eng. J. 2017, 327, 849–854. [Google Scholar] [CrossRef]
- Nanda, D.; Varshney, P.; Satapathy, M.; Mohapatra, S.; Bhushan, B.; Kumar, A. Single step method to fabricate durable superliquiphobic coating on aluminum surface with self-cleaning and anti-fogging properties. J. Colloid Interface Sci. 2017, 507, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Lomga, J.; Varshney, P.; Nanda, D.; Satapathy, M.; Mohapatra, S.; Kumar, A. Fabrication of durable and regenerable superhydrophobic coatings with excellent self-cleaning and anti-fogging properties for aluminium surfaces. J. Alloy. Compd. 2017, 702, 161–170. [Google Scholar] [CrossRef]
- Lobo, A.; Marciano, F.; Ramos, S.; Machado, M.; Corat, E.; Corat, M. Increasing mouse embryonic fibroblast cells adhesion on superhydrophilic vertically aligned carbon nanotube films. Mater. Sci. Eng. C 2011, 31, 1505–1511. [Google Scholar] [CrossRef]
- Antonioli, E.; Lobo, A.O.; Ferretti, M.; Cohen, M.; Marciano, F.R.; Corat, E.J.; Trava-Airoldi, V.J. An evaluation of chondrocyte morphology and gene expression on superhydrophilic vertically-aligned multi-walled carbon nanotube films. Mater. Sci. Eng. C 2012, 33, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Xu, X.; Liu, X.; Sun, T. Super hydrophilic PVDF based composite membrane for efficient separation of tetracycline. Chem. Eng. J. 2017, 308, 151–159. [Google Scholar] [CrossRef]
- Gorjanc, M.; Bukošek, V.; Gorenšek, M.; Vesel, A. The Influence of Water Vapor Plasma Treatment on Specific Properties of Bleached and Mercerized Cotton Fabric. Text. Res. J. 2009, 80, 557–567. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Liu, Y.; Wang, J.; Wang, S. Surface modification of NF membrane with zwitterionic polymer to improve anti-biofouling property. J. Membr. Sci. 2016, 514, 407–417. [Google Scholar] [CrossRef]
- Weng, X.; Ji, Y.; Ma, R.; Zhao, F.; An, Q.; Gao, C. Superhydrophilic and antibacterial zwitterionic polyamide nanofiltration membranes for antibiotics separation. J. Membr. Sci. 2016, 510, 122–130. [Google Scholar] [CrossRef]







| Material | Gas | Plasma Type | Working Conditions | Involves Elements/ Functional Groups | Morphology Change | Water Contact Angle | Ref. |
|---|---|---|---|---|---|---|---|
| Cellulose | C2F5H/Ar | RF | 120 °C, 1 Torr, 30 W, 13.56 MHz | F | No change | 0~104° | [60] |
| Cotton | HDTMS/N2 | Glow discharge | 3 × 10−3 mbar, 400 W, 13.56 MHz | -COO−, O-O− | Submicron particle | 148.2~161.3° | [61] |
| Cotton | n-Hexane/Ar | APPJ | V(DC) = 20 kv | O | Grain size larger | 0~154.7° | [62] |
| Faceted nonwovens | HMDSO/Ar | APP | 101 kPa, 300 V, 19 kHz | Si-C, Si-CH3 | NPs | 155° | [63] |
| Nanofibre pad | N2/He | DBD | 101 kPa, 9 W | N | No change | 57 ± 0.2°~ 10.6 ± 2° | [64] |
| Cellulose | N2/NH3 | DBD | 25 °C, 101 kPa, 1000 W | -NH2, -CONH2 | Roughness increase | 40~22° | [65] |
| Material | Gas | Plasma Type | Working Conditions | Involves Elements/ Functional Groups | Morphology Change | Water Contact Angle | Ref. |
|---|---|---|---|---|---|---|---|
| Porous aluminum | HMDSO/H2 | APPJ | 101.3 kPa, 25 °C, 15–25 kHz | Si-O-Si | Dendritic | <90°~ >150° | [51,67] |
| Porous aluminum | OTS/ Air | Glow discharge | 30–50 kPa, 150–250 W | Si, Cl | Nanopore size | 157.8° | [32] |
| Polystyrenefoam | O2 | Plasma treatment | 240 W, 40 kHz, 0.12 mbar | C=O, O-C=O, O-C(=O)-O | / | 86.0~15.13° | [68] |
| Porous fiber | Air | APP | 101 Kpa | -COOH, -OH | NPs | 81.3~72.5° | [69] |
| Porous silicon | O2 | Plasma treatment | 1 Torr, 10.5 W | Si-OH | No change | 64.5°~<0.5° | [70] |
| Filter paper | HMDSO/n-Hexane | PECVD | 80 W, 500 mToor | C-Hn | Double membrane | 0~141.5° | [71] |
| Material | Gas | Plasma Type | Working Conditions | Involves Elements/ Functional Groups | Morphology Change | Water Contact Angle | Ref. |
|---|---|---|---|---|---|---|---|
| Polyethylene film | O2 | RF | 90 °C, 1 Torr, 200 W | C=O, C-O | Nanostructures | 97.2~152.9° | [75] |
| Polyimide film | He | DBD | 80 kHz, 1.5 kV | C-O, C=O | Roughness increase | >70°~<30° | [46] |
| PTFE | O2 | RF | 6.66 Pa, 13.56 MHz, 20–70 W | O | Alveolar structure | 110~152.8° | [76] |
| PTFE | O2/Ar | RF | 150 W,3 h | / | Coronary structure | 110~178.9° | [50] |
| PTFE | Ar | APPJ | 4.4 kV,1.1 W | O | Roughness reduction | 100~28° | [28] |
| PTFE | Ar | APPJ | 9.6 kHz, 10 ± 2 °C, 26 kV | ·OH | Irregular bulge | 109~37° | [77] |
| Polyurethane foam | Aerosol/He | DBD | 22 kHz, 10–60 min | O | Spherical particle | 155 ± 5°~ 80 ± 2° | [52] |
| PTFE | CO2/N2 | DBD | 6.2 W, 19.4 kHz | C=O | Surface compactness | 140.9~48.6° | [26] |
| PTFE | O2 | CCP | 13.56 MHz, <70 s °C, 13 Pa | Roughness increase | \ | [78] | |
| PTFE | O2 | CCP | 13.56 MHz, 13 Pa | -CF2 | Roughness increase | \ | [79] |
| Ethylene propylene | O2, C4F8 | ICP, RIE | 1900 W, 0.75 Pa; 400 W, 10 mT | F | Roughness increase | 95~168° | [80] |
| Material | Gas | Plasma Type | Working Conditions | Involves Elements/ Functional Groups | Morphology Change | Water Contact Angle | Ref. |
|---|---|---|---|---|---|---|---|
| Chinese fir | HMDSO/Ar | DBD | 95 kHz, 10 kV | Si-C; Si-O-Si | Roughness reduction | <1°~137.7° | [83] |
| Wood | N2/N2O/Vapor crystallization solution of ZnO NPs | DBD | 1 bar, <50 °C | Si-OH | Spherical particle | 40~100° | [84] |
| Wood | O2 | RF | 13.56 MHz, 0.5 Toor | C=C, C-C | Roughness increase | 0~153° | [85] |
| Poplar | HMDSO | DBD | 60 W, 20 KPa, 75 s | Si-O-Si, Si-O-C | Acicular structure | 81~127.7° | [86] |
| Material | Gas | Plasma Type | Working Conditions | Involves Elements/ Functional Groups | Morphology Change | Water Contact Angle | Ref. |
|---|---|---|---|---|---|---|---|
| Organic glass | Air | DBD | 250 MHz, 30 kV | -CH3 | No change | 71~92° | [89] |
| Organic glass | CF4 | DBD | 1 × 105 Pa, 40 kV, 500 Hz, 2.25 W | F | Roughness increase | 68~99° | [55] |
| Glass | Ar/TMS | APPJ | 22 kHz | Si-O, Si-C | Uniformly convex | 67~110.3° | [90] |
| Slide | Aerosol/He | DBD | 22 kHz, 10–60 min | -COO, C-O | Spherical particle | 160~<5° | [52] |
| Organic glass | He | APPJ | 16 kV, 1 kHz | O=C-O, C-OH | Small peak | 27° | [91] |
| Glass | O2 | Low temperature plasma | 298 K | -OH, COOH | Roughness increase | 21.1~2.6° | [45] |
| Material | Gas | Plasma Type | Working Conditions | Involves Elements/ Functional Groups | Morphology Change | Water Contact Angle | Ref. |
|---|---|---|---|---|---|---|---|
| Clubmosses | O2/N2 | ICP | 10 MHz, 20 W, 0.8–40 Pa | O | No change | 140~60° | [96] |
| SiO2 | ppOD | ICP | 13.56 MHz, 0.7 Pa, 4–80 W | -CH− | Spherical particle | 37~>90° | [97] |
| Al2O3 | CO2/H2 | Plasma deposition | 13.56 MHz, 80 Pa | -OH, COOH | Clusters particles | 46~74° | [98] |
| PMMA | CF4 | RF | 10 Pa, 250 kHz | F | Small hills | 115.6~150.6° | [99] |
| PP | He | APP | 20 KHz, <28.9 °C | O-CO-O | / | 99~69° | [100] |
| Artificial sandstone | Air | microwave plasma | 25 °C, 1 kHz, 500 W | Si-OH | / | 123.6~58.5° | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Xu, K.; Zhang, Y. A Review on Research Progress in Plasma-Controlled Superwetting Surface Structure and Properties. Polymers 2022, 14, 3759. https://doi.org/10.3390/polym14183759
Li D, Xu K, Zhang Y. A Review on Research Progress in Plasma-Controlled Superwetting Surface Structure and Properties. Polymers. 2022; 14(18):3759. https://doi.org/10.3390/polym14183759
Chicago/Turabian StyleLi, Dayu, Kai Xu, and Yanjun Zhang. 2022. "A Review on Research Progress in Plasma-Controlled Superwetting Surface Structure and Properties" Polymers 14, no. 18: 3759. https://doi.org/10.3390/polym14183759
APA StyleLi, D., Xu, K., & Zhang, Y. (2022). A Review on Research Progress in Plasma-Controlled Superwetting Surface Structure and Properties. Polymers, 14(18), 3759. https://doi.org/10.3390/polym14183759






